How to Increase Alkyl Reaction Efficiency in Industry?
JUL 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Alkylation Background
Alkylation is a fundamental process in the petrochemical industry, playing a crucial role in the production of high-octane gasoline components and various other chemical products. The process involves the reaction of an alkene with an alkane in the presence of a strong acid catalyst, typically sulfuric acid or hydrofluoric acid. This reaction results in the formation of larger, branched hydrocarbons that are essential for improving fuel quality and producing valuable chemical intermediates.
The history of alkylation dates back to the 1930s when it was first developed to meet the growing demand for high-performance aviation fuel during World War II. Since then, the process has evolved significantly, becoming an integral part of modern refinery operations. The primary goal of alkylation is to convert low-value, light olefins and isobutane into high-value, branched paraffins, which are ideal components for gasoline blending due to their high octane ratings.
In recent years, the importance of alkylation has grown due to increasingly stringent environmental regulations and the demand for cleaner-burning fuels. The process allows refineries to produce gasoline with lower sulfur content and reduced emissions, aligning with global efforts to minimize environmental impact. Additionally, alkylation products serve as essential feedstocks for the production of various chemicals, including plastics, synthetic rubbers, and detergents.
The efficiency of alkylation reactions has become a critical focus for industry professionals, as it directly impacts production costs, product quality, and environmental sustainability. Improving alkylation efficiency involves optimizing various factors, including catalyst selection, reaction conditions, and process design. Researchers and engineers are continuously exploring innovative approaches to enhance reaction rates, increase selectivity, and reduce energy consumption in alkylation processes.
One of the key challenges in alkylation is managing the highly corrosive nature of the acid catalysts used in traditional processes. This has led to the development of alternative technologies, such as solid acid catalysts and ionic liquid-based systems, which aim to improve safety and reduce environmental risks associated with conventional alkylation methods. These emerging technologies also offer the potential for increased reaction efficiency and reduced operational costs.
As the industry evolves, there is a growing emphasis on developing more sustainable alkylation processes that minimize waste generation and energy consumption. This includes exploring the use of renewable feedstocks and implementing advanced process control systems to optimize reaction conditions in real-time. The quest for increased alkylation efficiency is driven not only by economic considerations but also by the broader goal of creating more environmentally friendly and sustainable industrial processes.
The history of alkylation dates back to the 1930s when it was first developed to meet the growing demand for high-performance aviation fuel during World War II. Since then, the process has evolved significantly, becoming an integral part of modern refinery operations. The primary goal of alkylation is to convert low-value, light olefins and isobutane into high-value, branched paraffins, which are ideal components for gasoline blending due to their high octane ratings.
In recent years, the importance of alkylation has grown due to increasingly stringent environmental regulations and the demand for cleaner-burning fuels. The process allows refineries to produce gasoline with lower sulfur content and reduced emissions, aligning with global efforts to minimize environmental impact. Additionally, alkylation products serve as essential feedstocks for the production of various chemicals, including plastics, synthetic rubbers, and detergents.
The efficiency of alkylation reactions has become a critical focus for industry professionals, as it directly impacts production costs, product quality, and environmental sustainability. Improving alkylation efficiency involves optimizing various factors, including catalyst selection, reaction conditions, and process design. Researchers and engineers are continuously exploring innovative approaches to enhance reaction rates, increase selectivity, and reduce energy consumption in alkylation processes.
One of the key challenges in alkylation is managing the highly corrosive nature of the acid catalysts used in traditional processes. This has led to the development of alternative technologies, such as solid acid catalysts and ionic liquid-based systems, which aim to improve safety and reduce environmental risks associated with conventional alkylation methods. These emerging technologies also offer the potential for increased reaction efficiency and reduced operational costs.
As the industry evolves, there is a growing emphasis on developing more sustainable alkylation processes that minimize waste generation and energy consumption. This includes exploring the use of renewable feedstocks and implementing advanced process control systems to optimize reaction conditions in real-time. The quest for increased alkylation efficiency is driven not only by economic considerations but also by the broader goal of creating more environmentally friendly and sustainable industrial processes.
Market Demand Analysis
The market demand for increasing alkyl reaction efficiency in industry has been steadily growing due to its wide-ranging applications across various sectors. Chemical manufacturers are constantly seeking ways to optimize their processes, reduce costs, and improve product quality, making efficient alkylation reactions a key focus area.
In the petrochemical industry, alkylation plays a crucial role in producing high-octane gasoline components. As global demand for cleaner-burning fuels continues to rise, refineries are under pressure to enhance their alkylation processes. This has led to a significant market for technologies that can increase alkyl reaction efficiency, reduce byproduct formation, and minimize catalyst consumption.
The pharmaceutical sector also presents a substantial market for improved alkylation techniques. Drug manufacturers rely on efficient alkylation reactions to synthesize various active pharmaceutical ingredients (APIs) and intermediates. As the industry moves towards more complex molecules and stricter regulatory requirements, there is a growing need for precise, high-yield alkylation processes that can operate under milder conditions.
In the agrochemical industry, alkylation reactions are essential for producing herbicides, pesticides, and other crop protection products. With the increasing global population and the need for sustainable agriculture, the demand for more efficient and environmentally friendly alkylation processes is on the rise. This has created opportunities for innovative catalysts and reactor designs that can improve reaction selectivity and reduce waste.
The polymer industry is another significant market driver for alkyl reaction efficiency improvements. Alkylation reactions are used in the production of various specialty polymers and plastics. As consumer demand for high-performance materials grows, manufacturers are seeking ways to optimize their alkylation processes to achieve better control over polymer properties and reduce production costs.
Environmental concerns and sustainability goals are also shaping the market demand for more efficient alkylation technologies. Industries are looking for solutions that can reduce energy consumption, minimize waste generation, and lower greenhouse gas emissions associated with alkylation reactions. This has led to increased interest in green chemistry approaches and catalytic systems that can operate under ambient conditions.
The market for alkylation efficiency improvements is further driven by the need for process intensification in chemical manufacturing. Companies are exploring continuous flow reactors, microreactor technologies, and other innovative approaches to increase throughput, improve product quality, and reduce equipment footprint. These advancements are particularly attractive for small-scale and specialty chemical producers looking to enhance their competitiveness.
In the petrochemical industry, alkylation plays a crucial role in producing high-octane gasoline components. As global demand for cleaner-burning fuels continues to rise, refineries are under pressure to enhance their alkylation processes. This has led to a significant market for technologies that can increase alkyl reaction efficiency, reduce byproduct formation, and minimize catalyst consumption.
The pharmaceutical sector also presents a substantial market for improved alkylation techniques. Drug manufacturers rely on efficient alkylation reactions to synthesize various active pharmaceutical ingredients (APIs) and intermediates. As the industry moves towards more complex molecules and stricter regulatory requirements, there is a growing need for precise, high-yield alkylation processes that can operate under milder conditions.
In the agrochemical industry, alkylation reactions are essential for producing herbicides, pesticides, and other crop protection products. With the increasing global population and the need for sustainable agriculture, the demand for more efficient and environmentally friendly alkylation processes is on the rise. This has created opportunities for innovative catalysts and reactor designs that can improve reaction selectivity and reduce waste.
The polymer industry is another significant market driver for alkyl reaction efficiency improvements. Alkylation reactions are used in the production of various specialty polymers and plastics. As consumer demand for high-performance materials grows, manufacturers are seeking ways to optimize their alkylation processes to achieve better control over polymer properties and reduce production costs.
Environmental concerns and sustainability goals are also shaping the market demand for more efficient alkylation technologies. Industries are looking for solutions that can reduce energy consumption, minimize waste generation, and lower greenhouse gas emissions associated with alkylation reactions. This has led to increased interest in green chemistry approaches and catalytic systems that can operate under ambient conditions.
The market for alkylation efficiency improvements is further driven by the need for process intensification in chemical manufacturing. Companies are exploring continuous flow reactors, microreactor technologies, and other innovative approaches to increase throughput, improve product quality, and reduce equipment footprint. These advancements are particularly attractive for small-scale and specialty chemical producers looking to enhance their competitiveness.
Current Challenges
The alkyl reaction efficiency in industrial processes faces several significant challenges that hinder its widespread adoption and optimization. One of the primary obstacles is the high energy consumption associated with traditional alkylation methods. Many current processes require elevated temperatures and pressures, leading to substantial energy costs and reduced overall efficiency.
Another critical challenge is the selectivity of alkylation reactions. Achieving high selectivity towards desired products while minimizing unwanted side reactions remains a persistent issue. This lack of selectivity often results in lower yields and increased purification costs, negatively impacting the economic viability of industrial-scale alkylation processes.
The use of homogeneous catalysts in alkylation reactions presents additional complications. While these catalysts often demonstrate high activity, their separation from the reaction mixture and subsequent recycling pose significant technical and economic challenges. This limitation not only increases production costs but also raises environmental concerns due to potential catalyst waste.
Catalyst deactivation is another major hurdle in maintaining consistent alkylation efficiency. Many industrial processes suffer from rapid catalyst degradation, necessitating frequent catalyst replacement or regeneration. This issue not only increases operational costs but also leads to production downtime and reduced overall process efficiency.
The handling and disposal of corrosive reagents, such as strong acids often used in alkylation reactions, present safety and environmental challenges. These reagents can cause equipment corrosion, require specialized handling procedures, and generate hazardous waste streams that need careful management and disposal.
Scale-up issues also pose significant challenges when transitioning from laboratory-scale to industrial-scale alkylation processes. Maintaining reaction efficiency and selectivity at larger scales often requires substantial process modifications and optimization, leading to increased development time and costs.
Furthermore, the availability and cost of raw materials, particularly high-quality alkylating agents, can significantly impact the economic feasibility of industrial alkylation processes. Fluctuations in feedstock prices and supply chain disruptions can introduce volatility into production costs and affect overall process viability.
Lastly, stringent environmental regulations and the push towards greener chemistry present additional challenges for traditional alkylation methods. There is a growing need to develop more sustainable processes that reduce waste generation, minimize the use of hazardous substances, and improve overall atom economy. Meeting these environmental standards while maintaining high efficiency and economic viability remains a significant challenge for the industry.
Another critical challenge is the selectivity of alkylation reactions. Achieving high selectivity towards desired products while minimizing unwanted side reactions remains a persistent issue. This lack of selectivity often results in lower yields and increased purification costs, negatively impacting the economic viability of industrial-scale alkylation processes.
The use of homogeneous catalysts in alkylation reactions presents additional complications. While these catalysts often demonstrate high activity, their separation from the reaction mixture and subsequent recycling pose significant technical and economic challenges. This limitation not only increases production costs but also raises environmental concerns due to potential catalyst waste.
Catalyst deactivation is another major hurdle in maintaining consistent alkylation efficiency. Many industrial processes suffer from rapid catalyst degradation, necessitating frequent catalyst replacement or regeneration. This issue not only increases operational costs but also leads to production downtime and reduced overall process efficiency.
The handling and disposal of corrosive reagents, such as strong acids often used in alkylation reactions, present safety and environmental challenges. These reagents can cause equipment corrosion, require specialized handling procedures, and generate hazardous waste streams that need careful management and disposal.
Scale-up issues also pose significant challenges when transitioning from laboratory-scale to industrial-scale alkylation processes. Maintaining reaction efficiency and selectivity at larger scales often requires substantial process modifications and optimization, leading to increased development time and costs.
Furthermore, the availability and cost of raw materials, particularly high-quality alkylating agents, can significantly impact the economic feasibility of industrial alkylation processes. Fluctuations in feedstock prices and supply chain disruptions can introduce volatility into production costs and affect overall process viability.
Lastly, stringent environmental regulations and the push towards greener chemistry present additional challenges for traditional alkylation methods. There is a growing need to develop more sustainable processes that reduce waste generation, minimize the use of hazardous substances, and improve overall atom economy. Meeting these environmental standards while maintaining high efficiency and economic viability remains a significant challenge for the industry.
Existing Solutions
01 Catalytic systems for alkyl reactions
Various catalytic systems are employed to enhance the efficiency of alkyl reactions. These systems often involve metal complexes or organometallic compounds that facilitate the formation or cleavage of carbon-carbon bonds. The choice of catalyst can significantly impact reaction rates, selectivity, and overall yield.- Catalysts for alkyl reactions: Various catalysts are employed to enhance the efficiency of alkyl reactions. These catalysts can include metal complexes, enzymes, or other chemical compounds that facilitate the reaction process, reduce activation energy, and improve overall yield. The choice of catalyst depends on the specific alkyl reaction and desired outcome.
- Reaction conditions optimization: Optimizing reaction conditions such as temperature, pressure, and solvent selection can significantly improve alkyl reaction efficiency. Careful control of these parameters can lead to increased reaction rates, higher yields, and improved selectivity. Researchers often employ design of experiments (DOE) techniques to determine optimal conditions.
- Novel reaction methodologies: Innovative reaction methodologies are developed to enhance alkyl reaction efficiency. These may include new synthetic routes, one-pot reactions, or the use of alternative energy sources such as microwave irradiation or photochemistry. Such approaches can lead to shorter reaction times, reduced waste, and improved overall efficiency.
- Reagent and substrate modifications: Modifying reagents or substrates can improve alkyl reaction efficiency. This may involve the use of activating groups, protecting groups, or the development of more reactive alkylating agents. Such modifications can enhance reactivity, selectivity, and overall yield of the desired alkyl products.
- Process intensification techniques: Process intensification techniques are employed to enhance alkyl reaction efficiency on an industrial scale. These may include continuous flow reactors, microreactors, or other innovative reactor designs that improve heat and mass transfer, leading to better reaction control and increased productivity.
02 Reaction conditions optimization
Optimizing reaction conditions such as temperature, pressure, solvent choice, and reactant ratios is crucial for improving alkyl reaction efficiency. Careful control of these parameters can lead to increased conversion rates, reduced side reactions, and improved product purity.Expand Specific Solutions03 Novel reagents and substrates
The development of new reagents and substrates can significantly enhance alkyl reaction efficiency. This includes the design of more reactive alkylating agents, the use of activated substrates, or the incorporation of directing groups to improve regioselectivity in alkylation reactions.Expand Specific Solutions04 Continuous flow and microreactor technology
Implementing continuous flow processes and microreactor technology can improve alkyl reaction efficiency by providing better control over reaction parameters, enhancing heat and mass transfer, and allowing for easier scale-up of reactions. These approaches often result in higher yields and improved product quality.Expand Specific Solutions05 Green chemistry approaches
Adopting green chemistry principles to improve alkyl reaction efficiency involves using environmentally friendly solvents, developing atom-economical processes, and employing renewable feedstocks. These approaches aim to reduce waste, improve energy efficiency, and minimize the environmental impact of alkyl reactions.Expand Specific Solutions
Key Industry Players
The alkyl reaction efficiency improvement in industry is currently in a mature development stage, with a substantial market size driven by the petrochemical and chemical sectors. The technology's maturity is evident from the involvement of major players like China Petroleum & Chemical Corp., BASF Corp., and PetroChina Co., Ltd. These companies have established research institutes and extensive R&D capabilities, indicating a high level of technological sophistication. The competitive landscape is diverse, with participation from global chemical giants, specialized research institutions, and academic centers, suggesting ongoing innovation and refinement of alkyl reaction processes. This multi-faceted approach to improving efficiency reflects the technology's critical importance in various industrial applications and its potential for further optimization.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a novel alkylation technology using ionic liquids as catalysts. This process, known as Sinopec Alkylation Technology (SAT), significantly improves reaction efficiency and product quality. The ionic liquid catalyst system allows for continuous operation at mild conditions, resulting in higher octane alkylate production[1]. The process operates at lower temperatures (10-20°C) compared to traditional sulfuric acid alkylation (0-10°C), reducing energy consumption. SAT also achieves a conversion rate of over 99% for isobutane and olefins, with alkylate yield exceeding 97%[2]. The technology has been successfully implemented in multiple refineries, demonstrating its industrial viability and scalability.
Strengths: Higher conversion rates, improved product quality, lower energy consumption, and reduced environmental impact. Weaknesses: Potential higher initial investment costs and the need for specialized handling of ionic liquids.
BASF Corp.
Technical Solution: BASF has developed an innovative Fixed-Bed Alkylation Technology (FBAT) to enhance alkyl reaction efficiency in industrial processes. This technology utilizes a solid catalyst in a fixed-bed reactor, offering significant advantages over traditional liquid acid catalysts. The FBAT process operates at higher temperatures (100-130°C) than conventional alkylation, allowing for improved reaction kinetics and higher throughput[3]. BASF's solid catalyst demonstrates excellent stability, with a lifespan of several years, reducing downtime and catalyst replacement costs. The process achieves high octane alkylate production with minimal byproduct formation, resulting in yields of up to 98%[4]. Additionally, BASF has implemented advanced process control systems and heat integration techniques to optimize energy efficiency and reduce operating costs.
Strengths: Long catalyst life, high product quality, improved safety due to solid catalyst, and reduced environmental impact. Weaknesses: Higher operating temperatures may increase energy requirements, and the technology may require significant modifications to existing alkylation units.
Innovative Catalysts
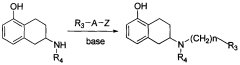
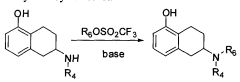
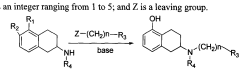
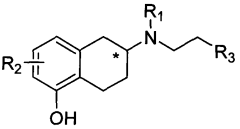
Method for industrially preparing nitrogen substituted amino-5,6,7,8-tetrahydronaphthol
PatentWO2013000273A1
Innovation
- A method involving the reaction of a compound of formula (II) with a compound of formula (III) under alkaline conditions and in the presence of a sulfite, which neutralizes acidic by-products and prevents oxidation, improving yield and reducing the need for excessive alkylating agents.
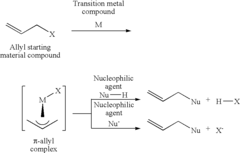
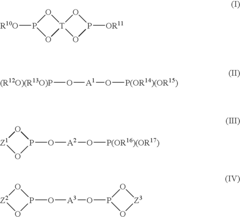
Method for producing allyl compound, and ether or ester compound produced thereby
PatentInactiveUS20060106181A1
Innovation
- A catalyst system comprising a multidentate phosphite compound and a transition metal compound from Group 8 to Group 10 of the Periodic Table is used to enhance the reactivity with oxygen nucleophilic agents, allowing for the efficient production of allyl compounds such as ethers and esters.
Process Optimization
Process optimization plays a crucial role in increasing alkyl reaction efficiency in industrial settings. By fine-tuning various parameters and implementing advanced control strategies, manufacturers can significantly enhance the overall performance of alkylation processes.
One key aspect of process optimization is the careful selection and adjustment of reaction conditions. Temperature control is paramount, as alkylation reactions are often highly sensitive to thermal variations. Implementing precise temperature regulation systems, such as advanced heat exchangers or jacketed reactors, can help maintain optimal reaction temperatures throughout the process. This ensures consistent product quality and maximizes conversion rates.
Pressure management is another critical factor in alkylation efficiency. Optimizing pressure conditions can drive the reaction equilibrium towards the desired products, improving yield and selectivity. The use of pressure-controlled reactors and advanced pressure relief systems can help maintain ideal pressure levels while ensuring process safety.
Catalyst optimization is a fundamental component of process improvement. Selecting the most suitable catalyst for the specific alkylation reaction and optimizing its performance can dramatically increase reaction rates and product yields. This may involve exploring novel catalyst formulations, fine-tuning catalyst particle size and distribution, or implementing catalyst regeneration techniques to extend catalyst life and maintain high activity levels.
Reactor design and configuration also play a significant role in process optimization. Employing advanced reactor technologies, such as continuous flow reactors or microreactors, can enhance mass and heat transfer, leading to improved reaction kinetics and product selectivity. Additionally, optimizing reactor geometry and internals can help minimize dead zones and ensure uniform mixing, further boosting reaction efficiency.
Process control and automation are essential for maintaining optimal operating conditions and responding to process variations in real-time. Implementing advanced process control systems, such as model predictive control (MPC) or artificial intelligence-driven control algorithms, can help maintain process stability and maximize efficiency. These systems can continuously adjust process parameters based on real-time data, ensuring that the alkylation reaction operates at peak performance.
Feedstock purification and preparation are often overlooked aspects of process optimization. Implementing effective pretreatment steps to remove impurities or unwanted components from raw materials can significantly improve reaction efficiency and product quality. This may involve the use of advanced separation technologies or the implementation of in-line purification systems.
Finally, process integration and heat recovery strategies can contribute to overall efficiency improvements. By optimizing heat integration between process streams and implementing energy recovery systems, manufacturers can reduce energy consumption and operating costs while maintaining high reaction efficiency.
One key aspect of process optimization is the careful selection and adjustment of reaction conditions. Temperature control is paramount, as alkylation reactions are often highly sensitive to thermal variations. Implementing precise temperature regulation systems, such as advanced heat exchangers or jacketed reactors, can help maintain optimal reaction temperatures throughout the process. This ensures consistent product quality and maximizes conversion rates.
Pressure management is another critical factor in alkylation efficiency. Optimizing pressure conditions can drive the reaction equilibrium towards the desired products, improving yield and selectivity. The use of pressure-controlled reactors and advanced pressure relief systems can help maintain ideal pressure levels while ensuring process safety.
Catalyst optimization is a fundamental component of process improvement. Selecting the most suitable catalyst for the specific alkylation reaction and optimizing its performance can dramatically increase reaction rates and product yields. This may involve exploring novel catalyst formulations, fine-tuning catalyst particle size and distribution, or implementing catalyst regeneration techniques to extend catalyst life and maintain high activity levels.
Reactor design and configuration also play a significant role in process optimization. Employing advanced reactor technologies, such as continuous flow reactors or microreactors, can enhance mass and heat transfer, leading to improved reaction kinetics and product selectivity. Additionally, optimizing reactor geometry and internals can help minimize dead zones and ensure uniform mixing, further boosting reaction efficiency.
Process control and automation are essential for maintaining optimal operating conditions and responding to process variations in real-time. Implementing advanced process control systems, such as model predictive control (MPC) or artificial intelligence-driven control algorithms, can help maintain process stability and maximize efficiency. These systems can continuously adjust process parameters based on real-time data, ensuring that the alkylation reaction operates at peak performance.
Feedstock purification and preparation are often overlooked aspects of process optimization. Implementing effective pretreatment steps to remove impurities or unwanted components from raw materials can significantly improve reaction efficiency and product quality. This may involve the use of advanced separation technologies or the implementation of in-line purification systems.
Finally, process integration and heat recovery strategies can contribute to overall efficiency improvements. By optimizing heat integration between process streams and implementing energy recovery systems, manufacturers can reduce energy consumption and operating costs while maintaining high reaction efficiency.
Environmental Impact
The environmental impact of increasing alkyl reaction efficiency in industry is a critical consideration that extends beyond mere economic benefits. As industries strive to enhance their alkylation processes, the potential environmental consequences must be carefully evaluated and mitigated.
One of the primary environmental benefits of improved alkyl reaction efficiency is the reduction in energy consumption. More efficient reactions typically require less energy input, leading to decreased greenhouse gas emissions associated with power generation. This aligns with global efforts to combat climate change and reduce the carbon footprint of industrial operations.
Furthermore, increased efficiency often translates to a reduction in raw material usage. This not only conserves natural resources but also minimizes the environmental impact associated with the extraction, processing, and transportation of these materials. Less waste generation is another positive outcome, as more efficient reactions tend to produce fewer by-products and unwanted side reactions.
However, it is crucial to consider potential negative environmental impacts as well. Some methods of increasing alkyl reaction efficiency may involve the use of more potent catalysts or reagents, which could pose environmental risks if not properly managed. The disposal or recycling of these substances must be carefully addressed to prevent soil and water contamination.
Additionally, the pursuit of higher efficiency might lead to increased production volumes, potentially offsetting some of the environmental gains. This rebound effect must be carefully monitored and managed to ensure that efficiency improvements truly result in net environmental benefits.
Water usage is another important factor to consider. While more efficient reactions may reduce overall water consumption, any changes in the process that affect water quality or quantity must be thoroughly assessed. Proper wastewater treatment and recycling systems should be implemented to minimize the impact on local water resources.
The life cycle assessment (LCA) of the entire alkylation process, including raw material sourcing, production, use, and disposal, is essential for a comprehensive understanding of the environmental impact. This holistic approach helps identify areas where efficiency improvements can yield the most significant environmental benefits while highlighting potential trade-offs that need to be addressed.
In conclusion, while increasing alkyl reaction efficiency in industry holds great promise for environmental sustainability, it requires a balanced and thoughtful approach. The potential benefits of reduced energy consumption, resource conservation, and waste minimization must be weighed against possible risks associated with new processes or increased production. Ongoing research and development should focus not only on efficiency gains but also on environmentally friendly catalysts, green chemistry principles, and sustainable production methods to ensure that industrial progress aligns with environmental stewardship.
One of the primary environmental benefits of improved alkyl reaction efficiency is the reduction in energy consumption. More efficient reactions typically require less energy input, leading to decreased greenhouse gas emissions associated with power generation. This aligns with global efforts to combat climate change and reduce the carbon footprint of industrial operations.
Furthermore, increased efficiency often translates to a reduction in raw material usage. This not only conserves natural resources but also minimizes the environmental impact associated with the extraction, processing, and transportation of these materials. Less waste generation is another positive outcome, as more efficient reactions tend to produce fewer by-products and unwanted side reactions.
However, it is crucial to consider potential negative environmental impacts as well. Some methods of increasing alkyl reaction efficiency may involve the use of more potent catalysts or reagents, which could pose environmental risks if not properly managed. The disposal or recycling of these substances must be carefully addressed to prevent soil and water contamination.
Additionally, the pursuit of higher efficiency might lead to increased production volumes, potentially offsetting some of the environmental gains. This rebound effect must be carefully monitored and managed to ensure that efficiency improvements truly result in net environmental benefits.
Water usage is another important factor to consider. While more efficient reactions may reduce overall water consumption, any changes in the process that affect water quality or quantity must be thoroughly assessed. Proper wastewater treatment and recycling systems should be implemented to minimize the impact on local water resources.
The life cycle assessment (LCA) of the entire alkylation process, including raw material sourcing, production, use, and disposal, is essential for a comprehensive understanding of the environmental impact. This holistic approach helps identify areas where efficiency improvements can yield the most significant environmental benefits while highlighting potential trade-offs that need to be addressed.
In conclusion, while increasing alkyl reaction efficiency in industry holds great promise for environmental sustainability, it requires a balanced and thoughtful approach. The potential benefits of reduced energy consumption, resource conservation, and waste minimization must be weighed against possible risks associated with new processes or increased production. Ongoing research and development should focus not only on efficiency gains but also on environmentally friendly catalysts, green chemistry principles, and sustainable production methods to ensure that industrial progress aligns with environmental stewardship.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!