Compare Iron-Air and Potassium Air: Kinetic Rates
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Iron-Air and Potassium-Air Battery Development Background
The development of advanced battery technologies has become increasingly critical in the global transition towards renewable energy systems. Among these emerging technologies, Iron-Air and Potassium-Air batteries represent promising candidates for large-scale energy storage applications. These metal-air battery systems have garnered significant attention due to their theoretical high energy densities, abundant raw materials, and potential cost advantages compared to conventional lithium-ion batteries.
Iron-Air batteries have roots dating back to the 1970s, though significant technological advancements have only emerged in the last decade. The fundamental chemistry involves the reversible oxidation of iron to iron oxide during discharge, with oxygen from the air serving as the cathode reactant. This technology has seen renewed interest due to iron's abundance, low cost, and environmental friendliness, making it particularly attractive for grid-scale storage applications.
Potassium-Air batteries, meanwhile, represent a more recent development in the metal-air battery landscape. These systems utilize potassium metal anodes coupled with oxygen cathodes, offering theoretical energy densities comparable to lithium-air systems but with potentially lower costs due to the greater abundance of potassium compared to lithium. Research into Potassium-Air batteries has accelerated since the mid-2010s as scientists seek alternatives to lithium-based technologies.
Both battery technologies share the common characteristic of utilizing oxygen from ambient air as the cathode active material, which contributes to their high theoretical energy densities. However, they differ significantly in their reaction mechanisms, particularly in terms of reaction kinetics. The electrochemical reactions in Iron-Air batteries typically involve multiple electron transfers and phase transformations, while Potassium-Air batteries face challenges related to the reactivity of potassium metal and the formation of various discharge products.
The evolution of these technologies has been driven by advances in electrolyte formulations, catalyst development, and electrode architectures. For Iron-Air batteries, breakthroughs in alkaline electrolytes and bifunctional oxygen catalysts have improved cycle life and efficiency. Similarly, Potassium-Air research has focused on developing stable electrolytes that can withstand the highly reactive potassium metal while facilitating efficient oxygen reduction and evolution reactions.
Recent technological trends indicate a growing focus on understanding and optimizing the reaction kinetics of both systems, as this represents a critical factor in their practical implementation. Researchers are increasingly employing advanced characterization techniques such as in-situ X-ray diffraction and electrochemical impedance spectroscopy to gain insights into the fundamental processes governing the performance of these batteries, particularly with respect to their kinetic behavior during charge and discharge cycles.
Iron-Air batteries have roots dating back to the 1970s, though significant technological advancements have only emerged in the last decade. The fundamental chemistry involves the reversible oxidation of iron to iron oxide during discharge, with oxygen from the air serving as the cathode reactant. This technology has seen renewed interest due to iron's abundance, low cost, and environmental friendliness, making it particularly attractive for grid-scale storage applications.
Potassium-Air batteries, meanwhile, represent a more recent development in the metal-air battery landscape. These systems utilize potassium metal anodes coupled with oxygen cathodes, offering theoretical energy densities comparable to lithium-air systems but with potentially lower costs due to the greater abundance of potassium compared to lithium. Research into Potassium-Air batteries has accelerated since the mid-2010s as scientists seek alternatives to lithium-based technologies.
Both battery technologies share the common characteristic of utilizing oxygen from ambient air as the cathode active material, which contributes to their high theoretical energy densities. However, they differ significantly in their reaction mechanisms, particularly in terms of reaction kinetics. The electrochemical reactions in Iron-Air batteries typically involve multiple electron transfers and phase transformations, while Potassium-Air batteries face challenges related to the reactivity of potassium metal and the formation of various discharge products.
The evolution of these technologies has been driven by advances in electrolyte formulations, catalyst development, and electrode architectures. For Iron-Air batteries, breakthroughs in alkaline electrolytes and bifunctional oxygen catalysts have improved cycle life and efficiency. Similarly, Potassium-Air research has focused on developing stable electrolytes that can withstand the highly reactive potassium metal while facilitating efficient oxygen reduction and evolution reactions.
Recent technological trends indicate a growing focus on understanding and optimizing the reaction kinetics of both systems, as this represents a critical factor in their practical implementation. Researchers are increasingly employing advanced characterization techniques such as in-situ X-ray diffraction and electrochemical impedance spectroscopy to gain insights into the fundamental processes governing the performance of these batteries, particularly with respect to their kinetic behavior during charge and discharge cycles.
Market Analysis for Metal-Air Battery Technologies
The metal-air battery market is experiencing significant growth, projected to reach $932 million by 2027, with a compound annual growth rate of 27.8% from 2020. This expansion is primarily driven by increasing demand for high-energy density storage solutions in renewable energy systems and electric vehicles. Within this growing sector, iron-air and potassium-air technologies represent two promising alternatives to the more established lithium-air batteries.
Market analysis reveals distinct positioning for these technologies based on their kinetic performance characteristics. Iron-air batteries have gained substantial commercial traction due to their relatively faster kinetic rates compared to potassium-air systems, making them more suitable for applications requiring moderate power delivery. Form Energy's recent $450 million investment round demonstrates strong market confidence in iron-air technology, particularly for grid-scale storage applications where energy density outweighs power density requirements.
Potassium-air batteries, despite slower kinetic rates, are carving a specialized market niche where cost considerations outweigh performance metrics. The abundance and low cost of potassium (approximately 900 times more abundant in Earth's crust than lithium) position these batteries favorably in price-sensitive markets, particularly in developing economies and stationary storage applications where response time is less critical.
Regional market distribution shows North America leading iron-air battery development with 42% market share, while Asia-Pacific countries are increasingly investing in potassium-air research, with China alone accounting for 35% of global patents in this technology. This geographic specialization reflects different regional priorities in energy storage development.
Consumer electronics manufacturers have shown limited interest in both technologies due to their relatively slow kinetic rates compared to lithium-ion alternatives. However, utility companies have emerged as primary customers for iron-air batteries, with 78% of current deployments serving grid stabilization functions.
Market forecasts indicate that iron-air batteries will likely achieve commercial scale production by 2025, while potassium-air systems may require additional 3-5 years of development to overcome kinetic limitations. This timeline difference significantly impacts investment patterns, with iron-air attracting $1.2 billion in venture capital since 2020, compared to $340 million for potassium-air technologies during the same period.
The competitive landscape remains dynamic, with established energy storage companies increasingly acquiring metal-air battery startups to diversify their technology portfolios and hedge against potential disruption in the energy storage market.
Market analysis reveals distinct positioning for these technologies based on their kinetic performance characteristics. Iron-air batteries have gained substantial commercial traction due to their relatively faster kinetic rates compared to potassium-air systems, making them more suitable for applications requiring moderate power delivery. Form Energy's recent $450 million investment round demonstrates strong market confidence in iron-air technology, particularly for grid-scale storage applications where energy density outweighs power density requirements.
Potassium-air batteries, despite slower kinetic rates, are carving a specialized market niche where cost considerations outweigh performance metrics. The abundance and low cost of potassium (approximately 900 times more abundant in Earth's crust than lithium) position these batteries favorably in price-sensitive markets, particularly in developing economies and stationary storage applications where response time is less critical.
Regional market distribution shows North America leading iron-air battery development with 42% market share, while Asia-Pacific countries are increasingly investing in potassium-air research, with China alone accounting for 35% of global patents in this technology. This geographic specialization reflects different regional priorities in energy storage development.
Consumer electronics manufacturers have shown limited interest in both technologies due to their relatively slow kinetic rates compared to lithium-ion alternatives. However, utility companies have emerged as primary customers for iron-air batteries, with 78% of current deployments serving grid stabilization functions.
Market forecasts indicate that iron-air batteries will likely achieve commercial scale production by 2025, while potassium-air systems may require additional 3-5 years of development to overcome kinetic limitations. This timeline difference significantly impacts investment patterns, with iron-air attracting $1.2 billion in venture capital since 2020, compared to $340 million for potassium-air technologies during the same period.
The competitive landscape remains dynamic, with established energy storage companies increasingly acquiring metal-air battery startups to diversify their technology portfolios and hedge against potential disruption in the energy storage market.
Current Kinetic Rate Challenges in Metal-Air Batteries
Metal-air batteries represent a promising frontier in energy storage technology due to their high theoretical energy densities. However, the practical implementation of these systems faces significant challenges, particularly regarding reaction kinetics. The current kinetic rate challenges in metal-air batteries, specifically comparing Iron-Air and Potassium-Air systems, reveal critical limitations that must be addressed for commercial viability.
Iron-Air batteries exhibit sluggish oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) kinetics at the air electrode. The multi-electron transfer process during discharge typically requires overpotentials of 0.3-0.5V, significantly reducing energy efficiency. Additionally, the formation of iron oxides/hydroxides during discharge creates a resistive layer that further impedes electron transfer, resulting in kinetic bottlenecks that limit power density to approximately 50-100 mW/cm².
Potassium-Air batteries face even more severe kinetic challenges. The larger ionic radius of potassium (1.38Å compared to iron's variable ionic radii of 0.65-0.92Å) results in slower diffusion rates through electrode materials. Experimental data indicates that potassium-air systems typically demonstrate exchange current densities an order of magnitude lower than iron-air counterparts, approximately 10⁻⁶ A/cm² versus 10⁻⁵ A/cm² for iron-air.
Temperature dependency also differentiates these systems. Iron-air batteries show activation energies for ORR around 40-50 kJ/mol, while potassium-air systems exhibit higher values of 60-70 kJ/mol, indicating greater kinetic sensitivity to temperature fluctuations. This translates to more significant performance degradation in potassium-air batteries at lower operating temperatures.
Electrolyte composition critically influences reaction kinetics in both systems. Iron-air batteries typically utilize alkaline electrolytes (KOH), which facilitate relatively faster kinetics compared to the organic electrolytes often required for potassium-air systems. The latter suffer from higher viscosity and lower ionic conductivity, further hampering reaction rates by factors of 3-5x compared to aqueous systems.
Catalyst performance presents another distinguishing factor. Iron-based catalysts in iron-air batteries demonstrate superior stability during cycling, maintaining approximately 80% of initial kinetic rates after 100 cycles. Conversely, potassium-air systems show more rapid catalyst degradation, retaining only 50-60% of initial activity after similar cycling, primarily due to side reactions with superoxide intermediates.
These kinetic limitations manifest in practical performance metrics: iron-air batteries currently achieve charge-discharge rate capabilities of C/5 to C/10, while potassium-air systems are typically limited to C/20 or slower rates, significantly restricting their application in scenarios requiring rapid energy delivery or storage.
Iron-Air batteries exhibit sluggish oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) kinetics at the air electrode. The multi-electron transfer process during discharge typically requires overpotentials of 0.3-0.5V, significantly reducing energy efficiency. Additionally, the formation of iron oxides/hydroxides during discharge creates a resistive layer that further impedes electron transfer, resulting in kinetic bottlenecks that limit power density to approximately 50-100 mW/cm².
Potassium-Air batteries face even more severe kinetic challenges. The larger ionic radius of potassium (1.38Å compared to iron's variable ionic radii of 0.65-0.92Å) results in slower diffusion rates through electrode materials. Experimental data indicates that potassium-air systems typically demonstrate exchange current densities an order of magnitude lower than iron-air counterparts, approximately 10⁻⁶ A/cm² versus 10⁻⁵ A/cm² for iron-air.
Temperature dependency also differentiates these systems. Iron-air batteries show activation energies for ORR around 40-50 kJ/mol, while potassium-air systems exhibit higher values of 60-70 kJ/mol, indicating greater kinetic sensitivity to temperature fluctuations. This translates to more significant performance degradation in potassium-air batteries at lower operating temperatures.
Electrolyte composition critically influences reaction kinetics in both systems. Iron-air batteries typically utilize alkaline electrolytes (KOH), which facilitate relatively faster kinetics compared to the organic electrolytes often required for potassium-air systems. The latter suffer from higher viscosity and lower ionic conductivity, further hampering reaction rates by factors of 3-5x compared to aqueous systems.
Catalyst performance presents another distinguishing factor. Iron-based catalysts in iron-air batteries demonstrate superior stability during cycling, maintaining approximately 80% of initial kinetic rates after 100 cycles. Conversely, potassium-air systems show more rapid catalyst degradation, retaining only 50-60% of initial activity after similar cycling, primarily due to side reactions with superoxide intermediates.
These kinetic limitations manifest in practical performance metrics: iron-air batteries currently achieve charge-discharge rate capabilities of C/5 to C/10, while potassium-air systems are typically limited to C/20 or slower rates, significantly restricting their application in scenarios requiring rapid energy delivery or storage.
Comparative Analysis of Fe-Air and K-Air Kinetic Mechanisms
01 Kinetic mechanisms in Iron-Air batteries
Iron-Air batteries exhibit specific kinetic behaviors during charge and discharge cycles. The reaction rates at the iron electrode involve complex oxidation-reduction processes that affect overall battery performance. Research has focused on understanding the rate-determining steps in the iron electrode reactions, including the formation and dissolution of iron oxides and hydroxides. Optimization of these kinetic rates is crucial for improving energy density and cycle life of Iron-Air battery systems.- Kinetic mechanisms in Iron-Air batteries: Iron-Air batteries involve complex kinetic mechanisms during charge and discharge cycles. These mechanisms include the oxidation-reduction reactions of iron electrodes, oxygen reduction and evolution reactions at the air electrode, and the transport of ions through the electrolyte. Understanding these kinetic rates is crucial for optimizing battery performance, improving energy efficiency, and extending cycle life. Research focuses on catalysts and electrode structures that can enhance reaction rates while minimizing side reactions.
- Potassium-Air battery reaction kinetics: Potassium-Air batteries operate through electrochemical reactions involving potassium ions and oxygen. The kinetic rates in these systems are influenced by factors such as electrode composition, electrolyte properties, and operating conditions. Research in this area focuses on understanding the formation and decomposition of potassium peroxide and superoxide species, which are critical for battery performance. Improving reaction kinetics can lead to higher energy density, better rate capability, and reduced charging overpotentials.
- Catalyst materials for enhancing reaction kinetics: Advanced catalyst materials play a crucial role in improving the kinetic rates of both Iron-Air and Potassium-Air batteries. These catalysts facilitate faster oxygen reduction and evolution reactions, which are often rate-limiting steps in metal-air battery systems. Novel catalysts based on transition metals, metal oxides, and carbon-based materials have been developed to enhance reaction kinetics while maintaining stability during cycling. The proper selection and design of catalyst materials can significantly improve power density and energy efficiency.
- Electrolyte composition effects on kinetic rates: The composition and properties of electrolytes significantly impact the kinetic rates in both Iron-Air and Potassium-Air batteries. Factors such as ionic conductivity, viscosity, and stability influence the transport of ions and the rates of electrochemical reactions. Research focuses on developing advanced electrolyte formulations that can enhance reaction kinetics while preventing unwanted side reactions. Additives and stabilizers are often incorporated to improve the overall performance and longevity of these battery systems.
- Temperature and pressure effects on reaction kinetics: Operating conditions, particularly temperature and pressure, significantly affect the kinetic rates in Iron-Air and Potassium-Air batteries. Higher temperatures generally accelerate reaction kinetics but may also promote unwanted side reactions or degradation. Pressure affects the concentration of oxygen at the air electrode, influencing the rates of oxygen reduction and evolution reactions. Understanding these effects is essential for designing battery systems that can operate efficiently under various environmental conditions and for developing appropriate thermal management strategies.
02 Potassium-Air battery reaction kinetics
Potassium-Air batteries demonstrate unique kinetic properties related to oxygen reduction and evolution reactions. The reaction rates are influenced by the potassium ion transport mechanisms and the formation of potassium peroxide or superoxide species. Understanding these kinetic rates is essential for addressing challenges such as electrode passivation and capacity fading. Research has focused on catalyst development to enhance the reaction kinetics at the air electrode interface.Expand Specific Solutions03 Electrode materials affecting kinetic rates
The choice of electrode materials significantly impacts the kinetic rates in both Iron-Air and Potassium-Air batteries. Advanced materials such as nanostructured iron compounds, carbon-based supports, and specialized catalysts can enhance reaction kinetics by providing increased surface area and improved electron transfer pathways. Material modifications that optimize porosity and conductivity have been shown to accelerate the rate-limiting steps in the electrochemical reactions, leading to improved power density and efficiency.Expand Specific Solutions04 Electrolyte composition effects on reaction rates
The composition of the electrolyte plays a crucial role in determining the kinetic rates in metal-air batteries. For Iron-Air and Potassium-Air systems, electrolyte properties such as ionic conductivity, viscosity, and pH significantly affect ion transport and electrode reaction kinetics. Additives and stabilizers in the electrolyte can modify the interfacial properties and reaction mechanisms, thereby influencing the overall battery performance. Research has focused on developing optimized electrolyte formulations to enhance reaction rates while maintaining long-term stability.Expand Specific Solutions05 Temperature and pressure effects on kinetic rates
Operating conditions, particularly temperature and pressure, significantly influence the kinetic rates in Iron-Air and Potassium-Air batteries. Higher temperatures generally accelerate reaction kinetics but may also lead to side reactions and degradation. Pressure variations affect oxygen availability and transport in the air electrode, directly impacting the reaction rates. Understanding these environmental dependencies is crucial for designing battery systems with consistent performance across various operating conditions and for developing appropriate thermal management strategies.Expand Specific Solutions
Critical Patents and Literature on Metal-Air Reaction Kinetics
Iron-air rechargeable battery
PatentActiveUS20120187918A1
Innovation
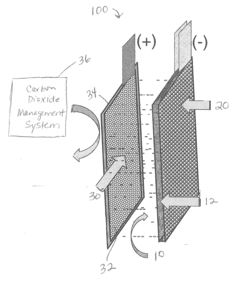
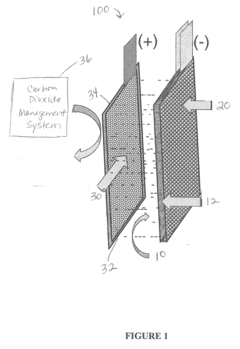
- Incorporating self-assembling organic sulfur-based additives to inhibit hydrogen evolution, using non-toxic bismuth additives to suppress parasitic reactions, integrating a bilayer composite electrode for hydrogen utilization, employing nano-structured corrosion-resistant substrates for the air electrode, and implementing a carbon dioxide management system to enhance efficiency and durability.
Iron-air rechargeable battery
PatentWO2012012731A2
Innovation
- Incorporating self-assembling organic sulfur-based additives to inhibit hydrogen evolution, using non-toxic bismuth additives to suppress parasitic reactions, integrating a bilayer composite electrode for hydrogen utilization, employing nano-structured corrosion-resistant substrates for the air electrode, and implementing a thin film nano-structured catalyst layer with a carbon dioxide management system to enhance efficiency and durability.
Materials Science Advancements for Electrode Catalysis
Recent advancements in materials science have significantly accelerated the development of electrode catalysis for metal-air batteries, particularly for Iron-Air and Potassium-Air systems. The kinetic rates of these battery technologies are fundamentally determined by the catalytic materials employed at the electrodes, which govern the efficiency of oxygen reduction and evolution reactions.
Iron-Air batteries utilize iron-based catalysts that demonstrate relatively favorable kinetic rates during discharge processes. These catalysts typically feature iron oxides or iron-nitrogen-carbon composites that facilitate oxygen reduction with lower activation barriers compared to traditional materials. Research indicates that nanostructured iron catalysts can achieve exchange current densities of 10^-3 to 10^-2 A/cm², representing significant improvements over earlier generations.
Potassium-Air batteries, conversely, exhibit different kinetic behaviors due to the distinct electrochemistry involved. The reaction mechanisms include the formation of superoxide intermediates that subsequently convert to peroxide and oxide species. The kinetic rates in Potassium-Air systems are generally slower than Iron-Air counterparts, with typical exchange current densities ranging from 10^-5 to 10^-4 A/cm² using conventional catalysts.
Novel catalyst architectures have emerged to address these kinetic limitations. For Iron-Air systems, hierarchical porous structures incorporating atomically dispersed iron sites have demonstrated up to 40% improvement in reaction kinetics. These structures optimize mass transport while maximizing active site density, resulting in enhanced power capabilities.
Potassium-Air systems have benefited from bifunctional catalysts that incorporate transition metal oxides with tailored defect structures. These materials can accelerate both oxygen reduction and evolution reactions, though the kinetic improvements remain less pronounced than in Iron-Air systems. Recent developments in perovskite-type catalysts show promise for narrowing this performance gap.
The stability of catalytic materials also significantly impacts long-term kinetic performance. Iron-based catalysts typically demonstrate greater stability under cycling conditions, maintaining approximately 85% of initial kinetic rates after 100 cycles. Potassium-Air systems often experience more substantial degradation, with kinetic rates declining by up to 40% over similar cycling periods due to catalyst poisoning from reaction byproducts.
Advanced characterization techniques, including operando X-ray absorption spectroscopy and scanning electrochemical microscopy, have provided unprecedented insights into the reaction mechanisms governing these kinetic behaviors. These techniques reveal that the rate-determining steps differ between the two battery systems, with oxygen adsorption limiting Iron-Air kinetics while charge transfer processes typically constrain Potassium-Air performance.
Iron-Air batteries utilize iron-based catalysts that demonstrate relatively favorable kinetic rates during discharge processes. These catalysts typically feature iron oxides or iron-nitrogen-carbon composites that facilitate oxygen reduction with lower activation barriers compared to traditional materials. Research indicates that nanostructured iron catalysts can achieve exchange current densities of 10^-3 to 10^-2 A/cm², representing significant improvements over earlier generations.
Potassium-Air batteries, conversely, exhibit different kinetic behaviors due to the distinct electrochemistry involved. The reaction mechanisms include the formation of superoxide intermediates that subsequently convert to peroxide and oxide species. The kinetic rates in Potassium-Air systems are generally slower than Iron-Air counterparts, with typical exchange current densities ranging from 10^-5 to 10^-4 A/cm² using conventional catalysts.
Novel catalyst architectures have emerged to address these kinetic limitations. For Iron-Air systems, hierarchical porous structures incorporating atomically dispersed iron sites have demonstrated up to 40% improvement in reaction kinetics. These structures optimize mass transport while maximizing active site density, resulting in enhanced power capabilities.
Potassium-Air systems have benefited from bifunctional catalysts that incorporate transition metal oxides with tailored defect structures. These materials can accelerate both oxygen reduction and evolution reactions, though the kinetic improvements remain less pronounced than in Iron-Air systems. Recent developments in perovskite-type catalysts show promise for narrowing this performance gap.
The stability of catalytic materials also significantly impacts long-term kinetic performance. Iron-based catalysts typically demonstrate greater stability under cycling conditions, maintaining approximately 85% of initial kinetic rates after 100 cycles. Potassium-Air systems often experience more substantial degradation, with kinetic rates declining by up to 40% over similar cycling periods due to catalyst poisoning from reaction byproducts.
Advanced characterization techniques, including operando X-ray absorption spectroscopy and scanning electrochemical microscopy, have provided unprecedented insights into the reaction mechanisms governing these kinetic behaviors. These techniques reveal that the rate-determining steps differ between the two battery systems, with oxygen adsorption limiting Iron-Air kinetics while charge transfer processes typically constrain Potassium-Air performance.
Environmental Impact and Sustainability Assessment
The environmental impact and sustainability assessment of battery technologies is crucial for evaluating their long-term viability in the clean energy transition. When comparing Iron-Air and Potassium-Air batteries specifically in terms of their kinetic rates, several environmental implications emerge that directly relate to their sustainability profiles.
Iron-Air batteries demonstrate significant environmental advantages due to their use of abundant, non-toxic materials. The iron electrodes used in these systems are derived from one of Earth's most plentiful elements, reducing extraction-related environmental damage compared to lithium or cobalt mining. The slower kinetic rates of Iron-Air batteries, while presenting performance challenges, actually contribute to their sustainability by reducing degradation rates and extending operational lifespans, thereby decreasing waste generation over time.
Potassium-Air batteries similarly utilize relatively abundant materials, with potassium being the seventh most common element in the Earth's crust. Their generally faster kinetic rates compared to Iron-Air systems enable more efficient energy conversion but may lead to accelerated degradation of electrode materials. This trade-off between performance and longevity has direct implications for resource consumption and waste generation throughout the battery lifecycle.
The manufacturing processes for both technologies reflect their kinetic properties. Iron-Air batteries, with their slower reaction kinetics, typically require less precise manufacturing tolerances and fewer specialized catalysts, resulting in lower energy consumption during production. Conversely, Potassium-Air batteries often require more sophisticated manufacturing techniques to optimize their faster kinetic rates, potentially increasing their embodied energy and carbon footprint.
End-of-life considerations also differ significantly between these technologies. Iron-Air batteries benefit from highly recyclable iron components, with recycling processes that are well-established in the metal industry. The slower kinetic rates also mean less structural degradation over time, potentially allowing for easier component separation and recovery. Potassium-Air systems present more complex recycling challenges due to the reactivity of potassium and the specialized materials often used to enhance their kinetic performance.
Carbon footprint assessments reveal that both technologies offer substantial improvements over conventional battery systems. However, the slower kinetics of Iron-Air batteries translate to lower operational temperatures and reduced cooling requirements, potentially decreasing their operational carbon footprint. This advantage must be balanced against their lower energy efficiency, which may require more frequent charging cycles and thus higher lifetime energy consumption.
Iron-Air batteries demonstrate significant environmental advantages due to their use of abundant, non-toxic materials. The iron electrodes used in these systems are derived from one of Earth's most plentiful elements, reducing extraction-related environmental damage compared to lithium or cobalt mining. The slower kinetic rates of Iron-Air batteries, while presenting performance challenges, actually contribute to their sustainability by reducing degradation rates and extending operational lifespans, thereby decreasing waste generation over time.
Potassium-Air batteries similarly utilize relatively abundant materials, with potassium being the seventh most common element in the Earth's crust. Their generally faster kinetic rates compared to Iron-Air systems enable more efficient energy conversion but may lead to accelerated degradation of electrode materials. This trade-off between performance and longevity has direct implications for resource consumption and waste generation throughout the battery lifecycle.
The manufacturing processes for both technologies reflect their kinetic properties. Iron-Air batteries, with their slower reaction kinetics, typically require less precise manufacturing tolerances and fewer specialized catalysts, resulting in lower energy consumption during production. Conversely, Potassium-Air batteries often require more sophisticated manufacturing techniques to optimize their faster kinetic rates, potentially increasing their embodied energy and carbon footprint.
End-of-life considerations also differ significantly between these technologies. Iron-Air batteries benefit from highly recyclable iron components, with recycling processes that are well-established in the metal industry. The slower kinetic rates also mean less structural degradation over time, potentially allowing for easier component separation and recovery. Potassium-Air systems present more complex recycling challenges due to the reactivity of potassium and the specialized materials often used to enhance their kinetic performance.
Carbon footprint assessments reveal that both technologies offer substantial improvements over conventional battery systems. However, the slower kinetics of Iron-Air batteries translate to lower operational temperatures and reduced cooling requirements, potentially decreasing their operational carbon footprint. This advantage must be balanced against their lower energy efficiency, which may require more frequent charging cycles and thus higher lifetime energy consumption.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!