How to Teach Thermite Chemistry in Academia?
JUN 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Thermite Chemistry Background and Objectives
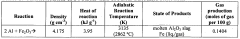
Thermite chemistry, a branch of inorganic chemistry and materials science, has a rich history dating back to the late 19th century. The thermite reaction, first discovered by German chemist Hans Goldschmidt in 1893, involves the reduction of a metal oxide by a more reactive metal, typically aluminum. This exothermic reaction produces intense heat and molten metal, making it a subject of significant interest in both academic and industrial settings.
The evolution of thermite chemistry has been marked by continuous advancements in understanding reaction mechanisms, developing new compositions, and expanding applications. From its initial use in welding railroad tracks, thermite reactions have found diverse applications in metallurgy, pyrotechnics, and even space exploration. The field has seen a surge in research interest over the past few decades, driven by the need for more efficient and controllable reactions.
In academia, teaching thermite chemistry presents unique opportunities and challenges. The primary objective is to provide students with a comprehensive understanding of the fundamental principles governing thermite reactions, including thermodynamics, kinetics, and materials science. This knowledge forms the foundation for exploring more advanced concepts and applications in the field.
Another crucial goal is to develop students' practical skills in handling and synthesizing thermite mixtures safely. Given the potentially hazardous nature of these reactions, instilling a strong sense of laboratory safety and proper experimental procedures is paramount. This hands-on experience is essential for bridging the gap between theoretical knowledge and real-world applications.
Furthermore, the teaching of thermite chemistry aims to foster critical thinking and problem-solving skills among students. By engaging with complex reaction systems, students learn to analyze variables, predict outcomes, and design experiments to test hypotheses. This approach not only enhances their understanding of thermite chemistry but also develops transferable skills applicable to broader scientific research.
An additional objective is to contextualize thermite chemistry within the larger framework of materials science and engineering. Students should gain insights into how thermite reactions contribute to the development of advanced materials, energy storage systems, and novel manufacturing processes. This perspective helps in appreciating the interdisciplinary nature of the field and its relevance to contemporary technological challenges.
Lastly, the teaching of thermite chemistry in academia strives to inspire innovation and creativity. By exposing students to cutting-edge research and emerging applications, educators aim to stimulate curiosity and encourage the exploration of new ideas. This forward-looking approach is crucial for preparing the next generation of scientists and engineers to tackle future challenges in the field of thermite chemistry and beyond.
The evolution of thermite chemistry has been marked by continuous advancements in understanding reaction mechanisms, developing new compositions, and expanding applications. From its initial use in welding railroad tracks, thermite reactions have found diverse applications in metallurgy, pyrotechnics, and even space exploration. The field has seen a surge in research interest over the past few decades, driven by the need for more efficient and controllable reactions.
In academia, teaching thermite chemistry presents unique opportunities and challenges. The primary objective is to provide students with a comprehensive understanding of the fundamental principles governing thermite reactions, including thermodynamics, kinetics, and materials science. This knowledge forms the foundation for exploring more advanced concepts and applications in the field.
Another crucial goal is to develop students' practical skills in handling and synthesizing thermite mixtures safely. Given the potentially hazardous nature of these reactions, instilling a strong sense of laboratory safety and proper experimental procedures is paramount. This hands-on experience is essential for bridging the gap between theoretical knowledge and real-world applications.
Furthermore, the teaching of thermite chemistry aims to foster critical thinking and problem-solving skills among students. By engaging with complex reaction systems, students learn to analyze variables, predict outcomes, and design experiments to test hypotheses. This approach not only enhances their understanding of thermite chemistry but also develops transferable skills applicable to broader scientific research.
An additional objective is to contextualize thermite chemistry within the larger framework of materials science and engineering. Students should gain insights into how thermite reactions contribute to the development of advanced materials, energy storage systems, and novel manufacturing processes. This perspective helps in appreciating the interdisciplinary nature of the field and its relevance to contemporary technological challenges.
Lastly, the teaching of thermite chemistry in academia strives to inspire innovation and creativity. By exposing students to cutting-edge research and emerging applications, educators aim to stimulate curiosity and encourage the exploration of new ideas. This forward-looking approach is crucial for preparing the next generation of scientists and engineers to tackle future challenges in the field of thermite chemistry and beyond.
Educational Demand Analysis
The demand for teaching thermite chemistry in academia has been steadily increasing due to its significant applications in various fields, including materials science, engineering, and pyrotechnics. Universities and research institutions are recognizing the importance of providing comprehensive education on this topic to prepare students for future careers in related industries.
The market for thermite chemistry education is primarily driven by the growing need for skilled professionals in industries such as metallurgy, welding, and advanced materials manufacturing. As these sectors continue to expand, there is a corresponding increase in the demand for graduates with a strong foundation in thermite reactions and their practical applications.
Educational institutions are facing challenges in meeting this demand due to the specialized nature of thermite chemistry and the need for appropriate safety measures in laboratory settings. This has led to a surge in the development of innovative teaching methods, including virtual simulations and remote learning platforms, to supplement traditional hands-on experiments.
The trend towards interdisciplinary education has also contributed to the rising interest in thermite chemistry. Many universities are integrating this topic into broader curricula, such as materials engineering, chemical engineering, and even forensic science programs. This approach not only enhances the relevance of thermite chemistry education but also expands its reach to a wider student population.
Industry partnerships play a crucial role in shaping the educational landscape for thermite chemistry. Collaborations between academic institutions and companies specializing in thermite applications have resulted in the development of more practical and industry-aligned course content. These partnerships often provide students with access to state-of-the-art equipment and real-world problem-solving opportunities.
The global emphasis on sustainable technologies has also influenced the demand for thermite chemistry education. Research into environmentally friendly thermite compositions and their applications in green energy solutions has created new avenues for academic exploration and student engagement.
As the field of nanotechnology advances, there is a growing interest in nano-thermite materials, which has further fueled the demand for specialized education in this area. Universities are increasingly incorporating nano-thermite concepts into their advanced chemistry and materials science courses to keep pace with this emerging field.
The safety aspects of handling and studying thermite reactions present both a challenge and an opportunity in academic settings. There is a significant demand for comprehensive safety training programs and protocols specific to thermite chemistry, which has led to the development of specialized courses and certifications in laboratory safety management.
The market for thermite chemistry education is primarily driven by the growing need for skilled professionals in industries such as metallurgy, welding, and advanced materials manufacturing. As these sectors continue to expand, there is a corresponding increase in the demand for graduates with a strong foundation in thermite reactions and their practical applications.
Educational institutions are facing challenges in meeting this demand due to the specialized nature of thermite chemistry and the need for appropriate safety measures in laboratory settings. This has led to a surge in the development of innovative teaching methods, including virtual simulations and remote learning platforms, to supplement traditional hands-on experiments.
The trend towards interdisciplinary education has also contributed to the rising interest in thermite chemistry. Many universities are integrating this topic into broader curricula, such as materials engineering, chemical engineering, and even forensic science programs. This approach not only enhances the relevance of thermite chemistry education but also expands its reach to a wider student population.
Industry partnerships play a crucial role in shaping the educational landscape for thermite chemistry. Collaborations between academic institutions and companies specializing in thermite applications have resulted in the development of more practical and industry-aligned course content. These partnerships often provide students with access to state-of-the-art equipment and real-world problem-solving opportunities.
The global emphasis on sustainable technologies has also influenced the demand for thermite chemistry education. Research into environmentally friendly thermite compositions and their applications in green energy solutions has created new avenues for academic exploration and student engagement.
As the field of nanotechnology advances, there is a growing interest in nano-thermite materials, which has further fueled the demand for specialized education in this area. Universities are increasingly incorporating nano-thermite concepts into their advanced chemistry and materials science courses to keep pace with this emerging field.
The safety aspects of handling and studying thermite reactions present both a challenge and an opportunity in academic settings. There is a significant demand for comprehensive safety training programs and protocols specific to thermite chemistry, which has led to the development of specialized courses and certifications in laboratory safety management.
Current Challenges in Thermite Education
Teaching thermite chemistry in academia presents several significant challenges that educators and institutions must address to ensure effective and safe learning experiences. One of the primary concerns is the inherent safety risks associated with thermite reactions. These reactions are highly exothermic and can produce temperatures exceeding 2500°C, posing potential fire hazards and risks of severe burns. Consequently, many educational institutions are hesitant to include hands-on thermite experiments in their curricula, limiting students' practical exposure to this important area of chemistry.
Another challenge lies in the regulatory and legal constraints surrounding the use of thermite materials. Many components used in thermite reactions, such as aluminum powder and certain metal oxides, are subject to strict regulations due to their potential for misuse. This often results in limited access to necessary materials for educational purposes, forcing instructors to rely heavily on theoretical teaching methods or simulations, which may not fully capture the essence of thermite chemistry.
The complexity of thermite reactions also presents a pedagogical challenge. These reactions involve multiple chemical processes occurring simultaneously, including redox reactions, heat transfer, and phase changes. Explaining these concepts in a way that is both comprehensive and accessible to students at various academic levels can be difficult. This complexity is further compounded by the rapid nature of thermite reactions, making it challenging to observe and analyze the reaction stages in real-time.
Resource limitations pose another significant hurdle in thermite education. Conducting thermite experiments, even on a small scale, requires specialized equipment and facilities that many educational institutions may not possess. This includes proper ventilation systems, fire suppression equipment, and personal protective gear. The cost associated with acquiring and maintaining these resources can be prohibitive for many schools and universities, particularly those with limited budgets.
Furthermore, there is a notable gap in standardized curriculum materials and teaching methodologies specific to thermite chemistry. Unlike more common chemical reactions, thermite reactions are often not covered extensively in standard textbooks or laboratory manuals. This lack of readily available, comprehensive teaching resources places an additional burden on instructors to develop their own materials, which can be time-consuming and may lead to inconsistencies in the quality and depth of education across different institutions.
Lastly, the interdisciplinary nature of thermite chemistry presents both an opportunity and a challenge. While it offers a unique platform to integrate concepts from chemistry, physics, and materials science, it also requires instructors to have a broad knowledge base. Ensuring that educators are adequately prepared to teach across these disciplines and can effectively communicate the interconnections to students is a significant challenge in current thermite education.
Another challenge lies in the regulatory and legal constraints surrounding the use of thermite materials. Many components used in thermite reactions, such as aluminum powder and certain metal oxides, are subject to strict regulations due to their potential for misuse. This often results in limited access to necessary materials for educational purposes, forcing instructors to rely heavily on theoretical teaching methods or simulations, which may not fully capture the essence of thermite chemistry.
The complexity of thermite reactions also presents a pedagogical challenge. These reactions involve multiple chemical processes occurring simultaneously, including redox reactions, heat transfer, and phase changes. Explaining these concepts in a way that is both comprehensive and accessible to students at various academic levels can be difficult. This complexity is further compounded by the rapid nature of thermite reactions, making it challenging to observe and analyze the reaction stages in real-time.
Resource limitations pose another significant hurdle in thermite education. Conducting thermite experiments, even on a small scale, requires specialized equipment and facilities that many educational institutions may not possess. This includes proper ventilation systems, fire suppression equipment, and personal protective gear. The cost associated with acquiring and maintaining these resources can be prohibitive for many schools and universities, particularly those with limited budgets.
Furthermore, there is a notable gap in standardized curriculum materials and teaching methodologies specific to thermite chemistry. Unlike more common chemical reactions, thermite reactions are often not covered extensively in standard textbooks or laboratory manuals. This lack of readily available, comprehensive teaching resources places an additional burden on instructors to develop their own materials, which can be time-consuming and may lead to inconsistencies in the quality and depth of education across different institutions.
Lastly, the interdisciplinary nature of thermite chemistry presents both an opportunity and a challenge. While it offers a unique platform to integrate concepts from chemistry, physics, and materials science, it also requires instructors to have a broad knowledge base. Ensuring that educators are adequately prepared to teach across these disciplines and can effectively communicate the interconnections to students is a significant challenge in current thermite education.
Existing Thermite Teaching Approaches
01 Thermite composition and reactions
Thermite reactions involve the reduction of metal oxides by aluminum, producing intense heat and molten metal. The composition typically includes aluminum powder and metal oxides like iron oxide. These reactions are used in various applications due to their exothermic nature and ability to produce high temperatures.- Thermite composition and reactions: Thermite reactions involve the reduction of metal oxides by aluminum, producing intense heat and molten metal. The composition typically includes aluminum powder and metal oxides like iron oxide. These reactions are used in various applications due to their exothermic nature and ability to produce high temperatures.
- Applications in welding and metal joining: Thermite reactions are utilized in welding and metal joining processes, particularly for large structures or in-situ repairs. The heat generated by the reaction can melt and fuse metal components together, making it useful in industries such as railway track welding and pipeline repair.
- Ignition systems for thermite reactions: Various ignition systems have been developed to initiate thermite reactions safely and efficiently. These systems may include electric igniters, chemical initiators, or specialized devices designed to withstand the high temperatures produced during the reaction.
- Thermite use in military and defense applications: Thermite reactions are employed in military and defense applications, such as incendiary devices, breaching charges, and ordnance disposal. The high heat and metal-cutting capabilities of thermite make it useful for penetrating armored targets or destroying sensitive equipment.
- Safety and control measures in thermite chemistry: Given the intense heat and potential hazards associated with thermite reactions, various safety and control measures have been developed. These include specialized containment vessels, reaction moderators, and precise mixing ratios to ensure controlled and safe use of thermite in industrial and research settings.
02 Applications in welding and metal joining
Thermite reactions are utilized in welding and metal joining processes, particularly for large structures or in-situ repairs. The heat generated by the reaction can melt and fuse metal components together, making it useful in industries such as railway track welding and pipeline repair.Expand Specific Solutions03 Ignition systems for thermite reactions
Various ignition systems have been developed to initiate thermite reactions safely and efficiently. These can include electric igniters, chemical initiators, or specialized devices designed to withstand the high temperatures produced during the reaction.Expand Specific Solutions04 Thermite use in military and defense applications
Thermite chemistry finds applications in military and defense technologies, including incendiary devices, armor-piercing munitions, and specialized demolition tools. The high heat and metal-cutting capabilities of thermite reactions make them valuable in these contexts.Expand Specific Solutions05 Safety and control measures in thermite handling
Given the intense heat and potential hazards associated with thermite reactions, various safety and control measures have been developed. These include specialized containment vessels, reaction moderators, and handling procedures to ensure safe use of thermite in industrial and research settings.Expand Specific Solutions
Key Institutions in Thermite Education
The field of thermite chemistry in academia is in a mature stage, with established research and teaching methodologies. The market size for educational materials and equipment in this area is relatively stable, primarily driven by academic institutions and research laboratories. Technologically, the field is well-developed, with companies like SGL Carbon SE, Arconic, Inc., and Elkem ASA providing advanced materials for thermite reactions. Lockheed Martin Corp. and Naval Research Laboratory contribute to cutting-edge applications, while universities such as Northeastern University and Shanghai Jiao Tong University focus on fundamental research and educational aspects. The competitive landscape is characterized by a mix of material suppliers, defense contractors, and academic institutions, each contributing to different aspects of thermite chemistry education and research.
Naval Research Laboratory
Technical Solution: The Naval Research Laboratory (NRL) has developed advanced methods for teaching thermite chemistry in academia. Their approach involves a combination of theoretical lectures and practical laboratory experiments. The theoretical component covers the fundamental principles of thermite reactions, including the role of metal oxides and reducing agents. In the practical sessions, students are guided through carefully controlled experiments demonstrating various thermite compositions and their reactions. NRL's method emphasizes safety protocols and proper handling of reactive materials. They have also incorporated computer simulations and high-speed video analysis to enhance understanding of the rapid exothermic reactions characteristic of thermites.
Strengths: Comprehensive approach combining theory and practice; emphasis on safety; use of advanced technology for visualization. Weaknesses: May require specialized equipment not available in all academic settings; potential safety concerns with hands-on experiments.
The Curators of the University of Missouri
Technical Solution: The University of Missouri has developed a multidisciplinary approach to teaching thermite chemistry. Their method integrates aspects of materials science, chemical engineering, and safety engineering. The curriculum includes in-depth study of the thermodynamics and kinetics of thermite reactions, as well as their applications in various industries. Students engage in project-based learning, designing and analyzing thermite compositions for specific applications. The university has also implemented virtual reality simulations to allow students to explore dangerous reactions safely. Additionally, they have partnerships with industry leaders to provide real-world context and potential internship opportunities for students studying thermite chemistry.
Strengths: Interdisciplinary approach; use of cutting-edge technology; industry partnerships. Weaknesses: May require significant resources to implement fully; could be too specialized for some general chemistry courses.
Core Thermite Reaction Principles
Solid-state thermite composition based heating device
PatentWO2010117857A2
Innovation
- A solid-state thermite reaction composition comprising a fuel component, primary oxidizer, initiating oxidizers, thermal diluent, and fluxing agents, integrated with a heating device featuring a reaction chamber and actuable trigger mechanism, allowing controlled thermite reactions for precise heat generation, with activation mechanisms like piezoelectric spark ignitors or exothermic couples to initiate the reaction safely.
Pyrotechnic thermite composition
PatentInactiveUS7632365B1
Innovation
- A thermite formulation comprising a magnesium-aluminum alloy as fuel, copper oxide (CuO) and molybdenum oxide (MoO3) as oxidizers, with a binder material, optimized to provide excellent material perforation with low toxicity starting and reaction products, specifically using a ratio of about 39.8% CuO, 33% MoO3, 24.2% magnalium, and 3% binder by weight.
Safety Protocols for Thermite Experiments
Safety protocols for thermite experiments in academic settings are paramount to ensure the well-being of students, instructors, and laboratory personnel. These protocols encompass a comprehensive set of guidelines and procedures designed to mitigate risks associated with the highly exothermic thermite reaction.
Proper personal protective equipment (PPE) is the first line of defense. All participants must wear flame-resistant lab coats, safety goggles, closed-toe shoes, and heat-resistant gloves. Long hair should be tied back, and loose clothing avoided. A face shield is recommended for those directly handling the thermite mixture or observing at close range.
The experimental setup requires careful consideration. Thermite reactions should be conducted in a designated area with a non-flammable surface, such as a sand bath or a fire-resistant ceramic dish. The surrounding area must be clear of combustible materials, and a fire extinguisher suitable for metal fires (Class D) should be readily accessible.
Preparation of the thermite mixture demands strict adherence to safety measures. Components should be stored separately and mixed only in the quantities required for the experiment. Mixing should occur in a controlled environment, preferably under a fume hood, to minimize the risk of accidental ignition and contain any potential dust.
Ignition procedures must be meticulously planned and executed. Remote ignition methods, such as using a magnesium ribbon or an electronic igniter, are preferred to maintain a safe distance. Students and observers should be positioned at a predetermined safe distance, with clear evacuation routes established.
Proper ventilation is crucial to manage the intense heat and fumes produced during the reaction. Experiments should be conducted outdoors or in a well-ventilated area with appropriate fume extraction systems in place. Air quality monitoring may be necessary for indoor setups.
Post-reaction safety measures are equally important. The reaction products remain extremely hot for an extended period and should not be approached or handled until completely cooled. Proper disposal methods for reaction products and any unused materials must be followed, adhering to institutional and local regulations.
Emergency response procedures should be clearly communicated to all participants before the experiment. This includes the location of emergency exits, eyewash stations, and safety showers. A detailed protocol for handling potential accidents, such as burns or fires, should be established and rehearsed.
Lastly, thorough documentation and record-keeping of safety procedures, incident reports, and near-misses are essential for continuous improvement of safety protocols. Regular safety audits and updates to procedures based on new information or experiences will help maintain a robust safety culture in thermite chemistry education.
Proper personal protective equipment (PPE) is the first line of defense. All participants must wear flame-resistant lab coats, safety goggles, closed-toe shoes, and heat-resistant gloves. Long hair should be tied back, and loose clothing avoided. A face shield is recommended for those directly handling the thermite mixture or observing at close range.
The experimental setup requires careful consideration. Thermite reactions should be conducted in a designated area with a non-flammable surface, such as a sand bath or a fire-resistant ceramic dish. The surrounding area must be clear of combustible materials, and a fire extinguisher suitable for metal fires (Class D) should be readily accessible.
Preparation of the thermite mixture demands strict adherence to safety measures. Components should be stored separately and mixed only in the quantities required for the experiment. Mixing should occur in a controlled environment, preferably under a fume hood, to minimize the risk of accidental ignition and contain any potential dust.
Ignition procedures must be meticulously planned and executed. Remote ignition methods, such as using a magnesium ribbon or an electronic igniter, are preferred to maintain a safe distance. Students and observers should be positioned at a predetermined safe distance, with clear evacuation routes established.
Proper ventilation is crucial to manage the intense heat and fumes produced during the reaction. Experiments should be conducted outdoors or in a well-ventilated area with appropriate fume extraction systems in place. Air quality monitoring may be necessary for indoor setups.
Post-reaction safety measures are equally important. The reaction products remain extremely hot for an extended period and should not be approached or handled until completely cooled. Proper disposal methods for reaction products and any unused materials must be followed, adhering to institutional and local regulations.
Emergency response procedures should be clearly communicated to all participants before the experiment. This includes the location of emergency exits, eyewash stations, and safety showers. A detailed protocol for handling potential accidents, such as burns or fires, should be established and rehearsed.
Lastly, thorough documentation and record-keeping of safety procedures, incident reports, and near-misses are essential for continuous improvement of safety protocols. Regular safety audits and updates to procedures based on new information or experiences will help maintain a robust safety culture in thermite chemistry education.
Ethical Considerations in Thermite Education
Teaching thermite chemistry in academia requires careful consideration of ethical implications to ensure responsible education and research practices. The potential dual-use nature of thermite reactions necessitates a balanced approach that promotes scientific understanding while mitigating risks associated with misuse.
Educators must prioritize safety protocols and emphasize the importance of responsible handling of reactive materials. This includes implementing strict laboratory procedures, providing appropriate personal protective equipment, and ensuring proper waste disposal methods. Clear guidelines should be established for the quantities of materials used in experiments, with a focus on using minimal amounts necessary for educational purposes.
Curriculum design should incorporate discussions on the ethical use of chemical knowledge and the potential societal impacts of thermite reactions. Students should be encouraged to critically evaluate the applications of thermite chemistry in both beneficial and potentially harmful contexts. This approach fosters a sense of scientific responsibility and helps students develop ethical decision-making skills.
Institutions should implement rigorous screening processes for students and researchers involved in thermite-related projects. Background checks and ongoing monitoring may be necessary to prevent unauthorized access to sensitive information or materials. Additionally, collaborations with relevant authorities and industry partners can help ensure that educational programs align with current safety standards and regulations.
Transparency in research objectives and methodologies is crucial when working with thermite reactions. Institutions should establish clear guidelines for publishing and sharing research findings, balancing the need for academic freedom with potential security concerns. This may involve implementing review processes for publications and presentations related to thermite chemistry.
Educators should also address the ethical considerations surrounding the potential environmental impacts of thermite reactions. Discussions on sustainable practices and the responsible use of resources in chemical processes can help students develop a holistic understanding of the broader implications of their work.
Integrating case studies that highlight both the beneficial applications and potential misuse of thermite chemistry can provide valuable context for students. This approach allows for in-depth discussions on ethical dilemmas and encourages students to consider the long-term consequences of their research and future professional activities.
By addressing these ethical considerations, academic institutions can ensure that the teaching of thermite chemistry contributes positively to scientific advancement while promoting responsible practices and minimizing potential risks to society.
Educators must prioritize safety protocols and emphasize the importance of responsible handling of reactive materials. This includes implementing strict laboratory procedures, providing appropriate personal protective equipment, and ensuring proper waste disposal methods. Clear guidelines should be established for the quantities of materials used in experiments, with a focus on using minimal amounts necessary for educational purposes.
Curriculum design should incorporate discussions on the ethical use of chemical knowledge and the potential societal impacts of thermite reactions. Students should be encouraged to critically evaluate the applications of thermite chemistry in both beneficial and potentially harmful contexts. This approach fosters a sense of scientific responsibility and helps students develop ethical decision-making skills.
Institutions should implement rigorous screening processes for students and researchers involved in thermite-related projects. Background checks and ongoing monitoring may be necessary to prevent unauthorized access to sensitive information or materials. Additionally, collaborations with relevant authorities and industry partners can help ensure that educational programs align with current safety standards and regulations.
Transparency in research objectives and methodologies is crucial when working with thermite reactions. Institutions should establish clear guidelines for publishing and sharing research findings, balancing the need for academic freedom with potential security concerns. This may involve implementing review processes for publications and presentations related to thermite chemistry.
Educators should also address the ethical considerations surrounding the potential environmental impacts of thermite reactions. Discussions on sustainable practices and the responsible use of resources in chemical processes can help students develop a holistic understanding of the broader implications of their work.
Integrating case studies that highlight both the beneficial applications and potential misuse of thermite chemistry can provide valuable context for students. This approach allows for in-depth discussions on ethical dilemmas and encourages students to consider the long-term consequences of their research and future professional activities.
By addressing these ethical considerations, academic institutions can ensure that the teaching of thermite chemistry contributes positively to scientific advancement while promoting responsible practices and minimizing potential risks to society.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!