Compare Iron-Air with Alkaline Air: Discharge Profile
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Battery Technology Background and Objectives
Battery technology has evolved significantly over the past century, with recent decades witnessing accelerated innovation in response to growing demands for sustainable energy storage solutions. Metal-air batteries have emerged as promising candidates for next-generation energy storage due to their theoretical high energy densities and potential cost advantages. Among these, Iron-Air and Alkaline Air batteries represent two distinct approaches that have garnered substantial research interest for grid-scale and potentially mobile applications.
The development trajectory of metal-air batteries dates back to the early 20th century, with zinc-air systems being the first commercially viable metal-air technology. However, the exploration of iron-air chemistry began in earnest during the 1970s energy crisis, while alkaline air systems saw significant development in the 1990s as researchers sought alternatives to acidic electrolytes. The technical evolution of these systems has accelerated dramatically in the past decade, driven by advances in materials science, electrochemistry, and computational modeling.
Iron-Air batteries leverage the redox reaction between iron and oxygen, offering theoretical energy densities of approximately 1,200 Wh/kg. These systems have attracted renewed attention due to iron's abundance, low cost, and environmental benignity. Conversely, Alkaline Air batteries typically employ various metals (including zinc, aluminum, or magnesium) in alkaline electrolytes, with theoretical energy densities ranging from 800-1,600 Wh/kg depending on the metal employed.
The discharge profile characteristics of these battery technologies represent a critical aspect of their performance evaluation and potential application domains. Understanding the differences in voltage stability, capacity utilization, rate capability, and cycle life between Iron-Air and Alkaline Air systems is essential for determining their suitability for various energy storage applications.
The primary technical objective of this research is to conduct a comprehensive comparative analysis of the discharge profiles between Iron-Air and Alkaline Air batteries, examining factors including discharge voltage plateaus, capacity retention, power density capabilities, and response to varying discharge rates. This analysis aims to identify the fundamental electrochemical processes governing discharge behavior, quantify performance differences, and establish correlations between material properties and discharge characteristics.
Additionally, this investigation seeks to evaluate how recent innovations in electrode structures, electrolyte formulations, and catalyst designs have influenced discharge performance in both systems. The ultimate goal is to provide actionable insights that can guide future research directions, accelerate technological maturation, and inform strategic decisions regarding the development and deployment of these promising battery technologies in renewable energy integration and other high-impact applications.
The development trajectory of metal-air batteries dates back to the early 20th century, with zinc-air systems being the first commercially viable metal-air technology. However, the exploration of iron-air chemistry began in earnest during the 1970s energy crisis, while alkaline air systems saw significant development in the 1990s as researchers sought alternatives to acidic electrolytes. The technical evolution of these systems has accelerated dramatically in the past decade, driven by advances in materials science, electrochemistry, and computational modeling.
Iron-Air batteries leverage the redox reaction between iron and oxygen, offering theoretical energy densities of approximately 1,200 Wh/kg. These systems have attracted renewed attention due to iron's abundance, low cost, and environmental benignity. Conversely, Alkaline Air batteries typically employ various metals (including zinc, aluminum, or magnesium) in alkaline electrolytes, with theoretical energy densities ranging from 800-1,600 Wh/kg depending on the metal employed.
The discharge profile characteristics of these battery technologies represent a critical aspect of their performance evaluation and potential application domains. Understanding the differences in voltage stability, capacity utilization, rate capability, and cycle life between Iron-Air and Alkaline Air systems is essential for determining their suitability for various energy storage applications.
The primary technical objective of this research is to conduct a comprehensive comparative analysis of the discharge profiles between Iron-Air and Alkaline Air batteries, examining factors including discharge voltage plateaus, capacity retention, power density capabilities, and response to varying discharge rates. This analysis aims to identify the fundamental electrochemical processes governing discharge behavior, quantify performance differences, and establish correlations between material properties and discharge characteristics.
Additionally, this investigation seeks to evaluate how recent innovations in electrode structures, electrolyte formulations, and catalyst designs have influenced discharge performance in both systems. The ultimate goal is to provide actionable insights that can guide future research directions, accelerate technological maturation, and inform strategic decisions regarding the development and deployment of these promising battery technologies in renewable energy integration and other high-impact applications.
Market Analysis for Metal-Air Battery Systems
The metal-air battery market is experiencing significant growth, projected to reach $932 million by 2027, with a compound annual growth rate of 27.8% from 2020. This expansion is primarily driven by increasing demand for renewable energy storage solutions and the push for cleaner transportation alternatives. Metal-air batteries, particularly iron-air and alkaline-air variants, are positioned as promising technologies due to their high theoretical energy densities and use of abundant materials.
Iron-air batteries have garnered substantial commercial interest, with major investments from companies like Form Energy, which secured $450 million in funding for grid-scale iron-air battery development. Their market potential is particularly strong in stationary energy storage applications, where energy density by weight is less critical than cost per kilowatt-hour. Current market pricing indicates iron-air systems can potentially achieve costs below $20/kWh, significantly undercutting lithium-ion alternatives.
Alkaline-air batteries, while less commercially developed, are finding niche applications in sectors requiring moderate energy density and lower cost than lithium-based systems. The market for these batteries is growing in portable electronics and backup power systems, with companies like Fluidic Energy and Phinergy leading development efforts. The global market share for alkaline-air systems remains under 5% of the total metal-air battery market but shows promising growth potential.
Regional market analysis reveals Asia-Pacific dominates metal-air battery manufacturing, accounting for approximately 62% of global production capacity. China leads with substantial government support for metal-air technology development, while North America shows increasing adoption rates in grid storage applications, particularly for iron-air systems. Europe follows with strong research initiatives but more limited commercial deployment.
Consumer electronics currently represent the largest application segment for metal-air batteries at 43% market share, followed by electric vehicles (27%) and grid storage (18%). However, the fastest growth is projected in the grid storage sector, with a 34.5% CAGR through 2027, where iron-air batteries show particular promise due to their discharge profile characteristics.
Market barriers include manufacturing scalability challenges, with current production capacities limited to megawatt-scale deployments rather than gigawatt-scale needed for widespread adoption. Additionally, the market faces competition from rapidly improving lithium-ion technologies and emerging alternatives like sodium-ion and flow batteries, which may offer comparable performance with fewer technical hurdles in certain applications.
Iron-air batteries have garnered substantial commercial interest, with major investments from companies like Form Energy, which secured $450 million in funding for grid-scale iron-air battery development. Their market potential is particularly strong in stationary energy storage applications, where energy density by weight is less critical than cost per kilowatt-hour. Current market pricing indicates iron-air systems can potentially achieve costs below $20/kWh, significantly undercutting lithium-ion alternatives.
Alkaline-air batteries, while less commercially developed, are finding niche applications in sectors requiring moderate energy density and lower cost than lithium-based systems. The market for these batteries is growing in portable electronics and backup power systems, with companies like Fluidic Energy and Phinergy leading development efforts. The global market share for alkaline-air systems remains under 5% of the total metal-air battery market but shows promising growth potential.
Regional market analysis reveals Asia-Pacific dominates metal-air battery manufacturing, accounting for approximately 62% of global production capacity. China leads with substantial government support for metal-air technology development, while North America shows increasing adoption rates in grid storage applications, particularly for iron-air systems. Europe follows with strong research initiatives but more limited commercial deployment.
Consumer electronics currently represent the largest application segment for metal-air batteries at 43% market share, followed by electric vehicles (27%) and grid storage (18%). However, the fastest growth is projected in the grid storage sector, with a 34.5% CAGR through 2027, where iron-air batteries show particular promise due to their discharge profile characteristics.
Market barriers include manufacturing scalability challenges, with current production capacities limited to megawatt-scale deployments rather than gigawatt-scale needed for widespread adoption. Additionally, the market faces competition from rapidly improving lithium-ion technologies and emerging alternatives like sodium-ion and flow batteries, which may offer comparable performance with fewer technical hurdles in certain applications.
Technical Challenges in Iron-Air and Alkaline-Air Batteries
Despite significant advancements in metal-air battery technologies, both Iron-Air and Alkaline-Air batteries face substantial technical challenges that impede their widespread commercial adoption. These challenges primarily stem from the complex electrochemical reactions occurring at the air electrode and the inherent properties of the metal components.
Iron-Air batteries encounter severe capacity fading during cycling due to the formation of irreversible iron oxides and hydroxides. The discharge profile typically shows a rapid initial voltage drop followed by a relatively stable plateau, but this stability deteriorates over multiple cycles. The iron electrode's tendency to passivate creates a barrier to electron transfer, resulting in increased internal resistance and diminished discharge capacity.
Oxygen reduction reaction (ORR) kinetics represent a significant bottleneck in both battery types. In Iron-Air systems, the sluggish ORR at the air cathode limits the discharge rate capability, manifesting as steeper voltage drops under higher current densities compared to theoretical predictions. This challenge is particularly evident when comparing discharge profiles at varying C-rates.
Alkaline-Air batteries suffer from carbonate formation when atmospheric CO2 reacts with the alkaline electrolyte. This parasitic reaction progressively reduces electrolyte conductivity and active material utilization, causing a gradual decline in the discharge voltage profile over time. The discharge curves of Alkaline-Air batteries typically exhibit more pronounced polarization than Iron-Air counterparts under similar discharge conditions.
Bifunctional oxygen electrocatalysts present another critical challenge. The oxygen evolution reaction (OER) during charging requires different optimal catalytic sites than ORR during discharge. This fundamental mismatch results in significant overpotentials and energy inefficiencies, visible as large hysteresis between charge and discharge profiles in both battery types.
Water management issues also differentiate the discharge behaviors. Iron-Air batteries show greater sensitivity to electrolyte drying, with discharge profiles exhibiting premature capacity cutoff when operated at low humidity conditions. Conversely, Alkaline-Air systems demonstrate flooding problems at high discharge rates, causing sudden voltage drops not observed in properly functioning Iron-Air cells.
Temperature dependency further complicates performance comparison. Iron-Air batteries maintain more consistent discharge profiles across temperature ranges (0-40°C), while Alkaline-Air systems show dramatic capacity and voltage profile variations with temperature fluctuations, particularly in the lower temperature regime where discharge capacity can decrease by up to 50%.
Iron-Air batteries encounter severe capacity fading during cycling due to the formation of irreversible iron oxides and hydroxides. The discharge profile typically shows a rapid initial voltage drop followed by a relatively stable plateau, but this stability deteriorates over multiple cycles. The iron electrode's tendency to passivate creates a barrier to electron transfer, resulting in increased internal resistance and diminished discharge capacity.
Oxygen reduction reaction (ORR) kinetics represent a significant bottleneck in both battery types. In Iron-Air systems, the sluggish ORR at the air cathode limits the discharge rate capability, manifesting as steeper voltage drops under higher current densities compared to theoretical predictions. This challenge is particularly evident when comparing discharge profiles at varying C-rates.
Alkaline-Air batteries suffer from carbonate formation when atmospheric CO2 reacts with the alkaline electrolyte. This parasitic reaction progressively reduces electrolyte conductivity and active material utilization, causing a gradual decline in the discharge voltage profile over time. The discharge curves of Alkaline-Air batteries typically exhibit more pronounced polarization than Iron-Air counterparts under similar discharge conditions.
Bifunctional oxygen electrocatalysts present another critical challenge. The oxygen evolution reaction (OER) during charging requires different optimal catalytic sites than ORR during discharge. This fundamental mismatch results in significant overpotentials and energy inefficiencies, visible as large hysteresis between charge and discharge profiles in both battery types.
Water management issues also differentiate the discharge behaviors. Iron-Air batteries show greater sensitivity to electrolyte drying, with discharge profiles exhibiting premature capacity cutoff when operated at low humidity conditions. Conversely, Alkaline-Air systems demonstrate flooding problems at high discharge rates, causing sudden voltage drops not observed in properly functioning Iron-Air cells.
Temperature dependency further complicates performance comparison. Iron-Air batteries maintain more consistent discharge profiles across temperature ranges (0-40°C), while Alkaline-Air systems show dramatic capacity and voltage profile variations with temperature fluctuations, particularly in the lower temperature regime where discharge capacity can decrease by up to 50%.
Current Discharge Profile Comparison Methods
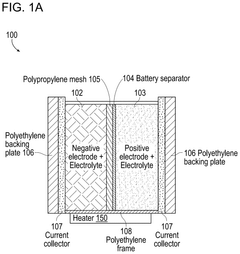
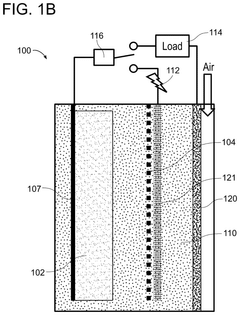
01 Iron-Air Battery Discharge Characteristics
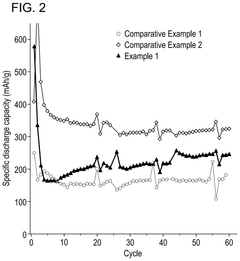
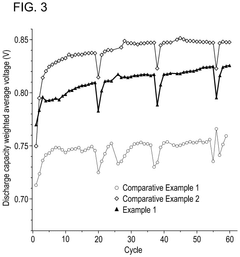
Iron-air batteries exhibit specific discharge profiles characterized by voltage plateaus during the oxidation of iron. These batteries typically demonstrate a stable discharge voltage around 1.0-1.2V with gradual capacity fade over multiple cycles. The discharge profile is influenced by the iron electrode composition, electrolyte concentration, and operating temperature. Iron-air batteries generally show good energy density but may suffer from capacity loss due to iron passivation during discharge.- Iron-Air Battery Discharge Characteristics: Iron-air batteries exhibit specific discharge profiles characterized by voltage plateaus during the reduction of iron oxides. These batteries typically show a discharge voltage around 1.0-1.2V with multiple plateaus corresponding to different iron oxidation states. The discharge capacity is influenced by the iron electrode composition, with higher iron content generally resulting in higher capacity. The discharge profile can be stabilized through proper electrolyte formulation and electrode design to minimize capacity fading during cycling.
- Alkaline Air Battery Performance: Alkaline air batteries utilize an alkaline electrolyte (typically KOH) and demonstrate distinct discharge characteristics. These batteries generally show a higher initial discharge voltage compared to neutral electrolyte systems, often in the range of 1.2-1.4V. The discharge profile typically exhibits a gradual voltage decline rather than sharp plateaus. Performance is significantly affected by air cathode design, with oxygen reduction reaction kinetics being a limiting factor. Humidity and carbon dioxide exposure can impact the long-term discharge stability of these systems.
- Comparative Analysis of Battery Technologies: When comparing iron-air and alkaline air batteries, several key differences in discharge profiles emerge. Iron-air batteries typically offer higher energy density but lower power density compared to alkaline air systems. Alkaline air batteries generally demonstrate better rate capability but may suffer from electrolyte carbonation issues during extended discharge. Iron-air systems show more distinct voltage plateaus during discharge, while alkaline air batteries tend to have smoother discharge curves. The selection between these technologies depends on specific application requirements such as discharge duration, power needs, and operating environment.
- Electrode Composition Effects on Discharge: The composition of electrodes significantly impacts the discharge profiles of both iron-air and alkaline air batteries. For iron electrodes, the addition of conductive additives and catalysts can improve discharge capacity and reduce polarization. In air electrodes, the type of carbon material, catalyst loading, and hydrophobic treatment affect oxygen reduction efficiency and discharge performance. Bifunctional catalysts in air electrodes can enhance both discharge and charge performance. The particle size and morphology of active materials also influence the discharge profile by affecting reaction kinetics and utilization efficiency.
- Advanced Electrolyte Systems for Enhanced Discharge: Innovative electrolyte formulations can significantly improve the discharge profiles of both battery types. Additives that suppress hydrogen evolution in iron-air batteries help maintain discharge efficiency. For alkaline air systems, electrolyte additives that reduce carbonation extend cycle life and stabilize the discharge voltage. Gel and polymer electrolytes offer advantages in terms of leakage prevention and discharge profile consistency. The concentration of alkaline electrolyte affects ionic conductivity and consequently the discharge capacity and voltage stability during high-rate discharge operations.
02 Alkaline Air Battery Performance
Alkaline air batteries utilize an alkaline electrolyte (typically KOH) and demonstrate distinctive discharge profiles with relatively flat voltage curves. These batteries show higher initial discharge voltages compared to neutral electrolyte systems, typically in the range of 1.2-1.4V. The discharge performance is affected by the concentration of the alkaline electrolyte, air cathode structure, and catalyst loading. Alkaline conditions enhance oxygen reduction kinetics but may lead to carbonation issues when exposed to air.Expand Specific Solutions03 Comparative Analysis of Iron-Air and Alkaline-Air Battery Discharge
When comparing iron-air and alkaline-air batteries, distinct differences in discharge profiles become apparent. Iron-air batteries typically show lower but more stable discharge voltages, while alkaline-air systems offer higher initial voltages with potentially steeper voltage decline. The discharge capacity of iron-air batteries is generally higher, but they may suffer from more pronounced voltage depression during high-rate discharge. Both systems show sensitivity to discharge rate, with performance degradation at higher current densities.Expand Specific Solutions04 Electrolyte Composition Effects on Discharge Profiles
The composition of the electrolyte significantly impacts the discharge profiles of both iron-air and alkaline-air batteries. Higher alkaline concentrations typically improve initial discharge performance but may accelerate degradation mechanisms. Additives such as lithium compounds, surfactants, and corrosion inhibitors can modify the discharge profile by affecting reaction kinetics and electrode surface properties. The presence of certain ions can either enhance capacity or lead to premature capacity fade, directly influencing the shape and stability of the discharge curve.Expand Specific Solutions05 Advanced Electrode Materials for Improved Discharge Performance
Recent developments in electrode materials have led to significant improvements in the discharge profiles of both battery types. Nano-structured iron particles, advanced carbon-based air cathodes, and novel catalysts have been shown to enhance discharge capacity and voltage stability. Composite electrodes incorporating multiple active materials can provide more consistent discharge profiles across a wider range of operating conditions. Surface modification techniques and protective coatings help mitigate degradation mechanisms that negatively affect discharge performance over multiple cycles.Expand Specific Solutions
Key Industry Players and Manufacturers
The metal-air battery market is currently in an early growth phase, with iron-air and alkaline-air technologies representing promising alternatives to conventional energy storage solutions. The global market for these technologies is expanding, driven by increasing demand for sustainable energy storage with projections reaching several billion dollars by 2030. Technologically, iron-air batteries developed by companies like Form Energy and Phinergy demonstrate longer discharge durations (100+ hours) but lower energy density compared to alkaline-air systems. Research institutions including MIT, Caltech, and Panasonic are advancing alkaline-air batteries with higher theoretical energy densities but facing challenges with rechargeability and cycle life. Toyota, Panasonic, and Cabot are leading commercial development efforts, while academic institutions like Wuhan University and IIT Madras are contributing significant research breakthroughs in electrode materials and electrolyte formulations.
Phinergy Ltd.
Technical Solution: Phinergy has developed advanced Iron-Air battery technology that utilizes atmospheric oxygen as a reactant. Their proprietary system features a unique electrode structure with high surface area iron particles and specialized catalysts that promote efficient oxygen reduction during discharge. The company's Iron-Air batteries demonstrate a flat discharge profile maintaining 1.0-1.2V for approximately 80% of discharge capacity, with gradual voltage decline only in the final discharge phase. This contrasts with their Alkaline-Air systems which show higher initial voltage (1.4-1.5V) but experience more pronounced voltage drop throughout discharge. Phinergy's Iron-Air technology incorporates patented electrolyte formulations that minimize self-discharge and hydrogen evolution, extending shelf life significantly compared to conventional alkaline air systems.
Strengths: Superior energy density (up to 400 Wh/kg), environmentally friendly materials, abundant and low-cost iron resources, and exceptional cycle stability. Weaknesses: Lower power density than alkaline-air alternatives, requires more sophisticated air management systems, and demonstrates slower response to dynamic load changes.
Panasonic Holdings Corp.
Technical Solution: Panasonic has developed both Iron-Air and Alkaline-Air battery technologies with distinctive discharge characteristics. Their Iron-Air batteries utilize nano-structured iron anodes with proprietary surface treatments to enhance reaction kinetics and prevent passivation. These batteries exhibit a relatively stable discharge voltage plateau around 1.0-1.1V for approximately 70-75% of the discharge curve, followed by a gradual decline. In contrast, their Alkaline-Air batteries feature a higher operating voltage (1.4-1.5V) but demonstrate a more sloping discharge profile throughout the discharge cycle. Panasonic's research indicates that their Iron-Air batteries maintain approximately 85% capacity retention after 100 cycles, while their Alkaline-Air alternatives show faster capacity degradation with cycling. The company has implemented advanced air cathode designs with specialized catalysts that significantly reduce the oxygen reduction overpotential in both battery types.
Strengths: Extensive manufacturing infrastructure, superior quality control processes, and established supply chains for mass production. Weaknesses: Iron-Air technology shows lower power density compared to their alkaline counterparts, and requires more complex moisture management systems to prevent premature degradation.
Critical Patents in Metal-Air Battery Discharge Technology
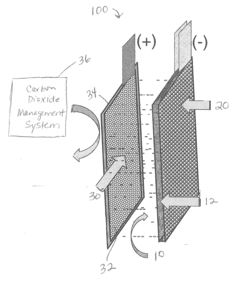
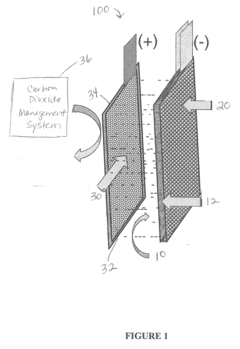
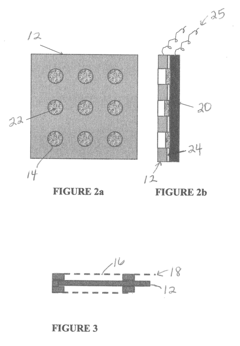
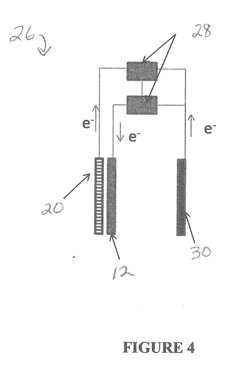
Iron-air rechargeable battery
PatentActiveUS20120187918A1
Innovation
- Incorporating self-assembling organic sulfur-based additives to inhibit hydrogen evolution, using non-toxic bismuth additives to suppress parasitic reactions, integrating a bilayer composite electrode for hydrogen utilization, employing nano-structured corrosion-resistant substrates for the air electrode, and implementing a carbon dioxide management system to enhance efficiency and durability.
Additive for iron-air batteries
PatentPendingUS20250140990A1
Innovation
- An alkaline electrolyte with a total hydroxide concentration greater than 1 molar, containing a trivalent element such as aluminum, sulfur, and tin, is used to improve the performance of iron-air batteries.
Environmental Impact Assessment
The environmental impact of battery technologies has become a critical consideration in the transition to sustainable energy systems. Iron-Air and Alkaline-Air batteries present distinct environmental profiles throughout their lifecycle, from raw material extraction to end-of-life management.
Iron-Air batteries utilize abundant, low-cost materials with iron being the primary active material. The environmental footprint of iron mining is relatively well-understood and generally less intensive compared to mining of rare metals used in other battery technologies. During operation, Iron-Air batteries generate iron hydroxide as they discharge, which is environmentally benign and can be fully recycled within the battery during charging cycles.
Alkaline-Air batteries, conversely, rely on potassium hydroxide electrolytes and typically incorporate zinc or other metals as anodes. The production of these materials involves more energy-intensive processes and potentially greater environmental disruption. The caustic nature of alkaline electrolytes presents additional handling and disposal challenges that must be carefully managed to prevent environmental contamination.
Both battery types offer significant environmental advantages over conventional lithium-ion batteries, particularly in their reduced reliance on critical minerals and rare earth elements. This characteristic decreases supply chain vulnerabilities and reduces the ecological damage associated with extractive industries in environmentally sensitive regions.
The discharge profiles of these technologies have direct environmental implications. Iron-Air batteries typically demonstrate more stable, consistent discharge patterns that can extend operational lifespans, potentially reducing waste generation through less frequent replacement requirements. Alkaline-Air systems may experience more variable discharge characteristics depending on environmental conditions, potentially leading to shorter effective lifespans in certain applications.
Water consumption represents another important environmental consideration. Iron-Air batteries require water as a reactant, which may present challenges in water-scarce regions. Alkaline-Air batteries generally have lower water requirements during operation but may necessitate more water during manufacturing processes.
End-of-life management reveals further distinctions. Iron-Air battery components are highly recyclable, with iron compounds being readily recovered and reprocessed. Alkaline-Air batteries present more complex recycling challenges due to their chemical composition, though advancements in recycling technologies are gradually improving recovery rates for these systems.
Carbon footprint assessments indicate that both technologies offer substantial greenhouse gas reductions compared to fossil fuel alternatives, with Iron-Air batteries potentially offering marginally better lifecycle emissions profiles due to their simpler chemistry and manufacturing requirements.
Iron-Air batteries utilize abundant, low-cost materials with iron being the primary active material. The environmental footprint of iron mining is relatively well-understood and generally less intensive compared to mining of rare metals used in other battery technologies. During operation, Iron-Air batteries generate iron hydroxide as they discharge, which is environmentally benign and can be fully recycled within the battery during charging cycles.
Alkaline-Air batteries, conversely, rely on potassium hydroxide electrolytes and typically incorporate zinc or other metals as anodes. The production of these materials involves more energy-intensive processes and potentially greater environmental disruption. The caustic nature of alkaline electrolytes presents additional handling and disposal challenges that must be carefully managed to prevent environmental contamination.
Both battery types offer significant environmental advantages over conventional lithium-ion batteries, particularly in their reduced reliance on critical minerals and rare earth elements. This characteristic decreases supply chain vulnerabilities and reduces the ecological damage associated with extractive industries in environmentally sensitive regions.
The discharge profiles of these technologies have direct environmental implications. Iron-Air batteries typically demonstrate more stable, consistent discharge patterns that can extend operational lifespans, potentially reducing waste generation through less frequent replacement requirements. Alkaline-Air systems may experience more variable discharge characteristics depending on environmental conditions, potentially leading to shorter effective lifespans in certain applications.
Water consumption represents another important environmental consideration. Iron-Air batteries require water as a reactant, which may present challenges in water-scarce regions. Alkaline-Air batteries generally have lower water requirements during operation but may necessitate more water during manufacturing processes.
End-of-life management reveals further distinctions. Iron-Air battery components are highly recyclable, with iron compounds being readily recovered and reprocessed. Alkaline-Air batteries present more complex recycling challenges due to their chemical composition, though advancements in recycling technologies are gradually improving recovery rates for these systems.
Carbon footprint assessments indicate that both technologies offer substantial greenhouse gas reductions compared to fossil fuel alternatives, with Iron-Air batteries potentially offering marginally better lifecycle emissions profiles due to their simpler chemistry and manufacturing requirements.
Energy Density and Cost Analysis
The energy density and cost analysis of Iron-Air and Alkaline Air batteries reveals significant differences that impact their commercial viability and application potential. Iron-Air batteries demonstrate theoretical energy densities ranging from 700-1200 Wh/kg, substantially higher than most conventional battery technologies. In practical implementations, current Iron-Air systems achieve 300-500 Wh/kg, which still represents a considerable advantage over lithium-ion batteries (150-260 Wh/kg) in energy density terms.
Alkaline Air batteries, by comparison, offer theoretical energy densities of 800-1100 Wh/kg. However, their practical energy densities typically fall between 200-400 Wh/kg due to challenges in cathode design and electrolyte management. This performance gap between theoretical and practical values represents a critical area for ongoing research and development.
From a cost perspective, Iron-Air batteries present compelling advantages. The raw material costs for iron are approximately $0.10-0.20/kg, significantly lower than lithium ($15-20/kg) or cobalt ($30-60/kg). This translates to potential cell-level costs of $50-80/kWh for Iron-Air systems at scale, compared to $100-150/kWh for current lithium-ion technologies.
Alkaline Air batteries also offer cost benefits with estimated cell-level costs of $60-100/kWh. However, their total cost of ownership is often higher due to shorter cycle life and more frequent replacement requirements. The alkaline electrolyte components are inexpensive, but the air cathodes typically require precious metal catalysts that increase overall system costs.
Lifecycle economic analysis indicates that Iron-Air batteries may achieve costs below $30/kWh for grid storage applications when accounting for their longer cycle life (1000-2000 cycles) compared to Alkaline Air systems (300-700 cycles). This difference becomes particularly significant in stationary storage applications where installation and replacement costs are substantial considerations.
Manufacturing scalability further differentiates these technologies. Iron-Air battery production can leverage existing manufacturing infrastructure with minimal adaptation, whereas Alkaline Air systems require more specialized production processes for their air cathodes. This manufacturing advantage contributes to the projected 40-60% lower production costs for Iron-Air systems at gigawatt-hour production scales.
Energy efficiency comparisons reveal that Iron-Air batteries typically operate at 50-65% round-trip efficiency, while Alkaline Air batteries demonstrate slightly lower efficiencies of 45-60%. These efficiency differences directly impact the levelized cost of storage, with each percentage point in efficiency potentially translating to 1-2% reduction in lifetime system costs.
Alkaline Air batteries, by comparison, offer theoretical energy densities of 800-1100 Wh/kg. However, their practical energy densities typically fall between 200-400 Wh/kg due to challenges in cathode design and electrolyte management. This performance gap between theoretical and practical values represents a critical area for ongoing research and development.
From a cost perspective, Iron-Air batteries present compelling advantages. The raw material costs for iron are approximately $0.10-0.20/kg, significantly lower than lithium ($15-20/kg) or cobalt ($30-60/kg). This translates to potential cell-level costs of $50-80/kWh for Iron-Air systems at scale, compared to $100-150/kWh for current lithium-ion technologies.
Alkaline Air batteries also offer cost benefits with estimated cell-level costs of $60-100/kWh. However, their total cost of ownership is often higher due to shorter cycle life and more frequent replacement requirements. The alkaline electrolyte components are inexpensive, but the air cathodes typically require precious metal catalysts that increase overall system costs.
Lifecycle economic analysis indicates that Iron-Air batteries may achieve costs below $30/kWh for grid storage applications when accounting for their longer cycle life (1000-2000 cycles) compared to Alkaline Air systems (300-700 cycles). This difference becomes particularly significant in stationary storage applications where installation and replacement costs are substantial considerations.
Manufacturing scalability further differentiates these technologies. Iron-Air battery production can leverage existing manufacturing infrastructure with minimal adaptation, whereas Alkaline Air systems require more specialized production processes for their air cathodes. This manufacturing advantage contributes to the projected 40-60% lower production costs for Iron-Air systems at gigawatt-hour production scales.
Energy efficiency comparisons reveal that Iron-Air batteries typically operate at 50-65% round-trip efficiency, while Alkaline Air batteries demonstrate slightly lower efficiencies of 45-60%. These efficiency differences directly impact the levelized cost of storage, with each percentage point in efficiency potentially translating to 1-2% reduction in lifetime system costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!