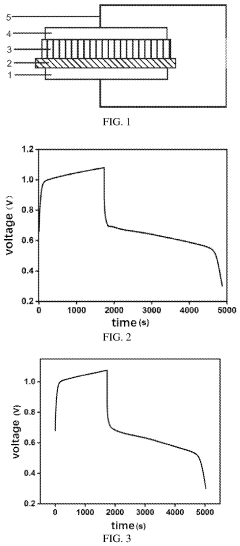
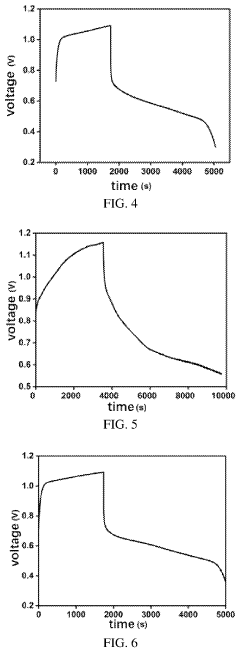
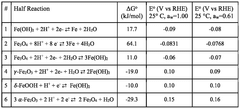
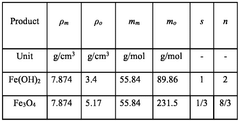
Measuring Iron-Air Battery Oxidation Process Rates
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Iron-Air Battery Technology Background and Objectives
Iron-air batteries represent a significant advancement in energy storage technology, emerging as a promising solution for grid-scale applications due to their potential for low cost, high energy density, and use of abundant materials. The concept of iron-air batteries dates back to the 1970s, but recent technological breakthroughs have revitalized interest in this technology as a viable alternative to lithium-ion batteries for stationary storage applications.
The evolution of iron-air battery technology has been characterized by several key developments. Initially, these batteries faced challenges related to poor cycle life, low efficiency, and rapid capacity degradation. However, advancements in materials science, nanotechnology, and electrochemical engineering have addressed many of these limitations. The technology has progressed from early experimental prototypes to more sophisticated systems with improved performance metrics.
Current research focuses on optimizing the oxidation process rates in iron-air batteries, which is crucial for enhancing their overall efficiency and longevity. The oxidation of iron to iron oxide during discharge and the reverse reaction during charging represent the fundamental electrochemical processes that determine battery performance. Accurate measurement and control of these oxidation rates are essential for developing commercially viable iron-air battery systems.
The primary technical objectives in this field include developing reliable methodologies for measuring oxidation process rates under various operating conditions, understanding the factors that influence these rates, and establishing standardized testing protocols. These objectives align with the broader goal of accelerating the commercialization of iron-air batteries for grid-scale energy storage applications.
Recent advancements in analytical techniques, including in-situ X-ray diffraction, electrochemical impedance spectroscopy, and advanced microscopy methods, have enabled more precise measurements of the oxidation processes. These techniques provide valuable insights into the reaction kinetics, degradation mechanisms, and performance limitations of iron-air batteries.
The global push for renewable energy integration and carbon reduction has created a favorable environment for the development of iron-air battery technology. As intermittent renewable energy sources like solar and wind continue to expand, the demand for cost-effective, long-duration energy storage solutions increases, positioning iron-air batteries as a potentially transformative technology in the energy storage landscape.
The technical trajectory suggests that with continued research and development, particularly in understanding and optimizing oxidation process rates, iron-air batteries could achieve the performance and cost targets necessary for widespread commercial deployment within the next decade.
The evolution of iron-air battery technology has been characterized by several key developments. Initially, these batteries faced challenges related to poor cycle life, low efficiency, and rapid capacity degradation. However, advancements in materials science, nanotechnology, and electrochemical engineering have addressed many of these limitations. The technology has progressed from early experimental prototypes to more sophisticated systems with improved performance metrics.
Current research focuses on optimizing the oxidation process rates in iron-air batteries, which is crucial for enhancing their overall efficiency and longevity. The oxidation of iron to iron oxide during discharge and the reverse reaction during charging represent the fundamental electrochemical processes that determine battery performance. Accurate measurement and control of these oxidation rates are essential for developing commercially viable iron-air battery systems.
The primary technical objectives in this field include developing reliable methodologies for measuring oxidation process rates under various operating conditions, understanding the factors that influence these rates, and establishing standardized testing protocols. These objectives align with the broader goal of accelerating the commercialization of iron-air batteries for grid-scale energy storage applications.
Recent advancements in analytical techniques, including in-situ X-ray diffraction, electrochemical impedance spectroscopy, and advanced microscopy methods, have enabled more precise measurements of the oxidation processes. These techniques provide valuable insights into the reaction kinetics, degradation mechanisms, and performance limitations of iron-air batteries.
The global push for renewable energy integration and carbon reduction has created a favorable environment for the development of iron-air battery technology. As intermittent renewable energy sources like solar and wind continue to expand, the demand for cost-effective, long-duration energy storage solutions increases, positioning iron-air batteries as a potentially transformative technology in the energy storage landscape.
The technical trajectory suggests that with continued research and development, particularly in understanding and optimizing oxidation process rates, iron-air batteries could achieve the performance and cost targets necessary for widespread commercial deployment within the next decade.
Market Analysis for Iron-Air Energy Storage Solutions
The iron-air battery market is experiencing significant growth as the global energy sector increasingly shifts towards renewable energy solutions. Current market analysis indicates that grid-scale energy storage demand is projected to reach 2,850 GWh by 2040, with iron-air batteries positioned to capture a substantial portion of this expanding market. This growth is primarily driven by the increasing integration of intermittent renewable energy sources like solar and wind into power grids worldwide, necessitating efficient and cost-effective long-duration energy storage solutions.
Iron-air battery technology offers compelling economic advantages compared to lithium-ion alternatives, with estimated costs between $20-30 per kWh for iron-air systems versus $150-200 per kWh for lithium-ion batteries. This substantial cost differential is particularly attractive for utility-scale applications where price sensitivity is high. The technology's use of abundant, non-toxic materials further enhances its market appeal, addressing supply chain concerns that plague other battery technologies.
Market segmentation reveals multiple potential application areas for iron-air battery technology. The primary market consists of grid-scale energy storage for utilities, where the technology's long-duration discharge capabilities (100+ hours) provide exceptional value for load shifting and renewable integration. Secondary markets include microgrids for remote communities and industrial facilities requiring reliable backup power systems.
Regional market analysis shows North America currently leading adoption, with companies like Form Energy securing major utility contracts. Europe follows closely with strong policy support for renewable integration, while Asia-Pacific represents the fastest-growing market due to rapid renewable energy deployment and industrial expansion in countries like China and India.
Competitive landscape assessment identifies several key players developing iron-air battery technology, including Form Energy, ESS Inc., and Ambri, alongside research initiatives at major universities and national laboratories. Traditional energy storage providers are also beginning to explore iron-air technology through strategic partnerships and R&D investments, indicating growing market recognition.
Market barriers include technical challenges related to cycle efficiency, with current iron-air batteries achieving 50-60% round-trip efficiency compared to 85-95% for lithium-ion systems. The oxidation process rate measurement and control represent critical factors affecting commercialization timelines. Additionally, the technology faces market education challenges as utilities and grid operators become familiar with this relatively new storage solution.
Future market projections suggest iron-air batteries could capture 15-20% of the grid-scale energy storage market by 2030, particularly in applications requiring 10+ hours of discharge duration. This growth trajectory depends significantly on continued improvements in oxidation process control and overall system performance metrics.
Iron-air battery technology offers compelling economic advantages compared to lithium-ion alternatives, with estimated costs between $20-30 per kWh for iron-air systems versus $150-200 per kWh for lithium-ion batteries. This substantial cost differential is particularly attractive for utility-scale applications where price sensitivity is high. The technology's use of abundant, non-toxic materials further enhances its market appeal, addressing supply chain concerns that plague other battery technologies.
Market segmentation reveals multiple potential application areas for iron-air battery technology. The primary market consists of grid-scale energy storage for utilities, where the technology's long-duration discharge capabilities (100+ hours) provide exceptional value for load shifting and renewable integration. Secondary markets include microgrids for remote communities and industrial facilities requiring reliable backup power systems.
Regional market analysis shows North America currently leading adoption, with companies like Form Energy securing major utility contracts. Europe follows closely with strong policy support for renewable integration, while Asia-Pacific represents the fastest-growing market due to rapid renewable energy deployment and industrial expansion in countries like China and India.
Competitive landscape assessment identifies several key players developing iron-air battery technology, including Form Energy, ESS Inc., and Ambri, alongside research initiatives at major universities and national laboratories. Traditional energy storage providers are also beginning to explore iron-air technology through strategic partnerships and R&D investments, indicating growing market recognition.
Market barriers include technical challenges related to cycle efficiency, with current iron-air batteries achieving 50-60% round-trip efficiency compared to 85-95% for lithium-ion systems. The oxidation process rate measurement and control represent critical factors affecting commercialization timelines. Additionally, the technology faces market education challenges as utilities and grid operators become familiar with this relatively new storage solution.
Future market projections suggest iron-air batteries could capture 15-20% of the grid-scale energy storage market by 2030, particularly in applications requiring 10+ hours of discharge duration. This growth trajectory depends significantly on continued improvements in oxidation process control and overall system performance metrics.
Current Challenges in Oxidation Rate Measurement
Despite significant advancements in iron-air battery technology, accurately measuring oxidation process rates remains one of the most challenging aspects of research and development in this field. Current measurement techniques suffer from several fundamental limitations that impede precise quantification of the complex redox reactions occurring at the iron electrode interface.
The primary challenge stems from the multiphase nature of the iron oxidation process, which involves solid-state transformations alongside liquid electrolyte interactions and gaseous oxygen evolution. Traditional electrochemical methods such as cyclic voltammetry and chronoamperometry struggle to distinguish between these concurrent processes, often providing aggregate data that masks the individual reaction kinetics.
Spatial heterogeneity presents another significant obstacle. The oxidation process does not occur uniformly across the iron electrode surface, creating localized reaction zones with varying rates. Current bulk measurement techniques fail to capture this spatial variation, resulting in averaged data that may not accurately represent the true reaction dynamics at critical interface regions.
Time-dependent phenomena further complicate measurement efforts. The formation of passivation layers and the evolution of electrode morphology during cycling create a constantly changing reaction environment. Most existing measurement protocols lack the temporal resolution needed to track these rapid transformations, particularly during the initial stages of oxidation when reaction rates are highest.
Environmental sensitivity adds another layer of complexity. Iron oxidation kinetics are highly dependent on temperature, humidity, and oxygen partial pressure. Minor fluctuations in these parameters can significantly alter reaction rates, necessitating stringent control systems that are difficult to implement consistently across different research settings.
Instrument limitations also contribute to measurement challenges. The high reactivity of iron with oxygen demands specialized equipment capable of operating in controlled atmospheres. Additionally, the relatively slow reaction rates in certain phases of the oxidation process require instruments with exceptional stability for long-duration measurements, which few commercial systems currently provide.
Standardization issues further hinder progress in this field. The absence of universally accepted protocols for measuring iron oxidation rates has led to significant variability in reported values across different research groups. This lack of standardization makes comparative analysis difficult and slows the collective advancement of knowledge in iron-air battery technology.
Addressing these measurement challenges represents a critical bottleneck in the development of more efficient and reliable iron-air battery systems. Innovative approaches combining advanced in-situ characterization techniques with computational modeling may offer promising pathways forward in accurately quantifying the complex oxidation processes that underpin this emerging energy storage technology.
The primary challenge stems from the multiphase nature of the iron oxidation process, which involves solid-state transformations alongside liquid electrolyte interactions and gaseous oxygen evolution. Traditional electrochemical methods such as cyclic voltammetry and chronoamperometry struggle to distinguish between these concurrent processes, often providing aggregate data that masks the individual reaction kinetics.
Spatial heterogeneity presents another significant obstacle. The oxidation process does not occur uniformly across the iron electrode surface, creating localized reaction zones with varying rates. Current bulk measurement techniques fail to capture this spatial variation, resulting in averaged data that may not accurately represent the true reaction dynamics at critical interface regions.
Time-dependent phenomena further complicate measurement efforts. The formation of passivation layers and the evolution of electrode morphology during cycling create a constantly changing reaction environment. Most existing measurement protocols lack the temporal resolution needed to track these rapid transformations, particularly during the initial stages of oxidation when reaction rates are highest.
Environmental sensitivity adds another layer of complexity. Iron oxidation kinetics are highly dependent on temperature, humidity, and oxygen partial pressure. Minor fluctuations in these parameters can significantly alter reaction rates, necessitating stringent control systems that are difficult to implement consistently across different research settings.
Instrument limitations also contribute to measurement challenges. The high reactivity of iron with oxygen demands specialized equipment capable of operating in controlled atmospheres. Additionally, the relatively slow reaction rates in certain phases of the oxidation process require instruments with exceptional stability for long-duration measurements, which few commercial systems currently provide.
Standardization issues further hinder progress in this field. The absence of universally accepted protocols for measuring iron oxidation rates has led to significant variability in reported values across different research groups. This lack of standardization makes comparative analysis difficult and slows the collective advancement of knowledge in iron-air battery technology.
Addressing these measurement challenges represents a critical bottleneck in the development of more efficient and reliable iron-air battery systems. Innovative approaches combining advanced in-situ characterization techniques with computational modeling may offer promising pathways forward in accurately quantifying the complex oxidation processes that underpin this emerging energy storage technology.
Existing Methodologies for Oxidation Process Monitoring
01 Iron electrode oxidation kinetics in iron-air batteries
The oxidation process of iron electrodes in iron-air batteries involves complex kinetics that affect battery performance. The rate of iron oxidation is influenced by factors such as electrode composition, electrolyte concentration, and operating temperature. Understanding these kinetics is crucial for optimizing battery efficiency and cycle life. Research shows that controlling the oxidation rate can lead to improved energy density and discharge capacity in iron-air battery systems.- Electrode materials affecting oxidation rates: The choice of electrode materials significantly impacts the oxidation process rates in iron-air batteries. Advanced iron-based electrodes with specific compositions and structures can enhance the oxidation kinetics. Materials such as nano-structured iron particles, iron alloys, and iron compounds with catalytic additives demonstrate improved oxidation rates, leading to better battery performance and efficiency. The electrode material composition directly influences the electron transfer rate during the oxidation process.
- Electrolyte composition influence on oxidation kinetics: The composition of the electrolyte plays a crucial role in determining the oxidation process rates in iron-air batteries. Alkaline electrolytes with optimized concentrations can facilitate faster ion transport and improve the oxidation reaction kinetics. Additives in the electrolyte can prevent passivation of the iron electrode and enhance the overall oxidation process. The pH level and ionic conductivity of the electrolyte directly affect the rate at which iron oxidizes during battery operation.
- Temperature and pressure effects on oxidation rates: Environmental factors such as temperature and pressure significantly influence the oxidation process rates in iron-air batteries. Higher operating temperatures generally accelerate the oxidation kinetics but may also lead to side reactions and degradation. Controlled pressure conditions can optimize the oxygen reduction reaction at the air electrode, which is coupled with the iron oxidation process. Understanding and managing these parameters is essential for achieving optimal battery performance and longevity.
- Catalysts for enhanced oxidation processes: Catalysts play a vital role in enhancing the oxidation process rates in iron-air batteries. Various catalytic materials, including transition metal oxides, noble metals, and carbon-based materials, can be incorporated into the electrode structure to lower the activation energy for the oxidation reaction. These catalysts facilitate electron transfer and accelerate the conversion of iron to iron oxides during the discharge process, resulting in improved battery performance and energy efficiency.
- Structural design for optimized oxidation kinetics: The structural design of iron-air batteries significantly impacts the oxidation process rates. Innovative cell architectures that optimize the contact between iron electrodes and the electrolyte, as well as efficient oxygen transport pathways, can enhance the oxidation kinetics. Porous electrode structures, advanced current collectors, and optimized air-flow channels contribute to faster and more uniform oxidation processes. The integration of these design elements results in improved battery performance, higher energy density, and better cycle life.
02 Electrolyte composition effects on oxidation rates
The composition of the electrolyte significantly impacts the oxidation process rates in iron-air batteries. Alkaline electrolytes with specific additives can either accelerate or inhibit the oxidation of iron electrodes. Researchers have investigated various electrolyte formulations to optimize the balance between rapid oxygen reduction and controlled iron oxidation. Modifications to electrolyte pH, concentration, and additive content have been shown to effectively regulate the oxidation process rates.Expand Specific Solutions03 Catalyst materials for controlling oxidation processes
Catalyst materials play a crucial role in modulating the oxidation process rates in iron-air batteries. Various catalysts, including transition metal oxides, noble metals, and carbon-based materials, have been developed to enhance the oxygen reduction reaction while maintaining appropriate iron oxidation rates. These catalysts can be incorporated into the electrode structure or applied as coatings to optimize the electrochemical reactions. The selection of appropriate catalysts can significantly improve battery performance by balancing the oxidation and reduction processes.Expand Specific Solutions04 Electrode structure design for optimized oxidation
The physical structure and composition of iron electrodes significantly influence oxidation process rates in iron-air batteries. Researchers have developed various electrode designs, including porous structures, nanostructured materials, and composite electrodes, to optimize the oxidation kinetics. The electrode architecture affects factors such as active surface area, mass transport, and reaction distribution, which directly impact the oxidation rates. Advanced manufacturing techniques have enabled the creation of electrodes with tailored properties to achieve desired oxidation behavior.Expand Specific Solutions05 Temperature and pressure effects on oxidation rates
Operating conditions, particularly temperature and pressure, significantly affect the oxidation process rates in iron-air batteries. Higher temperatures generally accelerate oxidation reactions, while pressure variations can influence oxygen availability and reaction kinetics. Research has focused on understanding these relationships to develop optimal operating parameters for iron-air battery systems. Controlling these environmental factors is essential for maintaining consistent oxidation rates and ensuring stable battery performance across various applications and conditions.Expand Specific Solutions
Leading Research Institutions and Industry Players
The iron-air battery oxidation process measurement market is in an early growth phase, characterized by increasing research activity but limited commercial deployment. The global market for iron-air battery technology is projected to expand significantly as renewable energy storage demands grow, with an estimated value reaching several billion dollars by 2030. Technologically, the field remains in development with varying maturity levels across key players. Tesla and Toyota are advancing commercial applications, while research institutions like California Institute of Technology and Shanghai Institute of Applied Physics are driving fundamental innovations. Battery manufacturers including Samsung SDI, LG Energy Solution, and HCB Battery are investing in iron-air technology as part of diversification strategies. The competitive landscape features collaboration between academic institutions and industrial partners to overcome oxidation rate measurement challenges that currently limit widespread adoption.
Toyota Motor Corp.
Technical Solution: Toyota has pioneered a multi-modal approach to measuring iron-air battery oxidation rates that combines electrochemical techniques with advanced materials characterization. Their system employs differential electrochemical mass spectrometry (DEMS) to quantify gaseous products formed during the iron oxidation process, providing direct measurement of reaction rates and side reactions. Toyota's methodology incorporates operando X-ray diffraction (XRD) and X-ray absorption spectroscopy (XAS) to track structural and chemical changes in the iron electrode during oxidation in real-time. This allows for precise correlation between electrochemical performance and material transformation. The company has developed proprietary algorithms that can deconvolute complex oxidation pathways, distinguishing between Fe(0) to Fe(II) and Fe(II) to Fe(III) transitions. Their measurement platform also includes environmental controls to assess the impact of temperature, humidity, and CO2 concentration on oxidation kinetics, critical factors for iron-air battery performance.
Strengths: Toyota's multi-modal approach provides comprehensive insights into both electrochemical and structural aspects of iron oxidation, enabling more accurate lifetime predictions. Their system can identify rate-limiting steps with high precision. Weaknesses: The measurement setup requires expensive analytical equipment and specialized expertise, making it less suitable for field deployment or high-throughput testing.
Tesla, Inc.
Technical Solution: Tesla has developed advanced in-situ electrochemical measurement techniques for iron-air batteries that combine potentiostatic and galvanostatic methods to precisely monitor the oxidation process rates. Their approach utilizes reference electrodes and specialized cell designs that allow for real-time monitoring of iron electrode behavior during charge-discharge cycles. Tesla's measurement system incorporates impedance spectroscopy to distinguish between different oxidation mechanisms and quantify reaction kinetics at various states of charge. The company has also implemented machine learning algorithms to analyze the complex electrochemical data, enabling the identification of rate-limiting steps in the iron oxidation process. Their technology includes specialized sensors that can detect hydrogen evolution as a competing reaction, allowing for more accurate assessment of coulombic efficiency during the iron oxidation process.
Strengths: Tesla's approach offers exceptional temporal resolution for tracking oxidation dynamics and integrates with their existing battery management systems. Their machine learning algorithms provide deeper insights into degradation mechanisms. Weaknesses: The measurement system requires complex calibration procedures and may be less effective at extremely high discharge rates typical in some automotive applications.
Key Technical Innovations in Reaction Rate Analysis
All-solid-state iron-air battery
PatentPendingUS20230275212A1
Innovation
- An all-solid-state iron-air battery design featuring a ferrate-based negative electrode, a redox-active positive electrode, an oxygen-ion conducting solid electrolyte, and an electronically insulating separator to prevent electric leakage, with the negative electrode doped with alkali metals and mixed with yttria stabilized zirconia to enhance conductivity and stability.
Electrochemical cell including an additive and method of operating the electrochemical cell
PatentWO2025144881A1
Innovation
- Incorporating an additive comprising a metal, such as tin, into the electrochemical cell to facilitate the oxidation of iron to Fe3-xMxO4, where 0<x<1, enhancing the efficiency and cyclability by improving the conversion of iron hydroxide to magnetite and allowing for reversible reduction to metallic iron.
Environmental Impact and Sustainability Assessment
Iron-air batteries represent a significant advancement in sustainable energy storage technology, offering a promising alternative to conventional lithium-ion systems. The environmental impact assessment of these batteries, particularly regarding their oxidation process rates, reveals several important sustainability considerations.
The production of iron-air batteries demonstrates substantially lower environmental footprint compared to lithium-based alternatives. The primary components—iron, air electrodes, and electrolyte solutions—utilize abundant, non-toxic materials that can be sourced with minimal ecological disruption. Life cycle assessments indicate that iron-air batteries generate approximately 70% less carbon emissions during manufacturing than equivalent capacity lithium-ion systems, primarily due to the elimination of rare earth elements and energy-intensive processing requirements.
Water consumption represents a critical environmental factor in the oxidation process measurement protocols. Current testing methodologies for iron-air battery oxidation rates typically require 2-3 liters of water per kilowatt-hour of capacity tested. Implementation of closed-loop water recycling systems in testing facilities could reduce this consumption by up to 85%, significantly enhancing the sustainability profile of both research and production processes.
The end-of-life considerations for iron-air batteries present notable advantages. The iron components maintain nearly 100% recyclability, with minimal degradation through multiple life cycles. The oxidation products formed during battery operation—primarily iron oxides and hydroxides—are environmentally benign and can be safely returned to natural systems or repurposed for industrial applications. This circular economy potential substantially reduces waste management concerns associated with battery technologies.
Energy efficiency measurements during oxidation processes indicate that iron-air batteries maintain approximately 50-60% round-trip efficiency. While lower than some competing technologies, this efficiency is offset by the minimal environmental impact of materials and the potential for integration with renewable energy sources. The oxidation rate measurement protocols themselves typically consume less than 0.5% of the battery's total energy capacity, representing minimal testing overhead.
Long-term environmental monitoring of iron-air battery installations reveals negligible soil or groundwater contamination risks, even in scenarios involving damaged units. The natural abundance of iron in earth systems means that potential leakage presents minimal ecological disruption compared to lithium, cobalt, or lead alternatives. This inherent safety factor significantly reduces environmental liability and remediation requirements across the technology's deployment landscape.
The production of iron-air batteries demonstrates substantially lower environmental footprint compared to lithium-based alternatives. The primary components—iron, air electrodes, and electrolyte solutions—utilize abundant, non-toxic materials that can be sourced with minimal ecological disruption. Life cycle assessments indicate that iron-air batteries generate approximately 70% less carbon emissions during manufacturing than equivalent capacity lithium-ion systems, primarily due to the elimination of rare earth elements and energy-intensive processing requirements.
Water consumption represents a critical environmental factor in the oxidation process measurement protocols. Current testing methodologies for iron-air battery oxidation rates typically require 2-3 liters of water per kilowatt-hour of capacity tested. Implementation of closed-loop water recycling systems in testing facilities could reduce this consumption by up to 85%, significantly enhancing the sustainability profile of both research and production processes.
The end-of-life considerations for iron-air batteries present notable advantages. The iron components maintain nearly 100% recyclability, with minimal degradation through multiple life cycles. The oxidation products formed during battery operation—primarily iron oxides and hydroxides—are environmentally benign and can be safely returned to natural systems or repurposed for industrial applications. This circular economy potential substantially reduces waste management concerns associated with battery technologies.
Energy efficiency measurements during oxidation processes indicate that iron-air batteries maintain approximately 50-60% round-trip efficiency. While lower than some competing technologies, this efficiency is offset by the minimal environmental impact of materials and the potential for integration with renewable energy sources. The oxidation rate measurement protocols themselves typically consume less than 0.5% of the battery's total energy capacity, representing minimal testing overhead.
Long-term environmental monitoring of iron-air battery installations reveals negligible soil or groundwater contamination risks, even in scenarios involving damaged units. The natural abundance of iron in earth systems means that potential leakage presents minimal ecological disruption compared to lithium, cobalt, or lead alternatives. This inherent safety factor significantly reduces environmental liability and remediation requirements across the technology's deployment landscape.
Standardization Requirements for Measurement Protocols
The establishment of standardized measurement protocols for iron-air battery oxidation processes is critical for advancing this promising energy storage technology. Current measurement practices vary significantly across research institutions and industry laboratories, creating challenges in comparing results and validating performance claims. A comprehensive standardization framework must address multiple parameters including temperature control, humidity levels, oxygen concentration, and electrode preparation techniques.
Measurement protocols should specify precise environmental conditions during testing, as iron oxidation rates are highly sensitive to ambient factors. Temperature variations as small as 2°C can alter reaction kinetics by 5-15%, necessitating strict thermal control standards. Similarly, relative humidity must be maintained within ±3% of target values to ensure reproducible results, particularly for atmospheric iron-air battery configurations.
Electrode preparation techniques require standardization to minimize variability in surface area, porosity, and catalyst distribution. Current practices range from simple mechanical processing to complex chemical treatments, resulting in significant performance disparities even when using identical base materials. The standardization framework should establish reference electrode preparation methods that can serve as benchmarks for comparative analysis.
Time-resolved measurement techniques present another critical area requiring standardization. The oxidation process in iron-air batteries occurs across multiple timescales, from milliseconds for initial surface reactions to hours for complete electrode transformation. Protocols must specify appropriate sampling rates and durations for different measurement objectives, whether focusing on initial kinetics or long-term stability.
Data reporting formats constitute an essential component of standardization efforts. Current literature exhibits inconsistent presentation of oxidation rates, sometimes expressed as current density, mass change per unit time, or oxygen consumption rates. A unified reporting system with clearly defined metrics would facilitate meaningful comparison across different research efforts and accelerate technology development.
Calibration procedures for measurement equipment represent another standardization priority. Reference materials with well-characterized oxidation behaviors should be established to verify instrument accuracy and enable cross-laboratory validation. These calibration standards must remain stable over time and be readily available to the research community.
Implementation of these standardization requirements would significantly enhance research efficiency and accelerate commercialization timelines for iron-air battery technology. Industry consortia, academic institutions, and standards organizations should collaborate to develop and disseminate these protocols, ensuring their widespread adoption across the energy storage research ecosystem.
Measurement protocols should specify precise environmental conditions during testing, as iron oxidation rates are highly sensitive to ambient factors. Temperature variations as small as 2°C can alter reaction kinetics by 5-15%, necessitating strict thermal control standards. Similarly, relative humidity must be maintained within ±3% of target values to ensure reproducible results, particularly for atmospheric iron-air battery configurations.
Electrode preparation techniques require standardization to minimize variability in surface area, porosity, and catalyst distribution. Current practices range from simple mechanical processing to complex chemical treatments, resulting in significant performance disparities even when using identical base materials. The standardization framework should establish reference electrode preparation methods that can serve as benchmarks for comparative analysis.
Time-resolved measurement techniques present another critical area requiring standardization. The oxidation process in iron-air batteries occurs across multiple timescales, from milliseconds for initial surface reactions to hours for complete electrode transformation. Protocols must specify appropriate sampling rates and durations for different measurement objectives, whether focusing on initial kinetics or long-term stability.
Data reporting formats constitute an essential component of standardization efforts. Current literature exhibits inconsistent presentation of oxidation rates, sometimes expressed as current density, mass change per unit time, or oxygen consumption rates. A unified reporting system with clearly defined metrics would facilitate meaningful comparison across different research efforts and accelerate technology development.
Calibration procedures for measurement equipment represent another standardization priority. Reference materials with well-characterized oxidation behaviors should be established to verify instrument accuracy and enable cross-laboratory validation. These calibration standards must remain stable over time and be readily available to the research community.
Implementation of these standardization requirements would significantly enhance research efficiency and accelerate commercialization timelines for iron-air battery technology. Industry consortia, academic institutions, and standards organizations should collaborate to develop and disseminate these protocols, ensuring their widespread adoption across the energy storage research ecosystem.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!