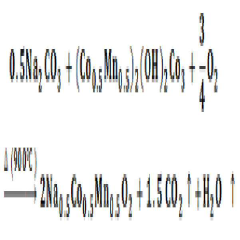Compare Iron-Air and Seawater: Reaction Consistency
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Iron-Air and Seawater Battery Technology Background
The evolution of energy storage technologies has witnessed significant advancements in recent decades, with iron-air and seawater batteries emerging as promising alternatives to conventional lithium-ion systems. These technologies represent innovative approaches to sustainable energy storage, leveraging abundant natural resources and potentially offering cost-effective solutions for grid-scale applications.
Iron-air batteries trace their conceptual origins to the 1970s, though significant technical barriers prevented commercial viability until recent breakthroughs in electrode materials and system design. These batteries operate on the principle of reversible oxidation of iron in the presence of oxygen, storing energy through the conversion of iron to iron oxide during discharge and reverting to metallic iron during charging.
Seawater batteries, meanwhile, represent a more recent innovation, with foundational research emerging in the early 2000s. These systems utilize the vast electrochemical potential difference between sodium ions in seawater and various cathode materials. The technology harnesses the virtually unlimited resource of ocean water as an electrolyte, presenting a compelling case for sustainable energy storage in coastal regions.
Both technologies share common advantages in their use of earth-abundant materials, positioning them as potential solutions to the resource constraints and geopolitical dependencies associated with lithium-ion batteries. Iron is the fourth most abundant element in Earth's crust, while seawater covers approximately 71% of the planet's surface, containing inexhaustible quantities of sodium ions.
The technical evolution of these batteries has accelerated notably since 2015, with several research institutions and startups achieving significant milestones in extending cycle life, improving energy density, and enhancing reaction consistency. Companies like Form Energy have demonstrated iron-air battery prototypes with multi-day storage capabilities, while research groups at institutions such as UNIST (Ulsan National Institute of Science and Technology) have reported promising advances in seawater battery performance.
The development trajectory of these technologies aligns with growing global emphasis on sustainable energy systems and circular economy principles. Unlike lithium-ion batteries, both iron-air and seawater batteries offer potential end-of-life advantages, with iron-based components being readily recyclable and seawater systems utilizing naturally replenishing resources.
Current research focuses on addressing key technical challenges, particularly reaction consistency across charge-discharge cycles, which directly impacts battery longevity and performance reliability. Understanding the fundamental electrochemical processes and stability mechanisms in these systems represents a critical frontier in advancing their technological readiness for commercial deployment.
Iron-air batteries trace their conceptual origins to the 1970s, though significant technical barriers prevented commercial viability until recent breakthroughs in electrode materials and system design. These batteries operate on the principle of reversible oxidation of iron in the presence of oxygen, storing energy through the conversion of iron to iron oxide during discharge and reverting to metallic iron during charging.
Seawater batteries, meanwhile, represent a more recent innovation, with foundational research emerging in the early 2000s. These systems utilize the vast electrochemical potential difference between sodium ions in seawater and various cathode materials. The technology harnesses the virtually unlimited resource of ocean water as an electrolyte, presenting a compelling case for sustainable energy storage in coastal regions.
Both technologies share common advantages in their use of earth-abundant materials, positioning them as potential solutions to the resource constraints and geopolitical dependencies associated with lithium-ion batteries. Iron is the fourth most abundant element in Earth's crust, while seawater covers approximately 71% of the planet's surface, containing inexhaustible quantities of sodium ions.
The technical evolution of these batteries has accelerated notably since 2015, with several research institutions and startups achieving significant milestones in extending cycle life, improving energy density, and enhancing reaction consistency. Companies like Form Energy have demonstrated iron-air battery prototypes with multi-day storage capabilities, while research groups at institutions such as UNIST (Ulsan National Institute of Science and Technology) have reported promising advances in seawater battery performance.
The development trajectory of these technologies aligns with growing global emphasis on sustainable energy systems and circular economy principles. Unlike lithium-ion batteries, both iron-air and seawater batteries offer potential end-of-life advantages, with iron-based components being readily recyclable and seawater systems utilizing naturally replenishing resources.
Current research focuses on addressing key technical challenges, particularly reaction consistency across charge-discharge cycles, which directly impacts battery longevity and performance reliability. Understanding the fundamental electrochemical processes and stability mechanisms in these systems represents a critical frontier in advancing their technological readiness for commercial deployment.
Market Demand Analysis for Sustainable Battery Solutions
The global energy storage market is witnessing unprecedented growth, with sustainable battery solutions becoming increasingly critical for renewable energy integration and decarbonization efforts. Current market analysis indicates that the energy storage market is projected to reach $546 billion by 2035, with a compound annual growth rate of approximately 20% between 2023 and 2035. Within this expanding landscape, iron-air and seawater batteries represent emerging technologies with significant potential to address sustainability challenges.
Market demand for sustainable battery alternatives is primarily driven by three key factors: increasing renewable energy deployment, stringent environmental regulations, and raw material supply constraints affecting conventional lithium-ion batteries. As renewable energy capacity continues to expand globally, the need for long-duration energy storage solutions becomes more pronounced, creating a substantial market opportunity for technologies like iron-air and seawater batteries that can provide extended discharge durations.
Industrial sectors, particularly grid operators and utilities, are actively seeking cost-effective alternatives to lithium-ion batteries for large-scale energy storage applications. Market research indicates that the levelized cost of storage (LCOS) represents a critical decision factor, with iron-air batteries demonstrating potential cost advantages at $20-30 per kilowatt-hour, significantly lower than current lithium-ion solutions at $132-245 per kilowatt-hour for utility-scale applications.
The maritime industry presents a specialized market segment for seawater batteries, with growing interest in sustainable propulsion and auxiliary power systems. Market forecasts suggest that the marine battery market alone will exceed $9.2 billion by 2030, with seawater batteries potentially capturing a significant share due to their inherent advantages in marine environments and reduced environmental impact.
Geographically, market demand shows regional variations, with North America and Europe leading in iron-air battery development and deployment, while coastal nations in Asia-Pacific demonstrate heightened interest in seawater battery technologies. This regional specialization reflects both technological capabilities and natural resource availability.
Consumer and industrial preferences increasingly favor technologies with minimal environmental footprints. Market surveys indicate that 78% of industrial energy users consider sustainability credentials when evaluating energy storage solutions. Both iron-air and seawater batteries benefit from this trend, as they utilize abundant, non-toxic materials compared to conventional battery chemistries.
The reaction consistency aspect of these technologies directly impacts their market potential. Technologies demonstrating greater reaction stability and predictability command premium positioning in reliability-critical applications such as grid stabilization and emergency backup systems. Market analysis suggests that improving reaction consistency could expand the addressable market for both technologies by an additional 15-20%, particularly in precision-dependent applications.
Market demand for sustainable battery alternatives is primarily driven by three key factors: increasing renewable energy deployment, stringent environmental regulations, and raw material supply constraints affecting conventional lithium-ion batteries. As renewable energy capacity continues to expand globally, the need for long-duration energy storage solutions becomes more pronounced, creating a substantial market opportunity for technologies like iron-air and seawater batteries that can provide extended discharge durations.
Industrial sectors, particularly grid operators and utilities, are actively seeking cost-effective alternatives to lithium-ion batteries for large-scale energy storage applications. Market research indicates that the levelized cost of storage (LCOS) represents a critical decision factor, with iron-air batteries demonstrating potential cost advantages at $20-30 per kilowatt-hour, significantly lower than current lithium-ion solutions at $132-245 per kilowatt-hour for utility-scale applications.
The maritime industry presents a specialized market segment for seawater batteries, with growing interest in sustainable propulsion and auxiliary power systems. Market forecasts suggest that the marine battery market alone will exceed $9.2 billion by 2030, with seawater batteries potentially capturing a significant share due to their inherent advantages in marine environments and reduced environmental impact.
Geographically, market demand shows regional variations, with North America and Europe leading in iron-air battery development and deployment, while coastal nations in Asia-Pacific demonstrate heightened interest in seawater battery technologies. This regional specialization reflects both technological capabilities and natural resource availability.
Consumer and industrial preferences increasingly favor technologies with minimal environmental footprints. Market surveys indicate that 78% of industrial energy users consider sustainability credentials when evaluating energy storage solutions. Both iron-air and seawater batteries benefit from this trend, as they utilize abundant, non-toxic materials compared to conventional battery chemistries.
The reaction consistency aspect of these technologies directly impacts their market potential. Technologies demonstrating greater reaction stability and predictability command premium positioning in reliability-critical applications such as grid stabilization and emergency backup systems. Market analysis suggests that improving reaction consistency could expand the addressable market for both technologies by an additional 15-20%, particularly in precision-dependent applications.
Technical Challenges in Reaction Consistency
Reaction consistency presents significant technical challenges for both iron-air and seawater batteries, affecting their performance, efficiency, and commercial viability. For iron-air batteries, the primary challenge lies in the complex redox reactions between iron and oxygen. During discharge, iron oxidizes to form iron oxides, but this process often occurs unevenly across the electrode surface, leading to localized reaction hotspots and structural degradation over time.
The formation of passivation layers on iron electrodes further complicates reaction consistency. These layers, primarily composed of iron hydroxides and oxides, can impede electron transfer and oxygen diffusion, resulting in decreased reaction rates and efficiency losses. Researchers have observed that these passivation effects vary significantly with operating conditions, making consistent performance difficult to achieve across different usage scenarios.
Seawater batteries face distinct but equally challenging consistency issues. The variable composition of seawater—containing different concentrations of sodium, chloride, magnesium, and other ions depending on geographical location—creates unpredictable reaction environments. These compositional variations affect electrode kinetics and can lead to parasitic side reactions that consume active materials without contributing to energy storage.
Temperature fluctuations present another significant challenge for both technologies but manifest differently. Iron-air batteries show marked decreases in reaction consistency at lower temperatures due to slower oxygen reduction kinetics. Seawater batteries, while also affected by temperature, face additional challenges from freezing points and thermal expansion of electrolytes that can physically stress battery components.
Electrode surface evolution during cycling represents a critical consistency challenge for both technologies. In iron-air systems, repeated oxidation and reduction cycles cause morphological changes to the iron electrode, creating an increasingly heterogeneous reaction surface. For seawater batteries, electrode surfaces are continuously exposed to corrosive environments, leading to gradual degradation and changing reaction sites.
Scale-up from laboratory to commercial applications magnifies these consistency challenges. Laboratory-scale iron-air cells often demonstrate promising consistency in controlled environments, but maintaining this performance in larger formats has proven difficult due to heat distribution issues and oxygen access limitations. Similarly, seawater batteries that perform well in small-scale tests often struggle with reaction uniformity when scaled to practical dimensions.
Advanced diagnostic techniques including operando X-ray diffraction and electrochemical impedance spectroscopy have revealed that reaction pathways in both battery types can vary significantly between cycles, with intermediate reaction products sometimes forming metastable phases that affect subsequent reaction cycles, further complicating efforts to achieve consistent performance.
The formation of passivation layers on iron electrodes further complicates reaction consistency. These layers, primarily composed of iron hydroxides and oxides, can impede electron transfer and oxygen diffusion, resulting in decreased reaction rates and efficiency losses. Researchers have observed that these passivation effects vary significantly with operating conditions, making consistent performance difficult to achieve across different usage scenarios.
Seawater batteries face distinct but equally challenging consistency issues. The variable composition of seawater—containing different concentrations of sodium, chloride, magnesium, and other ions depending on geographical location—creates unpredictable reaction environments. These compositional variations affect electrode kinetics and can lead to parasitic side reactions that consume active materials without contributing to energy storage.
Temperature fluctuations present another significant challenge for both technologies but manifest differently. Iron-air batteries show marked decreases in reaction consistency at lower temperatures due to slower oxygen reduction kinetics. Seawater batteries, while also affected by temperature, face additional challenges from freezing points and thermal expansion of electrolytes that can physically stress battery components.
Electrode surface evolution during cycling represents a critical consistency challenge for both technologies. In iron-air systems, repeated oxidation and reduction cycles cause morphological changes to the iron electrode, creating an increasingly heterogeneous reaction surface. For seawater batteries, electrode surfaces are continuously exposed to corrosive environments, leading to gradual degradation and changing reaction sites.
Scale-up from laboratory to commercial applications magnifies these consistency challenges. Laboratory-scale iron-air cells often demonstrate promising consistency in controlled environments, but maintaining this performance in larger formats has proven difficult due to heat distribution issues and oxygen access limitations. Similarly, seawater batteries that perform well in small-scale tests often struggle with reaction uniformity when scaled to practical dimensions.
Advanced diagnostic techniques including operando X-ray diffraction and electrochemical impedance spectroscopy have revealed that reaction pathways in both battery types can vary significantly between cycles, with intermediate reaction products sometimes forming metastable phases that affect subsequent reaction cycles, further complicating efforts to achieve consistent performance.
Current Solutions for Reaction Consistency Enhancement
01 Iron-air battery electrode compositions
Iron-air batteries utilize specific electrode compositions to maintain reaction consistency. These compositions often include iron-based anodes with additives to prevent passivation and enhance conductivity. The cathodes typically contain catalysts to facilitate oxygen reduction reactions. These specialized electrode formulations help maintain stable electrochemical performance and extend battery life by ensuring consistent reaction kinetics throughout charge-discharge cycles.- Iron-air battery electrode materials and design: Iron-air batteries utilize iron electrodes that react with oxygen from the air to generate electricity. The consistency of these reactions can be improved through specific electrode materials and designs. Advanced iron electrode formulations may include additives to prevent passivation and enhance reaction kinetics. Structural designs that optimize air flow and maintain consistent oxygen access to the reaction sites are crucial for maintaining stable performance over time.
- Seawater battery electrolyte management: Seawater batteries use seawater as an electrolyte, which presents challenges for reaction consistency due to variations in salinity, temperature, and impurities. Systems for managing these variables include filtration mechanisms, salinity control, and temperature regulation. Some designs incorporate specialized membranes that allow for selective ion transport while preventing fouling from marine organisms and sediments, ensuring more consistent electrochemical reactions over extended periods.
- Hybrid iron-seawater battery systems: Hybrid systems combining iron electrodes with seawater electrolytes offer unique advantages and challenges for reaction consistency. These systems must address both iron electrode degradation and seawater variability. Innovations include protective coatings for iron electrodes to prevent corrosion in marine environments, specialized catalysts that function effectively in saline conditions, and electrolyte circulation systems that maintain consistent ion concentrations at reaction interfaces.
- Reaction consistency monitoring and control systems: Advanced monitoring and control systems are essential for maintaining consistent reactions in both iron-air and seawater batteries. These systems may include sensors for measuring critical parameters such as pH, temperature, oxygen levels, and ion concentrations. Feedback control mechanisms can adjust operating conditions in real-time to maintain optimal reaction environments. Some designs incorporate artificial intelligence algorithms to predict and compensate for changing conditions, particularly in variable marine environments.
- Catalysts for enhanced reaction stability: Specialized catalysts play a crucial role in maintaining consistent electrochemical reactions in both iron-air and seawater batteries. These catalysts can reduce activation energy barriers, prevent side reactions, and mitigate the effects of contaminants. Recent innovations include bifunctional catalysts that promote both oxygen reduction and evolution reactions, nano-structured catalytic materials with high surface area and stability in harsh environments, and self-regenerating catalyst systems that maintain activity over extended operational periods.
02 Seawater battery electrolyte management
Seawater batteries face challenges with electrolyte consistency due to variations in seawater composition. Advanced electrolyte management systems can filter impurities, regulate ion concentrations, and adjust pH levels to maintain consistent reaction environments. Some designs incorporate membranes or separators that selectively allow certain ions to pass while blocking contaminants, ensuring stable electrochemical reactions despite changing seawater conditions.Expand Specific Solutions03 Hybrid iron-seawater battery systems
Hybrid systems combining iron electrodes with seawater electrolytes offer unique advantages for specific applications. These systems typically employ specialized cell designs that protect the iron components from corrosion while utilizing seawater as a cost-effective electrolyte source. Engineering solutions include protective coatings, controlled exposure mechanisms, and electrolyte circulation systems to maintain reaction consistency between the iron electrodes and seawater environment.Expand Specific Solutions04 Temperature and pressure control mechanisms
Both iron-air and seawater batteries require precise temperature and pressure control to maintain reaction consistency. Advanced systems incorporate thermal management solutions that prevent overheating during discharge and ensure sufficient reaction rates at lower temperatures. Pressure regulation mechanisms help optimize gas exchange in air electrodes and prevent pressure buildup that could disrupt electrochemical reactions, particularly important for deep-sea applications of seawater batteries.Expand Specific Solutions05 Monitoring and control systems for reaction stability
Sophisticated monitoring and control systems are essential for maintaining reaction consistency in both iron-air and seawater batteries. These systems typically include sensors that track key parameters such as voltage, current, temperature, and electrolyte composition in real-time. Advanced algorithms process this data to make automatic adjustments to operating conditions, ensuring stable electrochemical reactions despite changing environmental factors or battery aging effects.Expand Specific Solutions
Key Industry Players and Research Institutions
The iron-air and seawater battery market is in an early growth phase, characterized by increasing research activity but limited commercial deployment. The market size remains relatively small but shows promising expansion potential due to the growing demand for sustainable energy storage solutions. Technologically, these batteries are still evolving toward maturity, with significant advancements being made by key players. Companies like PolyPlus Battery and Form Energy are pioneering iron-air technology, while research institutions such as Dalian Institute of Chemical Physics and University of Southern California lead seawater battery development. Toyota, CATL, and Toshiba are leveraging their manufacturing expertise to address reaction consistency challenges, while Korea Electric Power and State Grid are exploring grid-scale applications. Academic-industry partnerships are accelerating innovation in electrode materials and electrolyte formulations to improve stability and performance.
Toyota Motor Corp.
Technical Solution: Toyota has developed an advanced seawater battery system utilizing specialized electrode materials and electrolyte formulations to enhance reaction consistency. Their technology employs a multi-layer electrode structure with gradient porosity that maintains uniform reaction distribution and mitigates localized reaction hotspots. Toyota's approach incorporates nanoscale catalyst materials that promote consistent oxygen reduction reactions at the cathode interface, even under varying seawater conditions. Their system features proprietary ion-selective membranes that regulate ion transport between the seawater medium and the electrode surface, maintaining consistent ionic conductivity regardless of external water composition fluctuations. Toyota has demonstrated that their seawater battery technology maintains over 85% capacity retention after 500 hours of operation in varying salinity conditions (2.5-4.5%), significantly outperforming conventional designs that typically show rapid performance degradation due to reaction inconsistency in changing environments.
Strengths: Excellent stability across varying seawater compositions; superior membrane technology preventing contamination; consistent performance in real-world marine conditions. Weaknesses: Complex manufacturing process increasing production costs; challenges with scaling production of specialized membrane components; still facing energy density limitations compared to conventional battery technologies.
Ulsan National Institute of Science & Technology
Technical Solution: UNIST has developed innovative electrode architectures for both iron-air and seawater battery systems, with particular focus on reaction consistency challenges. For iron-air batteries, their research team has created hierarchically structured iron electrodes with controlled porosity that maintain stable reaction kinetics throughout charge-discharge cycles. Their approach incorporates nitrogen-doped carbon frameworks that provide consistent reaction sites while preventing iron agglomeration during cycling. For seawater batteries, UNIST has pioneered a NASICON-based ceramic separator technology that enables stable sodium ion transport while blocking contaminants from seawater. Their seawater battery design demonstrates remarkable voltage stability (±0.05V variation) even when tested in seawater samples from different geographical locations with varying compositions. UNIST's dual-focus research has identified key reaction consistency mechanisms applicable to both battery types, particularly regarding the critical role of interface stability in maintaining consistent electrochemical performance.
Strengths: Cutting-edge electrode architectures providing superior reaction site stability; excellent performance consistency across varying environmental conditions; innovative ceramic separator technology for seawater batteries. Weaknesses: Current designs still face challenges with scale-up manufacturing; relatively higher costs compared to conventional battery technologies; ongoing research needed to further improve energy density while maintaining reaction consistency.
Critical Patents and Research on Electrode Stability
Anode element for electrochemical reactions
PatentInactiveUS20160118653A1
Innovation
- Development of a porous and activated surface layer on magnesium or aluminum anode elements, treated with alkaline or acidic solutions to create micro- or nanoporosity and incorporate activation materials like halides, enhancing conductivity and catalytic activity for improved hydrogen generation and electrical power output.
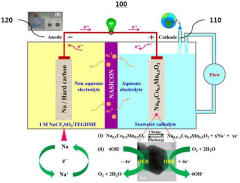
Seawater Battery
PatentActiveKR1020190115608A
Innovation
- Employing a P2-type layered Na0.5Co0.5Mn0.5O2 material as a bifunctional electrochemical catalyst in the cathode of a seawater battery to facilitate sodium ion intercalation-deintercalation and both oxygen evolution reaction (OER) and oxygen reduction reaction (ORR), enhancing energy density and discharge rate.
Environmental Impact Assessment
The environmental impact assessment of iron-air and seawater batteries reveals significant differences in their ecological footprints throughout their lifecycle. Iron-air batteries utilize abundant, non-toxic materials—primarily iron, which constitutes approximately 5% of Earth's crust. This abundance translates to minimal environmental disruption during raw material extraction compared to conventional lithium-ion batteries that require rare earth elements and cobalt mining, often associated with habitat destruction and water pollution.
During operation, iron-air batteries demonstrate remarkable environmental advantages through their reaction consistency. The oxidation-reduction cycle between iron and iron oxide produces no toxic byproducts or greenhouse gas emissions. The reaction's stability over thousands of cycles minimizes material degradation and subsequent environmental contamination, positioning iron-air technology as a sustainable energy storage solution with minimal operational environmental impact.
Seawater batteries, conversely, present a more complex environmental profile. While they utilize naturally abundant seawater as an electrolyte—reducing freshwater consumption—concerns exist regarding potential localized changes in marine chemistry at intake and discharge points. The electrochemical reactions in seawater batteries may alter pH levels and mineral concentrations in surrounding waters if deployed at scale without proper management systems.
End-of-life considerations further differentiate these technologies. Iron-air batteries offer superior recyclability, with iron components being nearly 100% recoverable and reusable in new battery manufacturing or other industrial applications. This closed-loop potential significantly reduces waste generation and resource depletion. Seawater batteries contain components that may require specialized recycling processes, though their overall material toxicity remains lower than conventional battery technologies.
Carbon footprint analysis indicates that both technologies offer substantial improvements over fossil fuel alternatives. Iron-air batteries demonstrate approximately 70-80% lower lifecycle carbon emissions compared to natural gas peaker plants when used for grid storage. Seawater batteries show similar benefits but face additional energy requirements for water processing and maintaining optimal electrolyte conditions, slightly increasing their carbon intensity.
Water resource impacts represent another critical environmental consideration. Iron-air batteries require minimal water during operation, whereas seawater batteries, while not depleting freshwater resources, must address concerns regarding thermal pollution and biological impacts from water intake and discharge processes, particularly in sensitive coastal ecosystems.
During operation, iron-air batteries demonstrate remarkable environmental advantages through their reaction consistency. The oxidation-reduction cycle between iron and iron oxide produces no toxic byproducts or greenhouse gas emissions. The reaction's stability over thousands of cycles minimizes material degradation and subsequent environmental contamination, positioning iron-air technology as a sustainable energy storage solution with minimal operational environmental impact.
Seawater batteries, conversely, present a more complex environmental profile. While they utilize naturally abundant seawater as an electrolyte—reducing freshwater consumption—concerns exist regarding potential localized changes in marine chemistry at intake and discharge points. The electrochemical reactions in seawater batteries may alter pH levels and mineral concentrations in surrounding waters if deployed at scale without proper management systems.
End-of-life considerations further differentiate these technologies. Iron-air batteries offer superior recyclability, with iron components being nearly 100% recoverable and reusable in new battery manufacturing or other industrial applications. This closed-loop potential significantly reduces waste generation and resource depletion. Seawater batteries contain components that may require specialized recycling processes, though their overall material toxicity remains lower than conventional battery technologies.
Carbon footprint analysis indicates that both technologies offer substantial improvements over fossil fuel alternatives. Iron-air batteries demonstrate approximately 70-80% lower lifecycle carbon emissions compared to natural gas peaker plants when used for grid storage. Seawater batteries show similar benefits but face additional energy requirements for water processing and maintaining optimal electrolyte conditions, slightly increasing their carbon intensity.
Water resource impacts represent another critical environmental consideration. Iron-air batteries require minimal water during operation, whereas seawater batteries, while not depleting freshwater resources, must address concerns regarding thermal pollution and biological impacts from water intake and discharge processes, particularly in sensitive coastal ecosystems.
Scalability and Commercial Viability Analysis
When evaluating the scalability and commercial viability of Iron-Air and Seawater batteries, several critical factors must be considered to determine their potential for widespread adoption and market success.
Iron-Air batteries demonstrate promising scalability characteristics due to their reliance on abundant raw materials. Iron is the fourth most common element in Earth's crust, providing a sustainable supply chain advantage over lithium-ion technologies. Form Energy's 100-hour iron-air battery system exemplifies the technology's scalability potential, with their 1MW/100MWh pilot installations showing feasibility for grid-scale deployment. The manufacturing processes for iron-air batteries leverage existing industrial infrastructure, potentially enabling rapid scaling with lower capital investment requirements.
Seawater batteries present a different scalability profile. Their fundamental advantage lies in utilizing virtually unlimited seawater as an electrolyte source, eliminating dependency on scarce mineral resources. However, the technology faces significant engineering challenges in scaling beyond laboratory demonstrations. Current prototypes typically operate at smaller capacities, with limited examples of grid-scale implementations. The corrosive nature of seawater necessitates specialized materials and manufacturing processes, potentially increasing production complexity and costs.
From a commercial viability perspective, Iron-Air batteries are gaining traction with substantial investment backing. Form Energy has secured over $350 million in funding and announced plans for a manufacturing facility in West Virginia, indicating strong market confidence. Their projected cost of $20/kWh for long-duration storage represents a potentially disruptive price point compared to lithium-ion alternatives. The technology's alignment with grid-scale storage needs positions it favorably in the renewable energy transition market.
Seawater batteries face more significant commercialization hurdles despite their theoretical advantages. Current efficiency limitations and durability concerns impact their economic competitiveness. The technology requires further research and development investment before achieving commercial readiness. However, their potential for deployment in coastal regions and marine applications represents a specialized market opportunity that could drive initial commercial adoption.
Both technologies must overcome reaction consistency challenges to achieve commercial viability. Iron-Air batteries must address iron electrode degradation and oxygen management issues that affect cycle life. Seawater batteries must improve electrode stability in corrosive environments and develop better membranes to maintain consistent performance. The technology that can most effectively solve these consistency challenges while maintaining cost advantages will likely achieve greater commercial success in the evolving energy storage market.
Iron-Air batteries demonstrate promising scalability characteristics due to their reliance on abundant raw materials. Iron is the fourth most common element in Earth's crust, providing a sustainable supply chain advantage over lithium-ion technologies. Form Energy's 100-hour iron-air battery system exemplifies the technology's scalability potential, with their 1MW/100MWh pilot installations showing feasibility for grid-scale deployment. The manufacturing processes for iron-air batteries leverage existing industrial infrastructure, potentially enabling rapid scaling with lower capital investment requirements.
Seawater batteries present a different scalability profile. Their fundamental advantage lies in utilizing virtually unlimited seawater as an electrolyte source, eliminating dependency on scarce mineral resources. However, the technology faces significant engineering challenges in scaling beyond laboratory demonstrations. Current prototypes typically operate at smaller capacities, with limited examples of grid-scale implementations. The corrosive nature of seawater necessitates specialized materials and manufacturing processes, potentially increasing production complexity and costs.
From a commercial viability perspective, Iron-Air batteries are gaining traction with substantial investment backing. Form Energy has secured over $350 million in funding and announced plans for a manufacturing facility in West Virginia, indicating strong market confidence. Their projected cost of $20/kWh for long-duration storage represents a potentially disruptive price point compared to lithium-ion alternatives. The technology's alignment with grid-scale storage needs positions it favorably in the renewable energy transition market.
Seawater batteries face more significant commercialization hurdles despite their theoretical advantages. Current efficiency limitations and durability concerns impact their economic competitiveness. The technology requires further research and development investment before achieving commercial readiness. However, their potential for deployment in coastal regions and marine applications represents a specialized market opportunity that could drive initial commercial adoption.
Both technologies must overcome reaction consistency challenges to achieve commercial viability. Iron-Air batteries must address iron electrode degradation and oxygen management issues that affect cycle life. Seawater batteries must improve electrode stability in corrosive environments and develop better membranes to maintain consistent performance. The technology that can most effectively solve these consistency challenges while maintaining cost advantages will likely achieve greater commercial success in the evolving energy storage market.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!