How to Minimize Risks Associated with Hypochlorous Acid Overexposure?
AUG 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hypochlorous Acid Safety Overview and Objectives
Hypochlorous acid (HOCl) is a powerful oxidizing agent widely used in various industries for disinfection and sanitation purposes. As its applications continue to expand, it becomes increasingly crucial to address the potential risks associated with overexposure. This technical research report aims to provide a comprehensive overview of hypochlorous acid safety and establish clear objectives for minimizing associated risks.
The primary goal of this research is to identify and analyze the potential hazards of hypochlorous acid overexposure, focusing on both acute and chronic effects on human health and the environment. By understanding these risks, we can develop effective strategies to mitigate them and ensure safer handling and application of HOCl in various settings.
One of the key objectives is to evaluate current safety protocols and guidelines for hypochlorous acid use across different industries. This assessment will help identify any gaps or inconsistencies in existing safety measures and provide a foundation for developing more robust and standardized safety practices.
Another critical aspect of this research is to investigate the latest technological advancements in HOCl production, storage, and application methods. By exploring innovative approaches, we aim to identify potential solutions that can inherently reduce the risk of overexposure without compromising the effectiveness of hypochlorous acid as a disinfectant.
Furthermore, this study will examine the regulatory landscape surrounding hypochlorous acid use and safety standards. Understanding current regulations and their effectiveness in preventing overexposure incidents will be crucial in formulating recommendations for policy improvements and industry-wide best practices.
The research will also focus on developing comprehensive training programs and educational materials for workers and end-users who handle hypochlorous acid. By improving awareness and knowledge about proper handling techniques and potential risks, we can significantly reduce the likelihood of overexposure incidents.
Additionally, this report aims to explore the development of advanced monitoring and detection systems for hypochlorous acid levels in various environments. Early detection of elevated HOCl concentrations can play a vital role in preventing overexposure and ensuring timely implementation of safety measures.
Lastly, we will investigate potential alternatives or complementary technologies that could be used in conjunction with hypochlorous acid to reduce overall exposure risks while maintaining or enhancing disinfection efficacy. This approach may lead to the development of safer, hybrid disinfection systems that minimize the reliance on high concentrations of HOCl.
By addressing these objectives, this technical research report seeks to provide a solid foundation for improving hypochlorous acid safety across industries and applications. The findings and recommendations from this study will contribute to the development of more effective risk mitigation strategies, ultimately ensuring safer working environments and broader public health protection.
The primary goal of this research is to identify and analyze the potential hazards of hypochlorous acid overexposure, focusing on both acute and chronic effects on human health and the environment. By understanding these risks, we can develop effective strategies to mitigate them and ensure safer handling and application of HOCl in various settings.
One of the key objectives is to evaluate current safety protocols and guidelines for hypochlorous acid use across different industries. This assessment will help identify any gaps or inconsistencies in existing safety measures and provide a foundation for developing more robust and standardized safety practices.
Another critical aspect of this research is to investigate the latest technological advancements in HOCl production, storage, and application methods. By exploring innovative approaches, we aim to identify potential solutions that can inherently reduce the risk of overexposure without compromising the effectiveness of hypochlorous acid as a disinfectant.
Furthermore, this study will examine the regulatory landscape surrounding hypochlorous acid use and safety standards. Understanding current regulations and their effectiveness in preventing overexposure incidents will be crucial in formulating recommendations for policy improvements and industry-wide best practices.
The research will also focus on developing comprehensive training programs and educational materials for workers and end-users who handle hypochlorous acid. By improving awareness and knowledge about proper handling techniques and potential risks, we can significantly reduce the likelihood of overexposure incidents.
Additionally, this report aims to explore the development of advanced monitoring and detection systems for hypochlorous acid levels in various environments. Early detection of elevated HOCl concentrations can play a vital role in preventing overexposure and ensuring timely implementation of safety measures.
Lastly, we will investigate potential alternatives or complementary technologies that could be used in conjunction with hypochlorous acid to reduce overall exposure risks while maintaining or enhancing disinfection efficacy. This approach may lead to the development of safer, hybrid disinfection systems that minimize the reliance on high concentrations of HOCl.
By addressing these objectives, this technical research report seeks to provide a solid foundation for improving hypochlorous acid safety across industries and applications. The findings and recommendations from this study will contribute to the development of more effective risk mitigation strategies, ultimately ensuring safer working environments and broader public health protection.
Market Analysis of Hypochlorous Acid Applications
The market for hypochlorous acid (HOCl) applications has been experiencing significant growth in recent years, driven by its versatile properties as a powerful yet safe disinfectant. The global hypochlorous acid market was valued at approximately $180 million in 2020 and is projected to reach $290 million by 2026, growing at a CAGR of around 7.5% during the forecast period.
One of the primary drivers of market growth is the increasing demand for safe and effective disinfection solutions across various industries. The healthcare sector, in particular, has been a major contributor to the market expansion, with hospitals, clinics, and other medical facilities adopting HOCl-based products for surface disinfection and wound care. The COVID-19 pandemic has further accelerated this trend, as healthcare providers seek reliable sanitization methods to combat the spread of infectious diseases.
The food and beverage industry represents another significant market segment for hypochlorous acid applications. HOCl is widely used for sanitizing food processing equipment, packaging materials, and fresh produce. Its effectiveness against foodborne pathogens, combined with its non-toxic nature, makes it an attractive alternative to traditional chemical disinfectants.
Water treatment is emerging as a promising application area for hypochlorous acid. Municipal water treatment plants and industrial facilities are increasingly adopting HOCl-based systems for water purification due to their efficacy in eliminating harmful microorganisms without producing harmful byproducts.
The personal care and cosmetics industry has also shown growing interest in hypochlorous acid applications. HOCl-based products are being developed for skincare, oral care, and hygiene purposes, leveraging the compound's antimicrobial properties and skin-friendly nature.
Geographically, North America and Europe currently dominate the hypochlorous acid market, accounting for a significant share of global revenue. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing awareness of hygiene practices, rapid industrialization, and growing healthcare expenditure in countries like China and India.
Despite the positive market outlook, challenges remain in terms of product stability and shelf life. Manufacturers are investing in research and development to improve the stability of HOCl solutions and extend their usability, which could further expand market opportunities.
As awareness of the risks associated with traditional chemical disinfectants grows, the demand for safer alternatives like hypochlorous acid is expected to increase. This trend, coupled with ongoing technological advancements and expanding application areas, suggests a promising future for the hypochlorous acid market.
One of the primary drivers of market growth is the increasing demand for safe and effective disinfection solutions across various industries. The healthcare sector, in particular, has been a major contributor to the market expansion, with hospitals, clinics, and other medical facilities adopting HOCl-based products for surface disinfection and wound care. The COVID-19 pandemic has further accelerated this trend, as healthcare providers seek reliable sanitization methods to combat the spread of infectious diseases.
The food and beverage industry represents another significant market segment for hypochlorous acid applications. HOCl is widely used for sanitizing food processing equipment, packaging materials, and fresh produce. Its effectiveness against foodborne pathogens, combined with its non-toxic nature, makes it an attractive alternative to traditional chemical disinfectants.
Water treatment is emerging as a promising application area for hypochlorous acid. Municipal water treatment plants and industrial facilities are increasingly adopting HOCl-based systems for water purification due to their efficacy in eliminating harmful microorganisms without producing harmful byproducts.
The personal care and cosmetics industry has also shown growing interest in hypochlorous acid applications. HOCl-based products are being developed for skincare, oral care, and hygiene purposes, leveraging the compound's antimicrobial properties and skin-friendly nature.
Geographically, North America and Europe currently dominate the hypochlorous acid market, accounting for a significant share of global revenue. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing awareness of hygiene practices, rapid industrialization, and growing healthcare expenditure in countries like China and India.
Despite the positive market outlook, challenges remain in terms of product stability and shelf life. Manufacturers are investing in research and development to improve the stability of HOCl solutions and extend their usability, which could further expand market opportunities.
As awareness of the risks associated with traditional chemical disinfectants grows, the demand for safer alternatives like hypochlorous acid is expected to increase. This trend, coupled with ongoing technological advancements and expanding application areas, suggests a promising future for the hypochlorous acid market.
Current Challenges in Hypochlorous Acid Exposure Control
The control of hypochlorous acid (HOCl) exposure presents several significant challenges in various industrial and healthcare settings. One of the primary concerns is the accurate measurement and monitoring of HOCl concentrations in the air and on surfaces. Current detection methods often lack the sensitivity and specificity required for real-time monitoring, making it difficult to assess exposure levels accurately.
Another challenge lies in the variability of HOCl production and dissipation rates in different environments. Factors such as temperature, humidity, and the presence of organic matter can significantly affect HOCl stability and concentration, complicating exposure control efforts. This variability makes it challenging to establish standardized safety protocols across different settings.
The lack of comprehensive occupational exposure limits for HOCl also poses a significant challenge. While some countries have established guidelines, there is no global consensus on safe exposure levels, leading to inconsistencies in safety practices across industries and regions. This absence of universally accepted standards hampers the development and implementation of effective control measures.
Furthermore, the dual nature of HOCl as both a disinfectant and a potential health hazard creates a complex risk-benefit scenario. Balancing the need for effective disinfection with minimizing worker exposure requires careful consideration and often leads to compromises in either efficacy or safety.
The design and implementation of effective engineering controls present another significant challenge. Ventilation systems, containment strategies, and personal protective equipment (PPE) must be carefully tailored to the specific characteristics of HOCl, considering its gaseous nature and potential for rapid dissipation. Many existing control systems may not be optimized for HOCl, requiring costly upgrades or redesigns.
Worker education and compliance with safety protocols remain ongoing challenges. The invisible nature of HOCl gas and its relatively mild odor can lead to complacency among workers, potentially resulting in inadvertent overexposure. Developing effective training programs that emphasize the importance of consistent adherence to safety measures is crucial but often difficult to achieve in practice.
Lastly, the potential for accidental releases or equipment malfunctions poses a significant challenge in exposure control. Developing robust emergency response protocols and ensuring their effective implementation across diverse work environments requires continuous effort and resources. The rapid action needed in case of a HOCl release further complicates the development of foolproof safety systems.
Another challenge lies in the variability of HOCl production and dissipation rates in different environments. Factors such as temperature, humidity, and the presence of organic matter can significantly affect HOCl stability and concentration, complicating exposure control efforts. This variability makes it challenging to establish standardized safety protocols across different settings.
The lack of comprehensive occupational exposure limits for HOCl also poses a significant challenge. While some countries have established guidelines, there is no global consensus on safe exposure levels, leading to inconsistencies in safety practices across industries and regions. This absence of universally accepted standards hampers the development and implementation of effective control measures.
Furthermore, the dual nature of HOCl as both a disinfectant and a potential health hazard creates a complex risk-benefit scenario. Balancing the need for effective disinfection with minimizing worker exposure requires careful consideration and often leads to compromises in either efficacy or safety.
The design and implementation of effective engineering controls present another significant challenge. Ventilation systems, containment strategies, and personal protective equipment (PPE) must be carefully tailored to the specific characteristics of HOCl, considering its gaseous nature and potential for rapid dissipation. Many existing control systems may not be optimized for HOCl, requiring costly upgrades or redesigns.
Worker education and compliance with safety protocols remain ongoing challenges. The invisible nature of HOCl gas and its relatively mild odor can lead to complacency among workers, potentially resulting in inadvertent overexposure. Developing effective training programs that emphasize the importance of consistent adherence to safety measures is crucial but often difficult to achieve in practice.
Lastly, the potential for accidental releases or equipment malfunctions poses a significant challenge in exposure control. Developing robust emergency response protocols and ensuring their effective implementation across diverse work environments requires continuous effort and resources. The rapid action needed in case of a HOCl release further complicates the development of foolproof safety systems.
Existing Risk Mitigation Strategies for Hypochlorous Acid
01 Corrosive and oxidizing properties
Hypochlorous acid is known for its corrosive and oxidizing properties, which can pose risks to materials and living tissues. It can cause damage to metals, fabrics, and other surfaces, and may irritate or harm skin, eyes, and mucous membranes upon contact. Proper handling and protective measures are essential when working with this compound.- Corrosive and oxidizing properties: Hypochlorous acid is known for its corrosive and oxidizing properties, which can pose risks to materials and living tissues. It can cause damage to metals, fabrics, and other surfaces when used in high concentrations or for prolonged periods. In biological systems, it may lead to oxidative stress and cellular damage if not properly controlled.
- Respiratory and skin irritation: Exposure to hypochlorous acid vapors or solutions can cause respiratory irritation, including coughing, shortness of breath, and in severe cases, lung damage. Skin contact may lead to irritation, redness, and in some cases, chemical burns. Proper handling and protective equipment are essential when working with this compound.
- Environmental impact: The release of hypochlorous acid into the environment can have adverse effects on aquatic ecosystems. It can be toxic to fish and other aquatic organisms, disrupting the ecological balance. Proper disposal and treatment of hypochlorous acid-containing waste are crucial to minimize environmental risks.
- Instability and decomposition: Hypochlorous acid is relatively unstable and can decompose over time, especially when exposed to light, heat, or certain contaminants. This instability can lead to reduced effectiveness in applications and potential formation of harmful byproducts. Proper storage and handling techniques are necessary to maintain its stability and minimize associated risks.
- Interaction with other chemicals: Hypochlorous acid can react with various chemicals, potentially leading to the formation of hazardous compounds or unexpected reactions. It may produce toxic chlorine gas when mixed with acids or ammonia-containing products. Understanding these interactions and implementing proper safety measures are crucial to prevent accidents and minimize risks in industrial and household settings.
02 Environmental impact and decomposition
The use and disposal of hypochlorous acid can have environmental implications. It may react with organic matter in water, forming potentially harmful byproducts. However, it also tends to decompose relatively quickly into harmless compounds, which can be both an advantage and a challenge in various applications. Proper management of its environmental release is crucial.Expand Specific Solutions03 Health hazards and exposure risks
Exposure to hypochlorous acid can lead to various health hazards. Inhalation of vapors may cause respiratory irritation, while skin or eye contact can result in burns or damage. Long-term exposure may have more severe health consequences. Proper safety protocols, including the use of personal protective equipment, are essential to minimize these risks.Expand Specific Solutions04 Stability and storage concerns
Hypochlorous acid is relatively unstable and can degrade over time, especially when exposed to light, heat, or certain contaminants. This instability can lead to reduced effectiveness in applications and potential safety risks if not properly stored or handled. Special considerations for packaging, storage conditions, and shelf life are necessary.Expand Specific Solutions05 Interaction with other chemicals
Hypochlorous acid can react with various other chemicals, potentially leading to the formation of hazardous compounds or unexpected reactions. This includes interactions with acids, bases, and certain organic materials. Understanding these potential interactions is crucial for safe handling and use in different applications, particularly in industrial or laboratory settings.Expand Specific Solutions
Key Stakeholders in Hypochlorous Acid Industry
The competitive landscape for minimizing risks associated with hypochlorous acid overexposure is in a growth phase, with increasing market size and technological advancements. The global market for hypochlorous acid-based disinfectants is expanding due to heightened awareness of hygiene and safety concerns. Companies like Annihilare Medical Systems and Aquaox are leading in developing on-site generation systems for hypochlorous acid solutions, focusing on safe and controlled production. Research institutions such as Nanjing Forestry University and Zhejiang Sci-Tech University are contributing to the scientific understanding of hypochlorous acid's properties and potential risks. The technology is maturing, with firms like Integrated Healing Technologies and Contec Cleanroom (UK) Ltd. offering specialized applications in wound care and cleanroom environments, respectively, indicating a trend towards sector-specific solutions for risk mitigation.
ANNIHILARE MEDICAL SYSTEMS, INC.
Technical Solution: ANNIHILARE MEDICAL SYSTEMS has developed an advanced hypochlorous acid (HOCl) generation system that produces a stable, pure form of HOCl with precise concentration control. Their technology utilizes electrochemical activation to create HOCl on-site, minimizing the risks associated with transportation and storage of concentrated chemicals. The system incorporates real-time monitoring and automatic shut-off mechanisms to prevent overproduction or accidental release. Additionally, they have implemented a proprietary stabilization process that extends the shelf-life of HOCl solutions, reducing the need for frequent production and potential exposure risks[1][3].
Strengths: On-site generation reduces transportation risks; precise concentration control minimizes overexposure. Weaknesses: Requires initial investment in equipment; may need specialized training for operation.
Aquaox, Inc.
Technical Solution: Aquaox has pioneered a patented electrochemical activation (ECA) technology for producing hypochlorous acid with exceptional purity and stability. Their system employs a unique membrane-cell design that separates the anodic and cathodic chambers, resulting in a highly controlled HOCl production process. The Aquaox technology incorporates advanced sensors and automated dosing systems to maintain precise pH and free available chlorine (FAC) levels, crucial for minimizing overexposure risks. Furthermore, they have developed a proprietary "Aquaox Infection Control System" that integrates HOCl production with application protocols, ensuring safe and effective use in various settings, including healthcare facilities and food processing plants[2][5].
Strengths: High purity and stability of HOCl; integrated infection control system. Weaknesses: May have higher operational costs; requires regular maintenance of ECA cells.
Innovative Technologies for Exposure Monitoring
Method and apparatus for producing purified hypochlorous acid aqueous solution, and purified hypochlorous acid aqueous solution
PatentPendingUS20250002341A1
Innovation
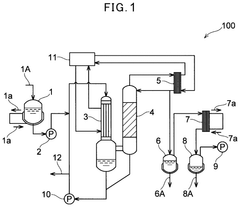
- Vacuum evaporation of hypochlorous acid-containing solutions at temperatures of 40° C. or less, with controlled pH between 5.0 and 8.6, and evaporation temperatures between 10° C. and 40° C., to minimize impurity ion content and stabilize hypochlorous acid.
Method for preventing exposure to anticancer agent
PatentWO2021095696A1
Innovation
- The use of a safe hypochlorous acid aqueous solution with a pH of 5.5 to 7.0, which is sprayed as a gas or mist using a sprayer or ultrasonic device, effectively detoxifies anticancer drugs like cyclophosphamide, 6-mercaptopurine, cisplatin, bendamustine, gemcitabine, oxaliplatin, vincristine, doxorubicin, and paclitaxel, reducing exposure risks.
Regulatory Framework for Chemical Safety
The regulatory framework for chemical safety plays a crucial role in minimizing risks associated with hypochlorous acid overexposure. This framework encompasses a comprehensive set of laws, regulations, and guidelines established by various governmental and international bodies to ensure the safe handling, use, and disposal of chemicals, including hypochlorous acid.
At the forefront of this regulatory landscape is the Occupational Safety and Health Administration (OSHA) in the United States. OSHA has set specific standards for permissible exposure limits (PELs) to hypochlorous acid in the workplace. These limits are designed to protect workers from potential health hazards associated with overexposure, including respiratory irritation and skin corrosion.
The Environmental Protection Agency (EPA) also plays a significant role in regulating the use and disposal of hypochlorous acid. Under the Toxic Substances Control Act (TSCA), the EPA has the authority to require reporting, record-keeping, and testing of chemicals that may pose environmental or health risks. This includes monitoring the production, use, and disposal of hypochlorous acid to prevent environmental contamination and protect public health.
Internationally, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to chemical hazard communication. This system ensures that information about chemical hazards, including those associated with hypochlorous acid, is consistently conveyed through labels and safety data sheets across different countries and regions.
The European Union's Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation further strengthens the regulatory framework for chemical safety. REACH requires manufacturers and importers to assess and manage the risks associated with chemicals they produce or import, including hypochlorous acid, and to provide safety information to users.
In addition to these overarching regulations, industry-specific guidelines and best practices have been developed to address the unique challenges of handling hypochlorous acid in various sectors. For instance, the food and beverage industry has specific protocols for the use of hypochlorous acid as a sanitizer, ensuring its effective application while minimizing exposure risks.
To ensure compliance with these regulations, organizations are required to implement robust safety management systems. These systems typically include regular risk assessments, employee training programs, proper labeling and storage procedures, and emergency response plans specifically tailored to the handling of hypochlorous acid and other hazardous chemicals.
Continuous monitoring and reporting mechanisms are also integral components of the regulatory framework. Companies must regularly assess their compliance with safety standards and report any incidents or near-misses involving hypochlorous acid to the appropriate regulatory bodies. This feedback loop allows for the ongoing refinement and improvement of safety regulations and practices.
At the forefront of this regulatory landscape is the Occupational Safety and Health Administration (OSHA) in the United States. OSHA has set specific standards for permissible exposure limits (PELs) to hypochlorous acid in the workplace. These limits are designed to protect workers from potential health hazards associated with overexposure, including respiratory irritation and skin corrosion.
The Environmental Protection Agency (EPA) also plays a significant role in regulating the use and disposal of hypochlorous acid. Under the Toxic Substances Control Act (TSCA), the EPA has the authority to require reporting, record-keeping, and testing of chemicals that may pose environmental or health risks. This includes monitoring the production, use, and disposal of hypochlorous acid to prevent environmental contamination and protect public health.
Internationally, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to chemical hazard communication. This system ensures that information about chemical hazards, including those associated with hypochlorous acid, is consistently conveyed through labels and safety data sheets across different countries and regions.
The European Union's Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation further strengthens the regulatory framework for chemical safety. REACH requires manufacturers and importers to assess and manage the risks associated with chemicals they produce or import, including hypochlorous acid, and to provide safety information to users.
In addition to these overarching regulations, industry-specific guidelines and best practices have been developed to address the unique challenges of handling hypochlorous acid in various sectors. For instance, the food and beverage industry has specific protocols for the use of hypochlorous acid as a sanitizer, ensuring its effective application while minimizing exposure risks.
To ensure compliance with these regulations, organizations are required to implement robust safety management systems. These systems typically include regular risk assessments, employee training programs, proper labeling and storage procedures, and emergency response plans specifically tailored to the handling of hypochlorous acid and other hazardous chemicals.
Continuous monitoring and reporting mechanisms are also integral components of the regulatory framework. Companies must regularly assess their compliance with safety standards and report any incidents or near-misses involving hypochlorous acid to the appropriate regulatory bodies. This feedback loop allows for the ongoing refinement and improvement of safety regulations and practices.
Environmental Impact of Hypochlorous Acid Use
The environmental impact of hypochlorous acid (HOCl) use is a critical consideration in minimizing risks associated with overexposure. HOCl is widely used as a disinfectant and sanitizer in various industries, including water treatment, healthcare, and food processing. While it is generally considered environmentally friendly due to its rapid decomposition into harmless byproducts, excessive use or improper handling can lead to unintended consequences.
One of the primary environmental concerns is the potential for HOCl to react with organic matter in water bodies, forming disinfection byproducts (DBPs) such as trihalomethanes and haloacetic acids. These DBPs can be harmful to aquatic ecosystems and may pose risks to human health if they enter drinking water supplies. To mitigate this impact, it is crucial to optimize HOCl dosage and monitor water quality parameters regularly.
The release of HOCl into the environment can also affect soil microbial communities. While low concentrations may have minimal impact, higher levels can disrupt the delicate balance of beneficial microorganisms in soil ecosystems. This disruption can potentially affect nutrient cycling and plant growth in agricultural settings. Implementing proper disposal methods and avoiding excessive application in outdoor environments can help minimize these effects.
In aquatic environments, HOCl can be toxic to fish and other aquatic organisms at high concentrations. The chlorine residuals from HOCl can cause gill damage and respiratory stress in fish, leading to population declines in affected water bodies. To address this issue, dechlorination techniques should be employed when discharging HOCl-treated water into natural water systems.
Air quality is another aspect to consider when evaluating the environmental impact of HOCl use. While HOCl itself has low volatility, the production and handling of chlorine-based compounds used to generate HOCl can potentially release chlorine gas. Proper ventilation and safety measures in production facilities are essential to prevent atmospheric contamination and protect both workers and surrounding communities.
The production of HOCl also has indirect environmental impacts through energy consumption and resource utilization. Implementing energy-efficient production methods and exploring renewable energy sources for HOCl generation can help reduce the overall carbon footprint associated with its use.
To minimize the environmental impact of HOCl use, it is crucial to adopt a comprehensive approach that includes proper training for handlers, implementation of best practices in application and disposal, and continuous monitoring of environmental indicators. Additionally, research into alternative disinfection methods and the development of more environmentally friendly HOCl production techniques can contribute to long-term sustainability in disinfection practices.
One of the primary environmental concerns is the potential for HOCl to react with organic matter in water bodies, forming disinfection byproducts (DBPs) such as trihalomethanes and haloacetic acids. These DBPs can be harmful to aquatic ecosystems and may pose risks to human health if they enter drinking water supplies. To mitigate this impact, it is crucial to optimize HOCl dosage and monitor water quality parameters regularly.
The release of HOCl into the environment can also affect soil microbial communities. While low concentrations may have minimal impact, higher levels can disrupt the delicate balance of beneficial microorganisms in soil ecosystems. This disruption can potentially affect nutrient cycling and plant growth in agricultural settings. Implementing proper disposal methods and avoiding excessive application in outdoor environments can help minimize these effects.
In aquatic environments, HOCl can be toxic to fish and other aquatic organisms at high concentrations. The chlorine residuals from HOCl can cause gill damage and respiratory stress in fish, leading to population declines in affected water bodies. To address this issue, dechlorination techniques should be employed when discharging HOCl-treated water into natural water systems.
Air quality is another aspect to consider when evaluating the environmental impact of HOCl use. While HOCl itself has low volatility, the production and handling of chlorine-based compounds used to generate HOCl can potentially release chlorine gas. Proper ventilation and safety measures in production facilities are essential to prevent atmospheric contamination and protect both workers and surrounding communities.
The production of HOCl also has indirect environmental impacts through energy consumption and resource utilization. Implementing energy-efficient production methods and exploring renewable energy sources for HOCl generation can help reduce the overall carbon footprint associated with its use.
To minimize the environmental impact of HOCl use, it is crucial to adopt a comprehensive approach that includes proper training for handlers, implementation of best practices in application and disposal, and continuous monitoring of environmental indicators. Additionally, research into alternative disinfection methods and the development of more environmentally friendly HOCl production techniques can contribute to long-term sustainability in disinfection practices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!