How to Mitigate Gas Bubble Formation in Iron-Air Cells
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Iron-Air Battery Technology Background and Objectives
Iron-air batteries represent a significant advancement in energy storage technology, with roots dating back to the 1970s. These batteries operate on the principle of reversible oxidation of iron to iron oxide during discharge, and reduction back to iron during charging, while utilizing oxygen from ambient air as the cathode active material. This technology has experienced renewed interest in recent years due to its potential to provide long-duration energy storage at significantly lower costs compared to lithium-ion batteries.
The evolution of iron-air battery technology has been marked by several key developments. Initially, these batteries suffered from poor cycle life and efficiency due to hydrogen evolution during charging. Subsequent research focused on improving iron electrode formulations and electrolyte compositions to mitigate these issues. By the early 2000s, advancements in materials science and electrochemistry led to significant improvements in performance metrics, though commercial viability remained elusive.
Current technological trends point toward the integration of nanotechnology and advanced catalyst materials to enhance reaction kinetics and reduce parasitic reactions. The formation of gas bubbles, particularly hydrogen and oxygen, represents one of the most significant challenges in iron-air cell development. These bubbles form during charging and discharging processes, leading to increased internal resistance, reduced active surface area, and ultimately diminished battery performance and lifespan.
The primary technical objectives for mitigating gas bubble formation include developing electrode structures that facilitate efficient gas management, designing electrolyte compositions that minimize hydrogen evolution, and creating cell architectures that allow for effective bubble release without compromising electrochemical performance. Researchers aim to achieve iron-air batteries with energy densities exceeding 300 Wh/kg, cycle lives of over 1,000 cycles, and round-trip efficiencies above 50%.
Global research efforts are increasingly focused on addressing the gas bubble formation issue through multidisciplinary approaches combining electrochemistry, fluid dynamics, materials science, and engineering. The ultimate goal is to enable iron-air batteries to serve as a cornerstone technology for grid-scale energy storage, providing multi-day backup power for renewable energy systems at costs below $20/kWh - approximately one-tenth the cost of current lithium-ion systems.
Success in mitigating gas bubble formation would position iron-air technology as a transformative solution for the renewable energy transition, offering sustainable, abundant, and low-cost energy storage at unprecedented scale. This would address one of the most critical challenges in the global shift toward carbon-neutral energy systems.
The evolution of iron-air battery technology has been marked by several key developments. Initially, these batteries suffered from poor cycle life and efficiency due to hydrogen evolution during charging. Subsequent research focused on improving iron electrode formulations and electrolyte compositions to mitigate these issues. By the early 2000s, advancements in materials science and electrochemistry led to significant improvements in performance metrics, though commercial viability remained elusive.
Current technological trends point toward the integration of nanotechnology and advanced catalyst materials to enhance reaction kinetics and reduce parasitic reactions. The formation of gas bubbles, particularly hydrogen and oxygen, represents one of the most significant challenges in iron-air cell development. These bubbles form during charging and discharging processes, leading to increased internal resistance, reduced active surface area, and ultimately diminished battery performance and lifespan.
The primary technical objectives for mitigating gas bubble formation include developing electrode structures that facilitate efficient gas management, designing electrolyte compositions that minimize hydrogen evolution, and creating cell architectures that allow for effective bubble release without compromising electrochemical performance. Researchers aim to achieve iron-air batteries with energy densities exceeding 300 Wh/kg, cycle lives of over 1,000 cycles, and round-trip efficiencies above 50%.
Global research efforts are increasingly focused on addressing the gas bubble formation issue through multidisciplinary approaches combining electrochemistry, fluid dynamics, materials science, and engineering. The ultimate goal is to enable iron-air batteries to serve as a cornerstone technology for grid-scale energy storage, providing multi-day backup power for renewable energy systems at costs below $20/kWh - approximately one-tenth the cost of current lithium-ion systems.
Success in mitigating gas bubble formation would position iron-air technology as a transformative solution for the renewable energy transition, offering sustainable, abundant, and low-cost energy storage at unprecedented scale. This would address one of the most critical challenges in the global shift toward carbon-neutral energy systems.
Market Analysis for Iron-Air Energy Storage Solutions
The global energy storage market is experiencing unprecedented growth, with iron-air battery technology emerging as a promising solution for grid-scale applications. Current market projections indicate that the grid energy storage market will reach approximately $15 billion by 2025, with a compound annual growth rate exceeding 20%. Within this expanding landscape, iron-air technology is positioned to capture significant market share due to its cost advantages and sustainability profile.
Iron-air energy storage solutions address a critical market need for long-duration energy storage systems that can effectively manage intermittent renewable energy sources. As renewable penetration increases globally, the demand for storage solutions that can provide 10+ hours of discharge duration is growing substantially. Iron-air batteries, with their theoretical ability to deliver energy for up to 100 hours, are uniquely positioned to serve this market segment.
The competitive landscape for iron-air technology remains relatively uncrowded compared to lithium-ion solutions. Form Energy has emerged as the market leader, securing over $800 million in funding and announcing commercial deployments with major utilities. Other players including ESS Tech and Ambri are developing alternative long-duration technologies that will compete in similar market segments.
Cost analysis reveals iron-air's significant market advantage, with projected levelized costs of storage potentially reaching below $20/kWh, compared to lithium-ion's $150-200/kWh floor. This dramatic cost differential is primarily driven by iron's abundance and low material costs, representing a fundamental shift in energy storage economics.
Market segmentation analysis indicates that iron-air technology will initially target utility-scale applications, particularly in regions with high renewable penetration. California, Texas, and international markets with aggressive decarbonization goals represent the most promising early adoption territories. The technology's long-duration capabilities make it particularly valuable for seasonal storage applications and grid resilience.
Customer demand signals are strengthening, with multiple utilities announcing pilot projects and procurement plans for long-duration storage. Recent regulatory developments, including investment tax credits for standalone storage and clean energy mandates, have significantly improved the market outlook for iron-air technology.
The addressable market for iron-air solutions is expected to grow substantially as the technology matures and production scales. By 2030, long-duration energy storage could represent a $50-100 billion market opportunity globally, with iron-air positioned to capture a significant portion if current technical challenges, including gas bubble formation issues, can be effectively addressed.
Iron-air energy storage solutions address a critical market need for long-duration energy storage systems that can effectively manage intermittent renewable energy sources. As renewable penetration increases globally, the demand for storage solutions that can provide 10+ hours of discharge duration is growing substantially. Iron-air batteries, with their theoretical ability to deliver energy for up to 100 hours, are uniquely positioned to serve this market segment.
The competitive landscape for iron-air technology remains relatively uncrowded compared to lithium-ion solutions. Form Energy has emerged as the market leader, securing over $800 million in funding and announcing commercial deployments with major utilities. Other players including ESS Tech and Ambri are developing alternative long-duration technologies that will compete in similar market segments.
Cost analysis reveals iron-air's significant market advantage, with projected levelized costs of storage potentially reaching below $20/kWh, compared to lithium-ion's $150-200/kWh floor. This dramatic cost differential is primarily driven by iron's abundance and low material costs, representing a fundamental shift in energy storage economics.
Market segmentation analysis indicates that iron-air technology will initially target utility-scale applications, particularly in regions with high renewable penetration. California, Texas, and international markets with aggressive decarbonization goals represent the most promising early adoption territories. The technology's long-duration capabilities make it particularly valuable for seasonal storage applications and grid resilience.
Customer demand signals are strengthening, with multiple utilities announcing pilot projects and procurement plans for long-duration storage. Recent regulatory developments, including investment tax credits for standalone storage and clean energy mandates, have significantly improved the market outlook for iron-air technology.
The addressable market for iron-air solutions is expected to grow substantially as the technology matures and production scales. By 2030, long-duration energy storage could represent a $50-100 billion market opportunity globally, with iron-air positioned to capture a significant portion if current technical challenges, including gas bubble formation issues, can be effectively addressed.
Gas Bubble Formation Challenges in Iron-Air Cells
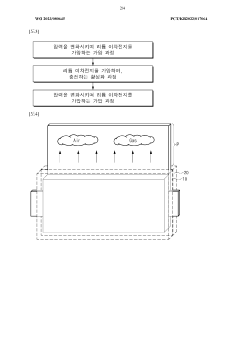
Gas bubble formation represents one of the most significant challenges in the development and operation of iron-air batteries. These bubbles primarily form during the charging process when oxygen evolution occurs at the air electrode and hydrogen evolution takes place at the iron electrode. The formation of these gas bubbles creates multiple detrimental effects that compromise both the performance and longevity of iron-air cells.
The primary issue with gas bubble formation is the creation of physical barriers that impede ion transport within the cell. When bubbles adhere to electrode surfaces, they block active reaction sites, effectively reducing the electrochemically active surface area. This phenomenon leads to increased cell resistance and diminished energy efficiency, as more energy must be applied to achieve the same level of charge.
Furthermore, gas bubbles can cause mechanical stress within the cell structure. As bubbles form and expand, they exert pressure on surrounding components, potentially leading to deformation of electrodes, separator damage, or even cell casing failures in severe cases. This mechanical degradation accelerates the overall aging process of the battery system.
The hydrogen evolution reaction (HER) occurring at the iron electrode during charging represents a significant parasitic reaction that consumes energy without contributing to the energy storage capacity. This parasitic reaction not only reduces coulombic efficiency but also creates safety concerns due to hydrogen accumulation within the cell.
On the oxygen side, bubble formation can lead to uneven current distribution across the air electrode, resulting in localized overcharging and accelerated degradation of catalyst materials. The oxygen evolution reaction (OER) also contributes to water loss from the electrolyte, gradually altering its composition and concentration over multiple charge-discharge cycles.
Temperature fluctuations further exacerbate bubble formation issues. Higher operating temperatures generally increase gas evolution rates while simultaneously decreasing gas solubility in the electrolyte, creating a compounding negative effect. This temperature sensitivity limits the operational range of iron-air batteries and complicates thermal management requirements.
Pressure management within iron-air cells presents another challenge. Without adequate pressure regulation mechanisms, gas accumulation can lead to dangerous pressure buildup or, conversely, create vacuum conditions during discharge that may draw in contaminants from the external environment, compromising cell chemistry and performance.
The primary issue with gas bubble formation is the creation of physical barriers that impede ion transport within the cell. When bubbles adhere to electrode surfaces, they block active reaction sites, effectively reducing the electrochemically active surface area. This phenomenon leads to increased cell resistance and diminished energy efficiency, as more energy must be applied to achieve the same level of charge.
Furthermore, gas bubbles can cause mechanical stress within the cell structure. As bubbles form and expand, they exert pressure on surrounding components, potentially leading to deformation of electrodes, separator damage, or even cell casing failures in severe cases. This mechanical degradation accelerates the overall aging process of the battery system.
The hydrogen evolution reaction (HER) occurring at the iron electrode during charging represents a significant parasitic reaction that consumes energy without contributing to the energy storage capacity. This parasitic reaction not only reduces coulombic efficiency but also creates safety concerns due to hydrogen accumulation within the cell.
On the oxygen side, bubble formation can lead to uneven current distribution across the air electrode, resulting in localized overcharging and accelerated degradation of catalyst materials. The oxygen evolution reaction (OER) also contributes to water loss from the electrolyte, gradually altering its composition and concentration over multiple charge-discharge cycles.
Temperature fluctuations further exacerbate bubble formation issues. Higher operating temperatures generally increase gas evolution rates while simultaneously decreasing gas solubility in the electrolyte, creating a compounding negative effect. This temperature sensitivity limits the operational range of iron-air batteries and complicates thermal management requirements.
Pressure management within iron-air cells presents another challenge. Without adequate pressure regulation mechanisms, gas accumulation can lead to dangerous pressure buildup or, conversely, create vacuum conditions during discharge that may draw in contaminants from the external environment, compromising cell chemistry and performance.
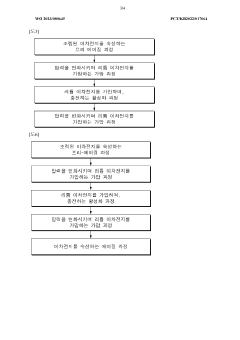
Current Gas Bubble Mitigation Strategies
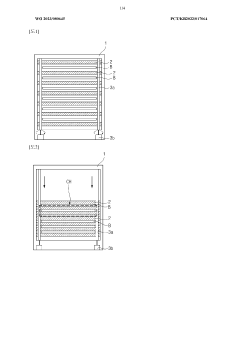
01 Gas bubble management in iron-air battery cells
Gas bubble formation is a critical issue in iron-air cells that affects performance and efficiency. Various techniques have been developed to manage gas bubbles, including specialized electrode designs and cell configurations that facilitate the controlled release or redirection of gas bubbles. These approaches help prevent bubble accumulation that could block active surfaces and reduce electrochemical reaction efficiency.- Gas bubble management in iron-air battery electrodes: Gas bubble formation and management in iron-air batteries is critical for optimal performance. Various electrode designs and structures can help control bubble formation, facilitate their release, and prevent blockage of active sites. Specialized electrode architectures with optimized porosity and hydrophobic/hydrophilic properties can effectively manage oxygen evolution during charging and oxygen reduction during discharging, improving overall battery efficiency and cycle life.
- Electrolyte composition for reducing gas bubble formation: The composition of electrolytes in iron-air cells significantly impacts gas bubble formation. Additives and specialized formulations can reduce surface tension, improve wettability, and facilitate bubble detachment from electrode surfaces. Modified electrolytes can also enhance oxygen solubility and transport, reducing the formation of large gas bubbles that could block reaction sites and impede electrochemical processes.
- Structural design for gas bubble removal: Specific structural designs in iron-air cells can facilitate the efficient removal of gas bubbles. These designs include flow channels, bubble guides, and specialized cell geometries that promote natural bubble migration away from active surfaces. Incorporating features such as sloped surfaces, bubble collection chambers, and strategic positioning of electrodes can significantly reduce the negative impact of gas bubbles on cell performance.
- Surface modification techniques to control bubble adhesion: Surface modification of electrodes and cell components can significantly influence gas bubble behavior in iron-air cells. Techniques include applying hydrophobic or hydrophilic coatings, creating micro/nano-textured surfaces, and incorporating specialized materials that reduce bubble adhesion. These modifications can control the size, distribution, and detachment rate of gas bubbles, improving overall cell efficiency and performance.
- Advanced monitoring and control systems for bubble formation: Monitoring and control systems can be implemented to detect and manage gas bubble formation in iron-air cells. These systems may include sensors to detect bubble formation, automated pressure regulation mechanisms, and feedback control algorithms that adjust operating parameters to minimize detrimental bubble effects. Real-time monitoring enables dynamic response to changing conditions, optimizing cell performance and extending operational life.
02 Electrode structure optimization for bubble mitigation
The design and structure of electrodes in iron-air cells significantly impact gas bubble formation and behavior. Porous electrode structures, surface treatments, and specialized materials can be incorporated to facilitate bubble detachment and prevent adhesion to electrode surfaces. These optimizations improve gas management and enhance the overall performance and longevity of iron-air cells.Expand Specific Solutions03 Electrolyte composition for controlling bubble formation
The composition of the electrolyte in iron-air cells plays a crucial role in gas bubble behavior. Additives and surfactants can be incorporated to modify surface tension and bubble characteristics, promoting smaller bubble formation and faster detachment. Optimized electrolyte formulations help manage gas evolution during charging and discharging cycles, improving cell efficiency and stability.Expand Specific Solutions04 Flow systems and circulation techniques for bubble removal
Active flow systems and circulation techniques can be implemented in iron-air cells to continuously remove gas bubbles from critical surfaces. These systems may include pumps, channels, or passive flow designs that utilize natural convection to sweep bubbles away from electrodes. Effective circulation prevents bubble accumulation and maintains consistent performance during operation.Expand Specific Solutions05 Pressure management and gas collection systems
Specialized pressure management and gas collection systems can be integrated into iron-air cells to handle the gases produced during operation. These systems may include pressure relief mechanisms, gas capture chambers, or recirculation pathways that prevent pressure buildup while maintaining cell integrity. Proper gas handling improves safety, extends cell lifetime, and maintains consistent performance under various operating conditions.Expand Specific Solutions
Leading Companies and Research Institutions in Iron-Air Battery Development
The iron-air battery market is in an early growth phase, characterized by increasing R&D investments but limited commercial deployment. The global market for this technology is projected to expand significantly as part of the $17.5 billion stationary energy storage sector, driven by renewable energy integration demands. Gas bubble formation remains a critical technical challenge affecting battery efficiency and longevity. Leading automotive manufacturers (Toyota, Tesla, Hyundai, BMW, Volkswagen) are actively developing solutions, while specialized companies like APB Corp focus on polymer-based alternatives. Research institutions such as Fraunhofer-Gesellschaft and Harbin Institute of Technology are advancing fundamental understanding of bubble dynamics. Technology leaders like Siemens and Bosch are leveraging their expertise in industrial systems to develop practical mitigation strategies through electrode design optimization and advanced membrane technologies.
Robert Bosch GmbH
Technical Solution: Bosch has developed an integrated approach to mitigate gas bubble formation in iron-air cells through their advanced materials engineering program. Their solution combines specially formulated electrode compositions with optimized microstructures that facilitate controlled gas release. Bosch's technology incorporates hydrophobic binding agents that create preferential pathways for gas evacuation while maintaining optimal ionic conductivity throughout the electrode. The company has pioneered the use of pulsed charging protocols that minimize gas evolution by avoiding potential regimes where oxygen evolution is accelerated. Additionally, Bosch has developed specialized cell housing designs with integrated pressure relief mechanisms and gas collection systems that prevent pressure buildup while enabling potential gas recirculation. Their approach also includes electrolyte formulations with surfactants that reduce bubble adhesion to electrode surfaces.
Strengths: Extensive experience in precision engineering and manufacturing; strong systems integration capabilities across multiple components. Weaknesses: Solutions may be more complex to implement in mass production; potentially higher initial development and tooling costs.
GM Global Technology Operations LLC
Technical Solution: GM has developed a comprehensive approach to mitigate gas bubble formation in iron-air cells through their advanced electrode engineering program. Their solution incorporates three-dimensional electrode architectures with gradient porosity that facilitates efficient gas release while maintaining optimal electrolyte contact. GM's technology utilizes specialized binder systems that create hydrophobic pathways within the electrode structure, allowing oxygen bubbles to coalesce and escape more efficiently. The company has also implemented ultrasonic agitation techniques during cell operation to dislodge bubbles from electrode surfaces before they can grow and cause performance issues. Additionally, GM's battery management systems incorporate pressure differential monitoring to detect early signs of problematic gas accumulation and adjust operating parameters accordingly.
Strengths: Extensive manufacturing expertise and scale-up capabilities; integration with comprehensive battery management systems. Weaknesses: Solutions may be optimized for automotive applications with less flexibility for other use cases; higher system complexity may increase costs.
Key Patents and Research on Gas Management in Metal-Air Batteries
Separators, batteries, systems, and methods for idle start stop vehicles
PatentActiveUS20220186029A1
Innovation
- The development of advanced lead acid battery separators incorporating Phosphate Induced Metal Stabilization (PIMS) minerals like fish bone powder and hydroxyapatite, which are integrated into the separator matrix to reduce lead ion concentration, enhance acid mixing, and improve cycle life by sequestering heavy metals and stabilizing acid distribution.
Method for activation of lithium secondary battery
PatentWO2023080645A1
Innovation
- A method involving multiple activation steps with varying pressure and charging rates, along with a degassing process to remove internal gas, is employed to prevent gas trapping and ensure uniform activation, using a formation device that pressurizes the battery at specific points during the activation process.
Environmental Impact and Sustainability of Iron-Air Battery Technology
Iron-air battery technology represents a significant advancement in sustainable energy storage solutions, offering environmental benefits that extend beyond traditional battery technologies. The environmental footprint of iron-air batteries is notably smaller than lithium-ion alternatives, primarily due to the abundance and non-toxic nature of iron as the primary material. Iron is the fourth most abundant element in Earth's crust, making it a highly sustainable resource with established mining and processing infrastructure that minimizes additional environmental disruption.
The production process for iron-air batteries generates significantly lower carbon emissions compared to conventional battery technologies. Research indicates that the lifecycle carbon footprint of iron-air batteries may be up to 40% lower than lithium-ion equivalents when accounting for manufacturing, operation, and end-of-life processing. This reduction stems from simpler production methods and lower energy requirements during manufacturing.
Water consumption represents another critical environmental consideration in battery production. Iron-air battery manufacturing requires approximately 50-60% less water than comparable energy storage technologies, contributing to conservation of this vital resource. Additionally, the absence of rare earth elements and toxic heavy metals in iron-air batteries eliminates concerns about environmental contamination from improper disposal or leakage.
End-of-life management of iron-air batteries presents substantial sustainability advantages. The primary components are highly recyclable, with iron being one of the most recycled materials globally. Recycling processes for iron-air batteries are less energy-intensive and more cost-effective than those for lithium-ion batteries, creating a more circular economy approach to energy storage solutions.
When addressing gas bubble formation in iron-air cells, environmental considerations must be integrated into mitigation strategies. Electrolyte additives used to reduce bubble formation should be environmentally benign and biodegradable. Similarly, electrode surface modifications must utilize eco-friendly materials and processes that minimize waste generation and toxic byproducts.
The long-term sustainability of iron-air battery technology depends on continuous improvement of operational efficiency, including effective management of gas evolution. By developing environmentally responsible approaches to mitigate bubble formation, the technology can maintain its green credentials while improving performance and reliability. This alignment of technical advancement with environmental stewardship positions iron-air batteries as a truly sustainable solution for grid-scale energy storage in the transition to renewable energy systems.
The production process for iron-air batteries generates significantly lower carbon emissions compared to conventional battery technologies. Research indicates that the lifecycle carbon footprint of iron-air batteries may be up to 40% lower than lithium-ion equivalents when accounting for manufacturing, operation, and end-of-life processing. This reduction stems from simpler production methods and lower energy requirements during manufacturing.
Water consumption represents another critical environmental consideration in battery production. Iron-air battery manufacturing requires approximately 50-60% less water than comparable energy storage technologies, contributing to conservation of this vital resource. Additionally, the absence of rare earth elements and toxic heavy metals in iron-air batteries eliminates concerns about environmental contamination from improper disposal or leakage.
End-of-life management of iron-air batteries presents substantial sustainability advantages. The primary components are highly recyclable, with iron being one of the most recycled materials globally. Recycling processes for iron-air batteries are less energy-intensive and more cost-effective than those for lithium-ion batteries, creating a more circular economy approach to energy storage solutions.
When addressing gas bubble formation in iron-air cells, environmental considerations must be integrated into mitigation strategies. Electrolyte additives used to reduce bubble formation should be environmentally benign and biodegradable. Similarly, electrode surface modifications must utilize eco-friendly materials and processes that minimize waste generation and toxic byproducts.
The long-term sustainability of iron-air battery technology depends on continuous improvement of operational efficiency, including effective management of gas evolution. By developing environmentally responsible approaches to mitigate bubble formation, the technology can maintain its green credentials while improving performance and reliability. This alignment of technical advancement with environmental stewardship positions iron-air batteries as a truly sustainable solution for grid-scale energy storage in the transition to renewable energy systems.
Safety Standards and Testing Protocols for Iron-Air Cells
The development of comprehensive safety standards and testing protocols for iron-air cells is essential for their commercial viability and widespread adoption. Currently, these standards are still evolving as the technology matures, with several key organizations leading the effort to establish unified guidelines.
The International Electrotechnical Commission (IEC) has begun incorporating iron-air cell specifications into their battery safety standards, focusing particularly on gas management systems. These standards mandate specific pressure relief mechanisms and recommend optimal cell designs that minimize bubble accumulation. The UL (Underwriters Laboratories) has also developed preliminary testing protocols specifically addressing gas evolution in metal-air batteries, requiring cells to demonstrate stable operation under various charging and discharging conditions.
Testing protocols for iron-air cells typically include accelerated life cycle testing under controlled conditions to evaluate gas bubble formation over time. These tests often involve operating cells at elevated temperatures (40-60°C) and varying charge rates to simulate worst-case scenarios for gas evolution. Pressure monitoring during these tests is critical, with standards typically requiring cells to maintain internal pressure below specified thresholds throughout their operational lifetime.
Safety certification for commercial iron-air cells requires passing rigorous abuse tests, including overcharging, external short circuit, and thermal stability tests. During these tests, cells must demonstrate controlled gas management without catastrophic failure. The National Renewable Energy Laboratory (NREL) has developed specialized protocols for evaluating the effectiveness of gas venting mechanisms in metal-air batteries, which are becoming industry benchmarks.
Regulatory frameworks in different regions present varying requirements for iron-air technology. The European Union, through its Battery Directive, has established specific safety parameters for metal-air batteries, while the U.S. Department of Energy has funded research to develop standardized testing methodologies for emerging battery technologies, including iron-air systems.
Industry consortia like the Battery Safety Council are working to harmonize these diverse standards into a cohesive framework specifically addressing the unique challenges of iron-air technology. Their recommendations include implementing real-time gas monitoring systems in large-scale installations and establishing maintenance protocols that account for the gradual evolution of hydrogen and oxygen during operation.
As iron-air technology advances toward commercialization, these safety standards and testing protocols will continue to evolve, with increasing focus on long-term reliability and integration with existing energy storage regulatory frameworks.
The International Electrotechnical Commission (IEC) has begun incorporating iron-air cell specifications into their battery safety standards, focusing particularly on gas management systems. These standards mandate specific pressure relief mechanisms and recommend optimal cell designs that minimize bubble accumulation. The UL (Underwriters Laboratories) has also developed preliminary testing protocols specifically addressing gas evolution in metal-air batteries, requiring cells to demonstrate stable operation under various charging and discharging conditions.
Testing protocols for iron-air cells typically include accelerated life cycle testing under controlled conditions to evaluate gas bubble formation over time. These tests often involve operating cells at elevated temperatures (40-60°C) and varying charge rates to simulate worst-case scenarios for gas evolution. Pressure monitoring during these tests is critical, with standards typically requiring cells to maintain internal pressure below specified thresholds throughout their operational lifetime.
Safety certification for commercial iron-air cells requires passing rigorous abuse tests, including overcharging, external short circuit, and thermal stability tests. During these tests, cells must demonstrate controlled gas management without catastrophic failure. The National Renewable Energy Laboratory (NREL) has developed specialized protocols for evaluating the effectiveness of gas venting mechanisms in metal-air batteries, which are becoming industry benchmarks.
Regulatory frameworks in different regions present varying requirements for iron-air technology. The European Union, through its Battery Directive, has established specific safety parameters for metal-air batteries, while the U.S. Department of Energy has funded research to develop standardized testing methodologies for emerging battery technologies, including iron-air systems.
Industry consortia like the Battery Safety Council are working to harmonize these diverse standards into a cohesive framework specifically addressing the unique challenges of iron-air technology. Their recommendations include implementing real-time gas monitoring systems in large-scale installations and establishing maintenance protocols that account for the gradual evolution of hydrogen and oxygen during operation.
As iron-air technology advances toward commercialization, these safety standards and testing protocols will continue to evolve, with increasing focus on long-term reliability and integration with existing energy storage regulatory frameworks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!