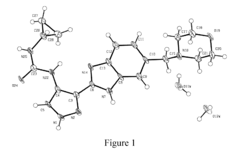
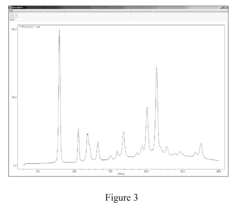
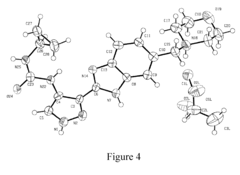
How to Test Tartaric Acid Efficacy in Antifungal Treatments
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Tartaric Acid Antifungal Testing Background and Objectives
Tartaric acid, a naturally occurring organic acid found predominantly in grapes and other fruits, has garnered increasing attention in the agricultural and pharmaceutical sectors for its potential antifungal properties. The historical trajectory of tartaric acid research dates back to the 18th century when it was first isolated, but its application as an antifungal agent represents a relatively recent development in the broader context of sustainable agricultural practices and natural pharmaceutical alternatives.
The evolution of antifungal treatments has progressed from traditional chemical fungicides with significant environmental impacts to more environmentally conscious solutions. This transition has been driven by growing concerns about fungicide resistance, environmental degradation, and consumer demand for reduced chemical residues in food products. Tartaric acid emerges within this context as a promising candidate for natural fungal control, potentially offering efficacy without the ecological footprint associated with conventional fungicides.
Current research indicates that tartaric acid may disrupt fungal cell membranes and interfere with essential metabolic pathways in pathogenic fungi. However, standardized methodologies for evaluating its antifungal efficacy remain underdeveloped, creating a significant gap in our ability to validate its practical applications. This technical gap necessitates the establishment of robust testing protocols that can reliably measure tartaric acid's effectiveness against various fungal pathogens under different environmental conditions.
The primary objective of this technical research is to develop and validate comprehensive testing methodologies for assessing tartaric acid's antifungal properties. These methodologies must address several critical parameters: minimum inhibitory concentration determination, spectrum of activity against diverse fungal species, mechanisms of action, stability under various environmental conditions, and comparative efficacy against conventional antifungal agents.
Secondary objectives include identifying potential synergistic combinations with other natural compounds, optimizing formulation parameters for enhanced efficacy, and evaluating potential resistance development in target fungi. Additionally, this research aims to establish clear correlations between laboratory testing results and field application outcomes, ensuring that laboratory findings translate effectively to real-world agricultural and pharmaceutical applications.
The anticipated outcomes of this technical investigation include standardized testing protocols that can be widely adopted by researchers and industry stakeholders, comprehensive efficacy profiles across different fungal species, and clear guidelines for formulation and application in various contexts. These outcomes will significantly contribute to the growing body of knowledge regarding natural antifungal alternatives and potentially facilitate the development of tartaric acid-based commercial products for sustainable fungal management.
The evolution of antifungal treatments has progressed from traditional chemical fungicides with significant environmental impacts to more environmentally conscious solutions. This transition has been driven by growing concerns about fungicide resistance, environmental degradation, and consumer demand for reduced chemical residues in food products. Tartaric acid emerges within this context as a promising candidate for natural fungal control, potentially offering efficacy without the ecological footprint associated with conventional fungicides.
Current research indicates that tartaric acid may disrupt fungal cell membranes and interfere with essential metabolic pathways in pathogenic fungi. However, standardized methodologies for evaluating its antifungal efficacy remain underdeveloped, creating a significant gap in our ability to validate its practical applications. This technical gap necessitates the establishment of robust testing protocols that can reliably measure tartaric acid's effectiveness against various fungal pathogens under different environmental conditions.
The primary objective of this technical research is to develop and validate comprehensive testing methodologies for assessing tartaric acid's antifungal properties. These methodologies must address several critical parameters: minimum inhibitory concentration determination, spectrum of activity against diverse fungal species, mechanisms of action, stability under various environmental conditions, and comparative efficacy against conventional antifungal agents.
Secondary objectives include identifying potential synergistic combinations with other natural compounds, optimizing formulation parameters for enhanced efficacy, and evaluating potential resistance development in target fungi. Additionally, this research aims to establish clear correlations between laboratory testing results and field application outcomes, ensuring that laboratory findings translate effectively to real-world agricultural and pharmaceutical applications.
The anticipated outcomes of this technical investigation include standardized testing protocols that can be widely adopted by researchers and industry stakeholders, comprehensive efficacy profiles across different fungal species, and clear guidelines for formulation and application in various contexts. These outcomes will significantly contribute to the growing body of knowledge regarding natural antifungal alternatives and potentially facilitate the development of tartaric acid-based commercial products for sustainable fungal management.
Market Analysis for Tartaric Acid-Based Antifungal Solutions
The global market for antifungal treatments has shown consistent growth over the past decade, with tartaric acid emerging as a promising natural alternative to conventional synthetic fungicides. Current market valuation for natural antifungal solutions stands at approximately $6.2 billion, with tartaric acid-based products representing a growing segment within this category. Agricultural applications dominate the market share, followed by food preservation and pharmaceutical formulations.
Consumer demand for eco-friendly and sustainable antifungal treatments has created significant market opportunities for tartaric acid-based solutions. This trend is particularly pronounced in organic farming sectors across North America and Europe, where regulatory restrictions on synthetic fungicides continue to tighten. Market research indicates that tartaric acid products command premium pricing compared to conventional alternatives, with consumers willing to pay 15-30% more for natural antifungal treatments.
The competitive landscape features both established agrochemical companies expanding their natural product portfolios and specialized biotech firms focused exclusively on organic solutions. Key market players include Novozymes, Marrone Bio Innovations, and Certis Biologicals, who have all introduced tartaric acid formulations in recent years. Regional market penetration varies significantly, with Europe leading adoption rates followed by North America and select Asian markets.
Market segmentation analysis reveals distinct application categories with varying growth potentials. Vineyard disease management represents the most mature market segment, leveraging tartaric acid's natural presence in grapes. Emerging applications in post-harvest fruit preservation show the highest growth rate, while pharmaceutical and cosmetic applications remain in early development stages but demonstrate promising profit margins.
Supply chain considerations significantly impact market dynamics, as tartaric acid production relies heavily on wine industry byproducts. This creates seasonal availability fluctuations and regional price variations. Countries with robust wine industries like Italy, France, and Spain currently dominate raw material supply, creating potential market entry barriers for regions without established wine production.
Regulatory frameworks play a decisive role in market development, with organic certification standards and food safety regulations directly influencing adoption rates. The EU's Farm to Fork Strategy and similar initiatives worldwide are creating favorable policy environments for natural antifungal solutions, accelerating market growth projections for tartaric acid-based products over the next five years.
Consumer education remains a critical market development factor, as awareness of tartaric acid's efficacy compared to conventional treatments varies significantly across regions and industry sectors. Marketing strategies emphasizing both environmental benefits and performance metrics will be essential for expanding market share beyond early adopters.
Consumer demand for eco-friendly and sustainable antifungal treatments has created significant market opportunities for tartaric acid-based solutions. This trend is particularly pronounced in organic farming sectors across North America and Europe, where regulatory restrictions on synthetic fungicides continue to tighten. Market research indicates that tartaric acid products command premium pricing compared to conventional alternatives, with consumers willing to pay 15-30% more for natural antifungal treatments.
The competitive landscape features both established agrochemical companies expanding their natural product portfolios and specialized biotech firms focused exclusively on organic solutions. Key market players include Novozymes, Marrone Bio Innovations, and Certis Biologicals, who have all introduced tartaric acid formulations in recent years. Regional market penetration varies significantly, with Europe leading adoption rates followed by North America and select Asian markets.
Market segmentation analysis reveals distinct application categories with varying growth potentials. Vineyard disease management represents the most mature market segment, leveraging tartaric acid's natural presence in grapes. Emerging applications in post-harvest fruit preservation show the highest growth rate, while pharmaceutical and cosmetic applications remain in early development stages but demonstrate promising profit margins.
Supply chain considerations significantly impact market dynamics, as tartaric acid production relies heavily on wine industry byproducts. This creates seasonal availability fluctuations and regional price variations. Countries with robust wine industries like Italy, France, and Spain currently dominate raw material supply, creating potential market entry barriers for regions without established wine production.
Regulatory frameworks play a decisive role in market development, with organic certification standards and food safety regulations directly influencing adoption rates. The EU's Farm to Fork Strategy and similar initiatives worldwide are creating favorable policy environments for natural antifungal solutions, accelerating market growth projections for tartaric acid-based products over the next five years.
Consumer education remains a critical market development factor, as awareness of tartaric acid's efficacy compared to conventional treatments varies significantly across regions and industry sectors. Marketing strategies emphasizing both environmental benefits and performance metrics will be essential for expanding market share beyond early adopters.
Current Testing Methodologies and Limitations
The evaluation of tartaric acid's antifungal efficacy currently relies on several established methodologies, each with specific advantages and limitations. In vitro disk diffusion assays represent the most commonly employed technique, where filter paper disks impregnated with tartaric acid solutions are placed on fungal-inoculated agar plates. While this method provides a visual indication of inhibition zones, it struggles to account for the complex interactions that occur in real-world applications, particularly in food preservation and agricultural settings.
Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC) determinations offer quantitative measurements of tartaric acid's antifungal properties. These broth dilution methods establish the lowest concentration required to inhibit visible fungal growth or achieve fungicidal effects. However, these techniques often yield variable results depending on the testing conditions, fungal strain variations, and medium composition, making standardization challenging across different laboratories.
Time-kill kinetic studies track fungal population dynamics over time when exposed to tartaric acid. While providing valuable information on the rate of antifungal activity, these methods are labor-intensive and time-consuming, typically requiring 24-72 hours for complete assessment. This extended timeframe limits their applicability in rapid screening applications or time-sensitive industrial quality control processes.
Molecular and biochemical assays examining tartaric acid's mechanism of action have emerged recently, including membrane integrity tests, metabolic inhibition assays, and gene expression analyses. These sophisticated approaches provide insights into how tartaric acid disrupts fungal cellular processes but require specialized equipment and expertise not readily available in many testing facilities.
Field trials and application-specific testing represent the most realistic evaluation methods but suffer from poor reproducibility due to environmental variables and biological diversity. The lack of standardized protocols for tartaric acid testing in specific applications (food preservation, crop protection, medical treatments) further complicates efficacy assessment across different use cases.
A significant limitation across all current methodologies is the insufficient correlation between laboratory results and real-world performance. pH variations, temperature fluctuations, and interactions with other compounds in complex matrices can dramatically alter tartaric acid's antifungal properties. Additionally, most testing protocols fail to account for adaptive resistance development in fungal populations during prolonged exposure to sub-lethal concentrations of tartaric acid.
The absence of internationally recognized standard testing protocols specifically designed for organic acids like tartaric acid creates challenges in comparing research findings and establishing definitive efficacy benchmarks. This gap highlights the need for developing more standardized, reproducible, and application-relevant testing methodologies to accurately assess tartaric acid's potential as an antifungal agent.
Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC) determinations offer quantitative measurements of tartaric acid's antifungal properties. These broth dilution methods establish the lowest concentration required to inhibit visible fungal growth or achieve fungicidal effects. However, these techniques often yield variable results depending on the testing conditions, fungal strain variations, and medium composition, making standardization challenging across different laboratories.
Time-kill kinetic studies track fungal population dynamics over time when exposed to tartaric acid. While providing valuable information on the rate of antifungal activity, these methods are labor-intensive and time-consuming, typically requiring 24-72 hours for complete assessment. This extended timeframe limits their applicability in rapid screening applications or time-sensitive industrial quality control processes.
Molecular and biochemical assays examining tartaric acid's mechanism of action have emerged recently, including membrane integrity tests, metabolic inhibition assays, and gene expression analyses. These sophisticated approaches provide insights into how tartaric acid disrupts fungal cellular processes but require specialized equipment and expertise not readily available in many testing facilities.
Field trials and application-specific testing represent the most realistic evaluation methods but suffer from poor reproducibility due to environmental variables and biological diversity. The lack of standardized protocols for tartaric acid testing in specific applications (food preservation, crop protection, medical treatments) further complicates efficacy assessment across different use cases.
A significant limitation across all current methodologies is the insufficient correlation between laboratory results and real-world performance. pH variations, temperature fluctuations, and interactions with other compounds in complex matrices can dramatically alter tartaric acid's antifungal properties. Additionally, most testing protocols fail to account for adaptive resistance development in fungal populations during prolonged exposure to sub-lethal concentrations of tartaric acid.
The absence of internationally recognized standard testing protocols specifically designed for organic acids like tartaric acid creates challenges in comparing research findings and establishing definitive efficacy benchmarks. This gap highlights the need for developing more standardized, reproducible, and application-relevant testing methodologies to accurately assess tartaric acid's potential as an antifungal agent.
Standard Tartaric Acid Antifungal Testing Methods
01 Tartaric acid in food and beverage applications
Tartaric acid is widely used in food and beverage applications due to its acidulant properties. It serves as a flavor enhancer, pH regulator, and preservative in various products. The acid contributes to the tartness and stability of beverages, confectioneries, and baked goods. Its natural occurrence in grapes makes it particularly suitable for wine production, where it helps maintain proper acidity levels and enhances flavor profiles.- Tartaric acid in food and beverage applications: Tartaric acid is widely used in food and beverage applications due to its acidulant properties. It serves as a flavor enhancer, pH regulator, and preservative in various products including wines, fruit juices, and confectioneries. The acid contributes to the tartness and stability of these products while also inhibiting microbial growth. Its natural occurrence in grapes makes it particularly suitable for wine production, where it helps maintain quality and taste profiles.
- Pharmaceutical and medicinal applications of tartaric acid: Tartaric acid and its derivatives have significant applications in pharmaceutical formulations. It functions as an excipient, stabilizing agent, and pH adjuster in various drug formulations. The acid also demonstrates antimicrobial properties that can enhance the efficacy of certain medications. Additionally, tartaric acid isomers are used as chiral agents in pharmaceutical synthesis, enabling the production of stereochemically pure compounds that are essential for drug efficacy and safety.
- Industrial and chemical synthesis applications: Tartaric acid serves as an important reagent and intermediate in various chemical synthesis processes. It is utilized in the production of chelating agents, catalysts, and as a chiral building block for asymmetric synthesis. The acid's stereochemical properties make it valuable for creating optically active compounds. In industrial applications, tartaric acid is employed in metal plating, textile dyeing, and as a complexing agent for metal ions, demonstrating its versatility beyond food and pharmaceutical uses.
- Tartaric acid in cosmetic and personal care products: Tartaric acid is incorporated into cosmetic and personal care formulations for its exfoliating and pH-adjusting properties. It functions as an alpha hydroxy acid (AHA) that promotes skin cell turnover and improves skin texture. The acid also helps stabilize formulations and enhance the efficacy of other active ingredients. In dental care products, tartaric acid contributes to cleaning efficacy and flavor profiles, while also providing mild antimicrobial benefits.
- Environmental and agricultural applications: Tartaric acid has emerging applications in environmental remediation and agricultural sectors. It can be used as a biodegradable chelating agent for heavy metal removal from contaminated soils and water. In agriculture, tartaric acid derivatives serve as plant growth regulators and components in eco-friendly pesticide formulations. The acid's natural origin and biodegradability make it an environmentally preferable alternative to synthetic chemicals in various applications, aligning with sustainable development goals.
02 Tartaric acid in pharmaceutical formulations
Tartaric acid and its derivatives play significant roles in pharmaceutical formulations. The acid is utilized as an excipient in drug delivery systems, providing pH adjustment and stability enhancement. It can improve the solubility and bioavailability of certain active pharmaceutical ingredients. Tartaric acid also serves as a chiral agent in the synthesis of pharmaceutically active compounds, enabling the production of specific enantiomers with desired therapeutic effects.Expand Specific Solutions03 Tartaric acid in chemical synthesis and industrial processes
Tartaric acid demonstrates significant efficacy in various chemical synthesis and industrial processes. It functions as a chelating agent, complexing with metal ions to form stable compounds used in catalysis. The acid serves as a chiral building block for asymmetric synthesis, enabling the production of optically active compounds. In industrial applications, tartaric acid is utilized in metal plating, textile dyeing, and as a precursor for other valuable chemicals.Expand Specific Solutions04 Tartaric acid in cosmetic and personal care products
Tartaric acid exhibits beneficial properties in cosmetic and personal care formulations. It functions as an alpha-hydroxy acid (AHA) that provides gentle exfoliation by breaking down the bonds between dead skin cells. This acid helps in skin renewal processes, improving texture and appearance. Additionally, tartaric acid serves as a pH adjuster in various cosmetic preparations, contributing to product stability and efficacy. Its natural origin makes it appealing for clean beauty formulations.Expand Specific Solutions05 Environmental and agricultural applications of tartaric acid
Tartaric acid demonstrates efficacy in environmental remediation and agricultural applications. In agriculture, it can function as a plant growth regulator and soil conditioner, helping to adjust soil pH and improve nutrient availability. The acid and its derivatives are used in eco-friendly cleaning formulations as alternatives to more aggressive chemicals. Additionally, tartaric acid plays a role in certain biodegradable polymers and sustainable materials, contributing to environmentally friendly product development.Expand Specific Solutions
Leading Research Institutions and Pharmaceutical Companies
The antifungal efficacy testing of tartaric acid is currently in an emerging research phase, with the market showing significant growth potential as natural antimicrobial solutions gain traction. The competitive landscape features a diverse mix of players across pharmaceutical, academic, and agricultural sectors. Research institutions like Nankai University, East China Normal University, and Peking University are advancing fundamental research, while pharmaceutical companies including Astex Therapeutics, PTC Therapeutics, and BioNTech are exploring clinical applications. Agricultural research entities such as Shanghai Academy of Agricultural Science are investigating crop protection uses. The technology remains in early-to-mid development stages, with companies like BASF and DuPont bringing industrial scale capabilities that could accelerate commercialization as efficacy testing protocols become standardized.
Astex Therapeutics Ltd.
Technical Solution: Astex Therapeutics has developed a structure-based approach to evaluating tartaric acid's antifungal properties, leveraging their expertise in fragment-based drug discovery. Their testing methodology begins with high-resolution crystallographic analysis of tartaric acid binding to key fungal target proteins, particularly those involved in cell wall synthesis and membrane integrity. Astex employs a cascade of biochemical assays to evaluate tartaric acid's inhibitory effects on specific fungal enzymes, including chitin synthase, β(1,3)-glucan synthase, and lanosterol 14α-demethylase. Their platform includes thermal shift assays to quantify binding affinity and cellular thermal shift assays (CETSA) to confirm target engagement in intact fungal cells. Astex has pioneered the use of NMR-based fragment screening to identify optimal tartaric acid derivatives with enhanced binding properties to fungal targets. Their testing framework incorporates advanced computational modeling to predict structure-activity relationships and guide rational modification of tartaric acid to improve antifungal potency. Astex also employs transcriptomic and proteomic analyses to characterize the broader cellular response to tartaric acid treatment in fungal pathogens, providing insights into resistance mechanisms and potential combination strategies.
Strengths: Sophisticated structural biology capabilities that provide molecular-level insights into tartaric acid's antifungal mechanisms. Strong integration of computational and experimental approaches for rational optimization of tartaric acid formulations. Weaknesses: Highly specialized approach may emphasize mechanistic understanding over practical application testing, potentially creating gaps in translating findings to field or clinical use.
Council of Scientific & Industrial Research
Technical Solution: CSIR has developed a multifaceted approach to testing tartaric acid's antifungal properties, with particular emphasis on indigenous applications for tropical fungal pathogens. Their methodology encompasses both traditional and advanced screening techniques, including agar well diffusion, broth microdilution, and time-kill kinetics studies. CSIR researchers have established standardized protocols for evaluating tartaric acid against dermatophytes, agricultural pathogens, and food spoilage fungi. Their testing framework includes evaluation of tartaric acid's mechanism of action through enzyme inhibition assays, particularly focusing on its effects on chitin synthase and ergosterol biosynthesis pathways critical for fungal cell wall integrity. CSIR has pioneered cost-effective methods for determining tartaric acid's minimum fungicidal concentration (MFC) using colorimetric indicators that can be deployed in resource-limited settings. Their research has demonstrated tartaric acid's efficacy against fluconazole-resistant Candida strains, suggesting potential applications in addressing antifungal resistance. CSIR also employs molecular docking studies to predict tartaric acid interactions with fungal target proteins, correlating computational predictions with experimental outcomes.
Strengths: Extensive experience with diverse fungal pathogens relevant to tropical and subtropical regions. Development of accessible testing methodologies suitable for implementation in developing countries. Weaknesses: Some testing protocols may lack standardization compared to pharmaceutical industry standards, potentially affecting reproducibility across different laboratory settings.
Key Research Findings on Tartaric Acid Mechanisms
Pyrazole derivatives having kinase modulating activity
PatentWO2006070202A1
Innovation
- Development of pyrazole derivatives that inhibit or modulate the activity of CDKs, Aurora kinases, and GSK3, offering a novel approach to treat diseases by administering these compounds as pharmaceutical compositions to target and regulate kinase activity.
Benzimidazole compounds that modulate the activity of CDK, GSK and aurora kinases
PatentInactiveEP2395000A1
Innovation
- Development of pyrazole compounds with specific inhibitory or modulating activity against CDK, Aurora kinases, and GSK, which are designed to inhibit or modulate the activity of these kinases, thereby providing a therapeutic approach for conditions such as cancer and leukemia.
Safety and Toxicity Considerations
The assessment of tartaric acid's safety profile is paramount when evaluating its efficacy as an antifungal treatment. Tartaric acid, while naturally occurring in many fruits, particularly grapes, requires comprehensive toxicological evaluation before widespread implementation in agricultural or clinical settings. Current research indicates that tartaric acid demonstrates relatively low toxicity in mammals, with an LD50 of approximately 5.3 g/kg in rats, positioning it as safer than many synthetic fungicides currently in use.
When conducting efficacy tests, researchers must carefully monitor for potential adverse effects on non-target organisms. Studies have shown minimal phytotoxicity at recommended application concentrations (typically 0.5-2%), though higher concentrations may cause leaf burning or fruit damage in sensitive plant species. Environmental impact assessments indicate that tartaric acid biodegrades rapidly in soil environments, with a half-life of approximately 7-14 days under standard conditions, significantly reducing concerns about environmental persistence.
Human exposure considerations must be thoroughly addressed in testing protocols. Dermal contact studies have demonstrated mild irritation potential, necessitating appropriate handling procedures during application. Respiratory exposure risks appear minimal at standard application concentrations, though aerosolization during spray applications warrants proper respiratory protection. Ingestion safety data supports tartaric acid's generally recognized as safe (GRAS) status by regulatory bodies, though concentrated formulations require appropriate labeling and handling precautions.
Residue analysis constitutes a critical component of safety testing. Current analytical methods, including high-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS), can detect tartaric acid residues at concentrations as low as 0.01 mg/kg. Maximum residue limits (MRLs) established by regulatory agencies typically range from 5-15 mg/kg, depending on the crop and intended use, providing adequate safety margins for consumers.
Regulatory compliance frameworks vary globally, with the FDA, EPA, and European Food Safety Authority (EFSA) each maintaining specific guidelines for natural acid-based fungicides. Efficacy testing protocols must incorporate these regulatory requirements, including standardized toxicity assessments and residue studies. The development of comprehensive safety data packages remains essential for registration and approval processes, particularly for novel applications or formulations of tartaric acid as an antifungal agent.
When conducting efficacy tests, researchers must carefully monitor for potential adverse effects on non-target organisms. Studies have shown minimal phytotoxicity at recommended application concentrations (typically 0.5-2%), though higher concentrations may cause leaf burning or fruit damage in sensitive plant species. Environmental impact assessments indicate that tartaric acid biodegrades rapidly in soil environments, with a half-life of approximately 7-14 days under standard conditions, significantly reducing concerns about environmental persistence.
Human exposure considerations must be thoroughly addressed in testing protocols. Dermal contact studies have demonstrated mild irritation potential, necessitating appropriate handling procedures during application. Respiratory exposure risks appear minimal at standard application concentrations, though aerosolization during spray applications warrants proper respiratory protection. Ingestion safety data supports tartaric acid's generally recognized as safe (GRAS) status by regulatory bodies, though concentrated formulations require appropriate labeling and handling precautions.
Residue analysis constitutes a critical component of safety testing. Current analytical methods, including high-performance liquid chromatography (HPLC) and gas chromatography-mass spectrometry (GC-MS), can detect tartaric acid residues at concentrations as low as 0.01 mg/kg. Maximum residue limits (MRLs) established by regulatory agencies typically range from 5-15 mg/kg, depending on the crop and intended use, providing adequate safety margins for consumers.
Regulatory compliance frameworks vary globally, with the FDA, EPA, and European Food Safety Authority (EFSA) each maintaining specific guidelines for natural acid-based fungicides. Efficacy testing protocols must incorporate these regulatory requirements, including standardized toxicity assessments and residue studies. The development of comprehensive safety data packages remains essential for registration and approval processes, particularly for novel applications or formulations of tartaric acid as an antifungal agent.
Regulatory Compliance Framework
The regulatory landscape for antifungal treatments containing tartaric acid requires comprehensive understanding and compliance with multiple frameworks across different jurisdictions. In the United States, the FDA regulates antifungal products through various pathways depending on their intended use - prescription antifungals fall under drug regulations requiring extensive clinical trials, while over-the-counter products may follow monograph systems with specific testing requirements for active ingredients like tartaric acid.
The Environmental Protection Agency (EPA) also plays a crucial role when tartaric acid is used in agricultural antifungal applications, requiring efficacy testing that demonstrates both fungicidal properties and environmental safety profiles. Testing protocols must adhere to Good Laboratory Practice (GLP) standards as outlined in 40 CFR Part 160, with specific attention to residue analysis and ecological impact assessments.
European regulatory frameworks present additional considerations through the European Medicines Agency (EMA) for medical applications and the European Food Safety Authority (EFSA) for food-related uses. The EU Biocidal Products Regulation (BPR) specifically addresses antifungal substances, requiring standardized efficacy testing against reference organisms under EN standards, with tartaric acid evaluations needing to demonstrate minimum inhibitory concentration (MIC) values against target fungi.
International Harmonization initiatives, particularly the International Council for Harmonisation (ICH) guidelines, provide standardized approaches for stability testing and impurity profiling of tartaric acid formulations. These guidelines establish uniform testing methodologies that facilitate global market access while ensuring consistent quality and efficacy standards across different regulatory environments.
Documentation requirements represent a significant compliance component, with detailed records needed for test methods validation, quality control procedures, and batch testing results. Regulatory submissions must include comprehensive data packages demonstrating tartaric acid's antifungal efficacy through standardized testing protocols such as CLSI M38 for filamentous fungi or EUCAST methodologies, with results expressed in statistically valid formats.
Emerging regulatory trends indicate increasing scrutiny of natural acid preservatives like tartaric acid, with growing emphasis on combination testing to evaluate synergistic effects with other antifungal agents. Regulatory bodies are also implementing more stringent requirements for resistance monitoring, necessitating testing protocols that evaluate tartaric acid's efficacy against resistant fungal strains and potential for resistance development through repeated exposure studies.
The Environmental Protection Agency (EPA) also plays a crucial role when tartaric acid is used in agricultural antifungal applications, requiring efficacy testing that demonstrates both fungicidal properties and environmental safety profiles. Testing protocols must adhere to Good Laboratory Practice (GLP) standards as outlined in 40 CFR Part 160, with specific attention to residue analysis and ecological impact assessments.
European regulatory frameworks present additional considerations through the European Medicines Agency (EMA) for medical applications and the European Food Safety Authority (EFSA) for food-related uses. The EU Biocidal Products Regulation (BPR) specifically addresses antifungal substances, requiring standardized efficacy testing against reference organisms under EN standards, with tartaric acid evaluations needing to demonstrate minimum inhibitory concentration (MIC) values against target fungi.
International Harmonization initiatives, particularly the International Council for Harmonisation (ICH) guidelines, provide standardized approaches for stability testing and impurity profiling of tartaric acid formulations. These guidelines establish uniform testing methodologies that facilitate global market access while ensuring consistent quality and efficacy standards across different regulatory environments.
Documentation requirements represent a significant compliance component, with detailed records needed for test methods validation, quality control procedures, and batch testing results. Regulatory submissions must include comprehensive data packages demonstrating tartaric acid's antifungal efficacy through standardized testing protocols such as CLSI M38 for filamentous fungi or EUCAST methodologies, with results expressed in statistically valid formats.
Emerging regulatory trends indicate increasing scrutiny of natural acid preservatives like tartaric acid, with growing emphasis on combination testing to evaluate synergistic effects with other antifungal agents. Regulatory bodies are also implementing more stringent requirements for resistance monitoring, necessitating testing protocols that evaluate tartaric acid's efficacy against resistant fungal strains and potential for resistance development through repeated exposure studies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!