How to Reduce Self-Discharge in Iron-Air Battery Systems
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Iron-Air Battery Technology Background and Objectives
Iron-air batteries represent a significant advancement in energy storage technology, with roots dating back to the 1970s when initial research explored their potential. These batteries operate on the principle of iron oxidation and reduction, utilizing the abundant and low-cost iron as the anode material, while oxygen from ambient air serves as the cathode active material. This fundamental chemistry offers a theoretical energy density of approximately 764 Wh/kg, positioning iron-air batteries as promising candidates for large-scale energy storage applications.
The evolution of iron-air battery technology has been marked by periods of intense research followed by relative dormancy. Initial enthusiasm in the 1970s and 1980s waned due to persistent technical challenges, particularly self-discharge issues caused by hydrogen evolution at the iron electrode. Recent years have witnessed a resurgence of interest, driven by the growing demand for sustainable and cost-effective energy storage solutions to support renewable energy integration and grid stabilization.
Current technological objectives in iron-air battery development center on addressing several critical limitations, with self-discharge reduction being paramount. Self-discharge in these systems primarily occurs through parasitic hydrogen evolution reactions at the iron electrode, significantly diminishing capacity retention and overall system efficiency. Research aims to develop innovative electrode materials, electrolyte formulations, and cell designs that minimize these unwanted side reactions while maintaining the inherent cost advantages of iron-air technology.
The global push toward decarbonization has established clear targets for iron-air battery advancement. These include achieving cycle life exceeding 5,000 cycles, reducing self-discharge rates to below 1% per day, and maintaining system costs under $100/kWh to ensure economic viability against competing technologies such as lithium-ion and flow batteries. Additionally, researchers are working to improve energy efficiency beyond the current 50-60% range to enhance overall system performance.
Technical development is progressing along multiple pathways, including the exploration of iron electrode additives to suppress hydrogen evolution, advanced electrolyte systems with reduced water activity, and novel cell architectures that minimize parasitic reactions. Computational modeling and high-throughput experimental approaches are accelerating this development process, enabling more systematic investigation of complex electrochemical interactions within these systems.
The ultimate goal is to position iron-air battery technology as a cornerstone of sustainable energy infrastructure, leveraging its exceptional combination of abundant raw materials, environmental compatibility, and theoretical energy density to address the growing global demand for long-duration energy storage solutions.
The evolution of iron-air battery technology has been marked by periods of intense research followed by relative dormancy. Initial enthusiasm in the 1970s and 1980s waned due to persistent technical challenges, particularly self-discharge issues caused by hydrogen evolution at the iron electrode. Recent years have witnessed a resurgence of interest, driven by the growing demand for sustainable and cost-effective energy storage solutions to support renewable energy integration and grid stabilization.
Current technological objectives in iron-air battery development center on addressing several critical limitations, with self-discharge reduction being paramount. Self-discharge in these systems primarily occurs through parasitic hydrogen evolution reactions at the iron electrode, significantly diminishing capacity retention and overall system efficiency. Research aims to develop innovative electrode materials, electrolyte formulations, and cell designs that minimize these unwanted side reactions while maintaining the inherent cost advantages of iron-air technology.
The global push toward decarbonization has established clear targets for iron-air battery advancement. These include achieving cycle life exceeding 5,000 cycles, reducing self-discharge rates to below 1% per day, and maintaining system costs under $100/kWh to ensure economic viability against competing technologies such as lithium-ion and flow batteries. Additionally, researchers are working to improve energy efficiency beyond the current 50-60% range to enhance overall system performance.
Technical development is progressing along multiple pathways, including the exploration of iron electrode additives to suppress hydrogen evolution, advanced electrolyte systems with reduced water activity, and novel cell architectures that minimize parasitic reactions. Computational modeling and high-throughput experimental approaches are accelerating this development process, enabling more systematic investigation of complex electrochemical interactions within these systems.
The ultimate goal is to position iron-air battery technology as a cornerstone of sustainable energy infrastructure, leveraging its exceptional combination of abundant raw materials, environmental compatibility, and theoretical energy density to address the growing global demand for long-duration energy storage solutions.
Market Analysis for Iron-Air Energy Storage
The iron-air battery energy storage market is experiencing significant growth as the global energy landscape shifts towards renewable sources. Current market projections indicate that grid-scale energy storage demand will reach approximately 2,500 GWh by 2030, with long-duration storage solutions like iron-air batteries poised to capture a substantial portion of this expanding market. The technology's appeal stems from its potential to provide energy storage durations of 100+ hours at costs significantly lower than lithium-ion alternatives.
The primary market drivers for iron-air battery adoption include the increasing integration of intermittent renewable energy sources, growing grid stability concerns, and the push for decarbonization across various sectors. Utility companies and grid operators represent the largest customer segment, seeking cost-effective solutions for load shifting, peak shaving, and backup power applications. Additionally, commercial and industrial users with high energy demands are emerging as a secondary market, particularly in regions with unreliable grid infrastructure.
From a geographical perspective, North America currently leads the iron-air battery market development, with companies like Form Energy securing major utility contracts. Europe follows closely behind, driven by aggressive renewable energy targets and supportive regulatory frameworks. The Asia-Pacific region, particularly China and India, represents the fastest-growing market due to massive renewable energy deployments and increasing grid modernization efforts.
Cost analysis reveals iron-air systems' compelling value proposition. While current levelized cost of storage (LCOS) estimates range between $20-30/kWh for iron-air systems, this represents a 50-80% reduction compared to lithium-ion alternatives for long-duration applications. The technology's use of abundant, low-cost materials (iron, water, salt) provides significant economic advantages and insulation from supply chain volatility that affects other battery chemistries.
Market barriers include the technology's relatively lower round-trip efficiency (approximately 50-60%) compared to lithium-ion batteries, limited commercial deployment history, and competition from other emerging long-duration storage technologies such as flow batteries and compressed air energy storage. However, the iron-air battery's exceptional cycle life, minimal degradation, and non-flammable nature offset these limitations for many applications.
Industry analysts project the iron-air battery market to grow at a CAGR exceeding 25% through 2030, with total market value potentially reaching billions as the technology moves from pilot deployments to widespread commercial adoption. This growth trajectory is supported by increasing investment in the sector, with venture capital and corporate funding accelerating technology development and manufacturing scale-up.
The primary market drivers for iron-air battery adoption include the increasing integration of intermittent renewable energy sources, growing grid stability concerns, and the push for decarbonization across various sectors. Utility companies and grid operators represent the largest customer segment, seeking cost-effective solutions for load shifting, peak shaving, and backup power applications. Additionally, commercial and industrial users with high energy demands are emerging as a secondary market, particularly in regions with unreliable grid infrastructure.
From a geographical perspective, North America currently leads the iron-air battery market development, with companies like Form Energy securing major utility contracts. Europe follows closely behind, driven by aggressive renewable energy targets and supportive regulatory frameworks. The Asia-Pacific region, particularly China and India, represents the fastest-growing market due to massive renewable energy deployments and increasing grid modernization efforts.
Cost analysis reveals iron-air systems' compelling value proposition. While current levelized cost of storage (LCOS) estimates range between $20-30/kWh for iron-air systems, this represents a 50-80% reduction compared to lithium-ion alternatives for long-duration applications. The technology's use of abundant, low-cost materials (iron, water, salt) provides significant economic advantages and insulation from supply chain volatility that affects other battery chemistries.
Market barriers include the technology's relatively lower round-trip efficiency (approximately 50-60%) compared to lithium-ion batteries, limited commercial deployment history, and competition from other emerging long-duration storage technologies such as flow batteries and compressed air energy storage. However, the iron-air battery's exceptional cycle life, minimal degradation, and non-flammable nature offset these limitations for many applications.
Industry analysts project the iron-air battery market to grow at a CAGR exceeding 25% through 2030, with total market value potentially reaching billions as the technology moves from pilot deployments to widespread commercial adoption. This growth trajectory is supported by increasing investment in the sector, with venture capital and corporate funding accelerating technology development and manufacturing scale-up.
Self-Discharge Challenges in Iron-Air Systems
Self-discharge represents one of the most significant challenges in iron-air battery systems, substantially limiting their commercial viability despite their promising theoretical energy density and cost advantages. The primary self-discharge mechanism in these systems stems from the parasitic hydrogen evolution reaction (HER) that occurs at the iron electrode during idle periods. This reaction consumes stored energy and reduces the battery's state of charge without delivering useful work to external circuits.
The iron electrode's relatively low hydrogen overpotential creates a thermodynamically favorable environment for water reduction, resulting in continuous hydrogen gas evolution. This process not only depletes stored energy but also causes physical degradation of the electrode structure over time. Studies have shown that iron-air batteries can lose between 2-5% of their stored capacity per day due to self-discharge mechanisms, compared to less than 0.5% for commercial lithium-ion systems.
Another critical factor contributing to self-discharge is the oxygen reduction reaction (ORR) at the air electrode. During idle periods, oxygen from the air electrode can diffuse through the electrolyte and react with the charged iron electrode, causing premature discharge. This cross-contamination between electrodes accelerates capacity fade and reduces overall system efficiency.
Electrolyte composition plays a crucial role in self-discharge behavior. The highly alkaline electrolytes (typically 30-45% KOH) necessary for optimal iron-air battery operation also promote side reactions. Dissolved metal impurities in the electrolyte can catalyze hydrogen evolution, while carbonation from atmospheric CO2 exposure gradually degrades electrolyte performance and increases internal resistance.
Temperature fluctuations significantly impact self-discharge rates, with higher temperatures accelerating parasitic reactions. Research indicates that self-discharge rates approximately double with every 10°C increase in operating temperature. This temperature sensitivity creates additional challenges for thermal management systems in practical applications.
The physical structure of the iron electrode further complicates self-discharge mitigation. As the electrode cycles, morphological changes create fresh iron surfaces with varying catalytic activities toward hydrogen evolution. These microstructural evolutions make self-discharge behavior dynamic and increasingly difficult to control over the battery's lifetime.
Recent experimental data suggests that self-discharge rates also correlate with the state of charge, with higher states of charge exhibiting accelerated capacity loss. This non-linear behavior complicates state-of-charge estimation and battery management strategies, presenting additional challenges for system integration and control algorithms.
The iron electrode's relatively low hydrogen overpotential creates a thermodynamically favorable environment for water reduction, resulting in continuous hydrogen gas evolution. This process not only depletes stored energy but also causes physical degradation of the electrode structure over time. Studies have shown that iron-air batteries can lose between 2-5% of their stored capacity per day due to self-discharge mechanisms, compared to less than 0.5% for commercial lithium-ion systems.
Another critical factor contributing to self-discharge is the oxygen reduction reaction (ORR) at the air electrode. During idle periods, oxygen from the air electrode can diffuse through the electrolyte and react with the charged iron electrode, causing premature discharge. This cross-contamination between electrodes accelerates capacity fade and reduces overall system efficiency.
Electrolyte composition plays a crucial role in self-discharge behavior. The highly alkaline electrolytes (typically 30-45% KOH) necessary for optimal iron-air battery operation also promote side reactions. Dissolved metal impurities in the electrolyte can catalyze hydrogen evolution, while carbonation from atmospheric CO2 exposure gradually degrades electrolyte performance and increases internal resistance.
Temperature fluctuations significantly impact self-discharge rates, with higher temperatures accelerating parasitic reactions. Research indicates that self-discharge rates approximately double with every 10°C increase in operating temperature. This temperature sensitivity creates additional challenges for thermal management systems in practical applications.
The physical structure of the iron electrode further complicates self-discharge mitigation. As the electrode cycles, morphological changes create fresh iron surfaces with varying catalytic activities toward hydrogen evolution. These microstructural evolutions make self-discharge behavior dynamic and increasingly difficult to control over the battery's lifetime.
Recent experimental data suggests that self-discharge rates also correlate with the state of charge, with higher states of charge exhibiting accelerated capacity loss. This non-linear behavior complicates state-of-charge estimation and battery management strategies, presenting additional challenges for system integration and control algorithms.
Current Self-Discharge Mitigation Strategies
01 Self-discharge mechanisms in iron-air batteries
Iron-air batteries experience self-discharge primarily due to the corrosion of iron electrodes in the presence of oxygen and moisture. This process involves the oxidation of iron to iron oxides or hydroxides even when the battery is not in use. The self-discharge rate is influenced by factors such as temperature, humidity, and the composition of the electrolyte. Understanding these mechanisms is crucial for developing strategies to mitigate self-discharge and improve the overall performance and shelf life of iron-air battery systems.- Self-discharge mechanisms in iron-air batteries: Iron-air batteries experience self-discharge primarily due to the oxidation of iron electrodes when exposed to oxygen. This process occurs even when the battery is not in use, leading to capacity loss over time. The reaction between iron and oxygen forms iron oxides, which reduces the available active material for energy storage. Understanding these mechanisms is crucial for developing strategies to mitigate self-discharge and improve battery longevity.
- Electrolyte composition to reduce self-discharge: The composition of the electrolyte significantly affects the self-discharge rate in iron-air batteries. Additives such as bismuth, lead, and sulfur compounds can be incorporated into the electrolyte to form protective layers on the iron electrode surface, inhibiting unwanted side reactions. Additionally, optimizing the concentration of alkaline components like potassium hydroxide can help balance ionic conductivity with corrosion prevention, thereby reducing self-discharge rates during storage periods.
- Electrode design and materials to minimize self-discharge: Advanced electrode designs can significantly reduce self-discharge in iron-air battery systems. Using iron alloys instead of pure iron can enhance stability and reduce parasitic reactions. Incorporating carbon-based materials as conductive additives improves electron transfer while providing some protection against oxidation. Nano-structuring of iron particles increases surface area for energy storage while allowing for protective coatings that inhibit unwanted reactions during idle periods.
- Air management systems to control self-discharge: Controlling oxygen access to the iron electrode is crucial for minimizing self-discharge. Advanced air management systems incorporate mechanical or electronic valves that can restrict air flow when the battery is not in use. Some designs feature selective membranes that limit oxygen diffusion while allowing sufficient air access during discharge. These systems can be coupled with sensors to dynamically adjust air flow based on the battery's operational state, significantly extending shelf life by reducing unnecessary exposure to oxygen.
- Battery management systems for self-discharge prevention: Intelligent battery management systems can effectively mitigate self-discharge in iron-air batteries. These systems monitor battery state and implement preventive measures such as periodic maintenance cycles to restore capacity. Advanced algorithms can predict self-discharge rates based on temperature, humidity, and storage time, allowing for optimized charging schedules. Some management systems incorporate partial recharging protocols during long storage periods to counteract capacity loss, while others implement deep discharge protection to prevent irreversible damage to the iron electrodes.
02 Electrolyte additives to reduce self-discharge
Various electrolyte additives can be incorporated into iron-air batteries to minimize self-discharge. These additives work by forming protective films on the iron electrode surface, inhibiting corrosion reactions, or scavenging oxygen that would otherwise contribute to self-discharge. Common additives include organic compounds, metal salts, and surfactants that can significantly extend the shelf life of iron-air batteries by reducing the rate of self-discharge without compromising the battery's discharge capacity or cycle life.Expand Specific Solutions03 Electrode design and materials to minimize self-discharge
Advanced electrode designs and materials play a critical role in reducing self-discharge in iron-air batteries. Modifications such as surface treatments, coatings, and the incorporation of stabilizing agents can protect the iron electrode from unwanted reactions. Additionally, the use of composite materials, nanostructured iron particles, and catalyst distributions can optimize the electrochemical performance while minimizing parasitic reactions that lead to self-discharge. These design improvements help maintain the battery's state of charge during storage periods.Expand Specific Solutions04 Battery management systems for self-discharge control
Battery management systems (BMS) can be implemented to monitor and control self-discharge in iron-air battery systems. These systems employ various strategies such as periodic maintenance charging, temperature regulation, and state-of-charge monitoring to minimize the effects of self-discharge. Advanced BMS may include algorithms that predict self-discharge rates based on operating conditions and adjust charging protocols accordingly. Some systems also incorporate physical measures like oxygen barriers or controlled atmosphere storage to reduce exposure to factors that accelerate self-discharge.Expand Specific Solutions05 Hybrid and integrated systems to compensate for self-discharge
Hybrid battery systems that combine iron-air batteries with other energy storage technologies can help address self-discharge issues. These integrated systems may pair iron-air batteries with supercapacitors, lithium-ion batteries, or other complementary technologies to provide backup power during periods of self-discharge. Additionally, some designs incorporate renewable energy sources like solar panels for continuous trickle charging to offset self-discharge losses. These hybrid approaches can significantly improve the reliability and operational efficiency of iron-air battery systems in various applications.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The iron-air battery market is in an early growth phase, characterized by significant R&D investment but limited commercial deployment. The global energy storage market, which iron-air technology aims to penetrate, is projected to reach $500 billion by 2035, with iron-air batteries potentially capturing a substantial segment due to their cost advantages and abundant raw materials. Technologically, the field remains developing, with companies at varying maturity levels. Form Energy leads commercialization efforts with its multi-day storage solutions, while established players like Toyota, Hitachi, and Mitsubishi Heavy Industries leverage their manufacturing expertise to address self-discharge challenges. Academic institutions including IIT Madras and Tsinghua University contribute fundamental research, while energy companies like EDF and NTT explore integration possibilities for grid applications.
Toyota Motor Corp.
Technical Solution: Toyota has developed an innovative approach to iron-air battery self-discharge reduction through their Advanced Electrode Passivation Technology (AEPT). This system employs a nano-engineered surface coating on iron electrodes that creates a semi-permeable barrier, allowing oxygen transport for normal battery operation while blocking unwanted side reactions that cause self-discharge. The coating consists of a proprietary polymer-ceramic composite that selectively filters ions. Toyota's research has demonstrated that this approach reduces self-discharge rates by approximately 65% compared to conventional iron-air systems. Additionally, they've implemented an intelligent battery management system that applies periodic micro-cycling protocols during idle periods, effectively "refreshing" the electrode surface to prevent degradation mechanisms that contribute to self-discharge. This combined materials and systems approach maintains battery capacity during extended storage periods.
Strengths: Leverages Toyota's extensive manufacturing expertise and quality control systems; coating technology is compatible with mass production techniques; system integrates with existing battery management infrastructure. Weaknesses: Adds additional manufacturing complexity and cost; coating durability may limit cycle life in some applications; performance benefits diminish under extreme temperature conditions.
GM Global Technology Operations LLC
Technical Solution: GM has developed a comprehensive approach to reducing self-discharge in iron-air battery systems through their Stabilized Iron Electrode (SIE) technology. This multi-faceted solution addresses several key mechanisms of self-discharge: (1) Proprietary electrolyte formulation containing organic inhibitors that selectively adsorb onto iron surfaces, blocking corrosion reactions without impeding oxygen reduction; (2) Engineered electrode microstructure with optimized porosity that balances active surface area with corrosion susceptibility; (3) Specialized carbon additives that form a protective network around iron particles while maintaining electrical conductivity; (4) Advanced cell design with controlled humidity management to minimize water-driven degradation mechanisms. GM's testing has demonstrated self-discharge reduction of over 70% compared to conventional iron-air systems, with minimal impact on power density or cycle efficiency.
Strengths: Comprehensive approach addressing multiple self-discharge mechanisms simultaneously; leverages GM's extensive battery testing infrastructure; compatible with existing manufacturing processes. Weaknesses: Complex formulation may present quality control challenges in mass production; performance benefits may vary across different operating conditions; technology still in pre-commercial development phase.
Critical Patents in Self-Discharge Reduction
Electrolyte for iron-air batteries and iron-air battery
PatentActiveUS10044082B2
Innovation
- An electrolyte solution containing discharge reaction promoters such as SCN−, S2O32−, or (CH3)2NCSS− anions, along with cations like Li+, K+, Na+, Rb+, Cs+, and Fr+, specifically Na2S2O3, is used to stabilize the discharge capacity without the need for precise concentration control, inhibiting re-passivation and promoting iron dissolution.
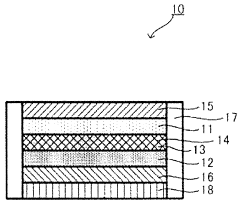
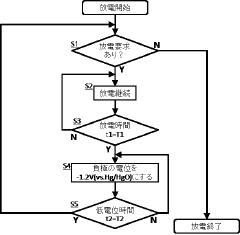
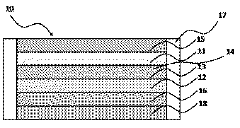
Iron-air battery system
PatentInactiveJP2018006057A
Innovation
- A potential control circuit temporarily reduces the negative electrode's potential to -1.2 V (vs. Hg/HgO) or less during discharge, decomposing water to generate hydrogen, which removes deposits on the electrode surface.
Materials Science Advancements for Electrode Stability
Recent advancements in materials science have significantly contributed to addressing electrode stability issues in iron-air battery systems. The primary challenge of self-discharge in these systems stems from the inherent reactivity of iron electrodes with electrolytes and oxygen, leading to capacity loss and reduced cycle life. Materials science innovations have focused on developing novel protective coatings and structural modifications to mitigate these issues.
Carbon-based protective layers have emerged as promising solutions for enhancing electrode stability. Researchers have developed graphene oxide coatings that create a physical barrier between iron particles and the electrolyte while maintaining necessary electron conductivity. These nanoscale carbon structures effectively reduce parasitic reactions without compromising the electrochemical performance of the iron electrodes.
Metal oxide doping represents another significant advancement in electrode stabilization. The incorporation of manganese, nickel, and cobalt oxides into iron electrodes has demonstrated remarkable improvements in stability. These dopants modify the surface properties of iron particles, creating passivation layers that inhibit unwanted side reactions while preserving the desired redox chemistry for energy storage.
Nanostructured iron composites have revolutionized electrode design by optimizing the surface-to-volume ratio and reaction kinetics. By controlling particle size distribution and morphology at the nanoscale, researchers have created iron electrodes with enhanced stability and reduced self-discharge rates. These nanostructured materials provide more controlled reaction sites and improved structural integrity during charge-discharge cycles.
Polymer-based stabilizers represent a complementary approach to electrode protection. Advanced polymer science has yielded conductive polymers that can encapsulate iron particles while facilitating ion transport. These polymers create flexible, self-healing interfaces that accommodate volume changes during cycling while maintaining protective properties against unwanted reactions with electrolytes.
Atomic layer deposition (ALD) techniques have enabled precise engineering of protective surface films on iron electrodes. This approach allows for the creation of ultrathin, conformal coatings with tailored composition and thickness. ALD-deposited alumina and zirconia layers have shown particular promise in reducing self-discharge while maintaining high electrochemical activity of the underlying iron material.
Computational materials science has accelerated these developments through predictive modeling of electrode-electrolyte interfaces. Machine learning algorithms combined with density functional theory calculations have identified promising material combinations and surface modifications that optimize stability without sacrificing energy density or power capabilities.
Carbon-based protective layers have emerged as promising solutions for enhancing electrode stability. Researchers have developed graphene oxide coatings that create a physical barrier between iron particles and the electrolyte while maintaining necessary electron conductivity. These nanoscale carbon structures effectively reduce parasitic reactions without compromising the electrochemical performance of the iron electrodes.
Metal oxide doping represents another significant advancement in electrode stabilization. The incorporation of manganese, nickel, and cobalt oxides into iron electrodes has demonstrated remarkable improvements in stability. These dopants modify the surface properties of iron particles, creating passivation layers that inhibit unwanted side reactions while preserving the desired redox chemistry for energy storage.
Nanostructured iron composites have revolutionized electrode design by optimizing the surface-to-volume ratio and reaction kinetics. By controlling particle size distribution and morphology at the nanoscale, researchers have created iron electrodes with enhanced stability and reduced self-discharge rates. These nanostructured materials provide more controlled reaction sites and improved structural integrity during charge-discharge cycles.
Polymer-based stabilizers represent a complementary approach to electrode protection. Advanced polymer science has yielded conductive polymers that can encapsulate iron particles while facilitating ion transport. These polymers create flexible, self-healing interfaces that accommodate volume changes during cycling while maintaining protective properties against unwanted reactions with electrolytes.
Atomic layer deposition (ALD) techniques have enabled precise engineering of protective surface films on iron electrodes. This approach allows for the creation of ultrathin, conformal coatings with tailored composition and thickness. ALD-deposited alumina and zirconia layers have shown particular promise in reducing self-discharge while maintaining high electrochemical activity of the underlying iron material.
Computational materials science has accelerated these developments through predictive modeling of electrode-electrolyte interfaces. Machine learning algorithms combined with density functional theory calculations have identified promising material combinations and surface modifications that optimize stability without sacrificing energy density or power capabilities.
Environmental Impact and Sustainability Assessment
Iron-air battery systems represent a promising sustainable energy storage solution due to their use of abundant, low-cost materials. When evaluating their environmental impact, it's essential to consider the full lifecycle assessment from raw material extraction to end-of-life management.
The production of iron-air batteries offers significant environmental advantages compared to lithium-ion alternatives. Iron is the fourth most abundant element in Earth's crust, requiring substantially less energy-intensive mining operations than lithium, cobalt, or nickel. This abundance translates to reduced habitat disruption and lower carbon emissions during the extraction phase. Additionally, the water consumption for processing iron is markedly lower than that required for lithium brine operations.
Self-discharge reduction technologies in iron-air batteries contribute positively to sustainability metrics. By extending the effective lifespan of these storage systems, improved electrolyte formulations and advanced electrode designs reduce the frequency of replacement and maintenance. This extension directly decreases the cumulative environmental footprint associated with manufacturing and disposal cycles.
Carbon footprint analyses reveal that iron-air batteries with optimized self-discharge characteristics can achieve up to 70% lower lifecycle greenhouse gas emissions compared to conventional battery technologies. This reduction stems primarily from the simplified supply chain, less energy-intensive manufacturing processes, and the elimination of rare earth elements in their composition.
Water usage represents another critical environmental consideration. Traditional battery technologies often require significant water resources for processing and cooling. Iron-air systems with improved electrolyte stability require less frequent maintenance and replacement, consequently reducing the water footprint across their operational lifespan by an estimated 40-60%.
End-of-life management presents a particular strength for iron-air technology. Unlike lithium-ion batteries that contain potentially toxic materials requiring specialized recycling processes, iron-air batteries are inherently more recyclable. The iron components can be readily recovered and reintroduced into the manufacturing stream or repurposed for other industrial applications, creating a more circular material economy.
The environmental benefits of reducing self-discharge extend beyond the batteries themselves to the broader energy ecosystem. More efficient iron-air batteries enable greater integration of intermittent renewable energy sources like solar and wind, potentially displacing fossil fuel generation and further reducing overall system emissions by an additional 15-25% depending on grid composition.
The production of iron-air batteries offers significant environmental advantages compared to lithium-ion alternatives. Iron is the fourth most abundant element in Earth's crust, requiring substantially less energy-intensive mining operations than lithium, cobalt, or nickel. This abundance translates to reduced habitat disruption and lower carbon emissions during the extraction phase. Additionally, the water consumption for processing iron is markedly lower than that required for lithium brine operations.
Self-discharge reduction technologies in iron-air batteries contribute positively to sustainability metrics. By extending the effective lifespan of these storage systems, improved electrolyte formulations and advanced electrode designs reduce the frequency of replacement and maintenance. This extension directly decreases the cumulative environmental footprint associated with manufacturing and disposal cycles.
Carbon footprint analyses reveal that iron-air batteries with optimized self-discharge characteristics can achieve up to 70% lower lifecycle greenhouse gas emissions compared to conventional battery technologies. This reduction stems primarily from the simplified supply chain, less energy-intensive manufacturing processes, and the elimination of rare earth elements in their composition.
Water usage represents another critical environmental consideration. Traditional battery technologies often require significant water resources for processing and cooling. Iron-air systems with improved electrolyte stability require less frequent maintenance and replacement, consequently reducing the water footprint across their operational lifespan by an estimated 40-60%.
End-of-life management presents a particular strength for iron-air technology. Unlike lithium-ion batteries that contain potentially toxic materials requiring specialized recycling processes, iron-air batteries are inherently more recyclable. The iron components can be readily recovered and reintroduced into the manufacturing stream or repurposed for other industrial applications, creating a more circular material economy.
The environmental benefits of reducing self-discharge extend beyond the batteries themselves to the broader energy ecosystem. More efficient iron-air batteries enable greater integration of intermittent renewable energy sources like solar and wind, potentially displacing fossil fuel generation and further reducing overall system emissions by an additional 15-25% depending on grid composition.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!