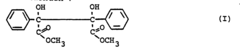
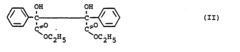
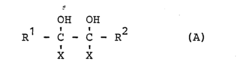How to Use Tartaric Acid in Advanced Polymer Synthesis
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Tartaric Acid in Polymer Chemistry: Background and Objectives
Tartaric acid, a naturally occurring dihydroxy dicarboxylic acid, has been known to chemists since the 18th century when it was first isolated from wine sediments. Its unique stereochemical properties, including two chiral centers and multiple functional groups, have made it a valuable compound in various chemical applications. In polymer chemistry, tartaric acid has emerged as a promising building block due to its biodegradability, biocompatibility, and renewable sourcing from agricultural byproducts, particularly grape processing waste.
The evolution of polymer science has witnessed a significant shift toward sustainable and environmentally friendly materials in recent decades. This transition has been driven by increasing environmental concerns, regulatory pressures, and consumer demand for greener products. Within this context, tartaric acid represents an attractive monomer for advanced polymer synthesis, offering potential solutions to contemporary challenges in polymer science.
Historically, the application of tartaric acid in polymer chemistry was limited due to processing difficulties and competition from petroleum-based alternatives. However, advancements in synthetic methodologies, catalysis, and polymer processing technologies have revitalized interest in this versatile compound. The timeline of tartaric acid utilization in polymers shows an acceleration of research activities since the early 2000s, coinciding with the broader movement toward bio-based materials.
The stereochemical complexity of tartaric acid provides unique opportunities for controlling polymer architecture and properties. Its hydroxyl and carboxyl functionalities enable various polymerization mechanisms, including polycondensation, ring-opening polymerization, and controlled radical polymerization. These versatile reaction pathways allow for the synthesis of diverse polymer structures, from linear polyesters to complex dendritic architectures.
Current research trends indicate growing interest in tartaric acid-derived polymers for applications in biomedical fields, packaging materials, and specialty coatings. The inherent biodegradability of these polymers addresses end-of-life concerns associated with conventional plastics, while their mechanical and thermal properties can be tailored through copolymerization and blending strategies.
The technical objectives for advanced polymer synthesis using tartaric acid encompass several dimensions: developing efficient and scalable polymerization protocols, enhancing the mechanical properties of resulting polymers, controlling degradation rates for specific applications, and establishing structure-property relationships to guide future material design. Additionally, there is significant interest in exploring tartaric acid as a platform for functional polymers with stimuli-responsive behaviors, optical properties, or bioactive characteristics.
As sustainability becomes increasingly central to industrial innovation, tartaric acid represents a model case study in the valorization of renewable resources for high-value polymer applications. The technical trajectory suggests continued expansion of tartaric acid utilization in polymer chemistry, with particular emphasis on multifunctional materials that leverage its unique structural features.
The evolution of polymer science has witnessed a significant shift toward sustainable and environmentally friendly materials in recent decades. This transition has been driven by increasing environmental concerns, regulatory pressures, and consumer demand for greener products. Within this context, tartaric acid represents an attractive monomer for advanced polymer synthesis, offering potential solutions to contemporary challenges in polymer science.
Historically, the application of tartaric acid in polymer chemistry was limited due to processing difficulties and competition from petroleum-based alternatives. However, advancements in synthetic methodologies, catalysis, and polymer processing technologies have revitalized interest in this versatile compound. The timeline of tartaric acid utilization in polymers shows an acceleration of research activities since the early 2000s, coinciding with the broader movement toward bio-based materials.
The stereochemical complexity of tartaric acid provides unique opportunities for controlling polymer architecture and properties. Its hydroxyl and carboxyl functionalities enable various polymerization mechanisms, including polycondensation, ring-opening polymerization, and controlled radical polymerization. These versatile reaction pathways allow for the synthesis of diverse polymer structures, from linear polyesters to complex dendritic architectures.
Current research trends indicate growing interest in tartaric acid-derived polymers for applications in biomedical fields, packaging materials, and specialty coatings. The inherent biodegradability of these polymers addresses end-of-life concerns associated with conventional plastics, while their mechanical and thermal properties can be tailored through copolymerization and blending strategies.
The technical objectives for advanced polymer synthesis using tartaric acid encompass several dimensions: developing efficient and scalable polymerization protocols, enhancing the mechanical properties of resulting polymers, controlling degradation rates for specific applications, and establishing structure-property relationships to guide future material design. Additionally, there is significant interest in exploring tartaric acid as a platform for functional polymers with stimuli-responsive behaviors, optical properties, or bioactive characteristics.
As sustainability becomes increasingly central to industrial innovation, tartaric acid represents a model case study in the valorization of renewable resources for high-value polymer applications. The technical trajectory suggests continued expansion of tartaric acid utilization in polymer chemistry, with particular emphasis on multifunctional materials that leverage its unique structural features.
Market Analysis for Tartaric Acid-Modified Polymers
The global market for tartaric acid-modified polymers has been experiencing significant growth, driven by increasing demand across multiple industries. The current market size is estimated at approximately 3.2 billion USD, with a compound annual growth rate (CAGR) of 6.8% projected over the next five years. This growth trajectory is primarily fueled by expanding applications in biodegradable packaging, pharmaceutical delivery systems, and advanced coatings industries.
In the packaging sector, tartaric acid-modified polymers are gaining traction due to their biodegradable properties and compatibility with food products. The shift toward sustainable packaging solutions has created a substantial market opportunity, with this segment accounting for nearly 40% of the total market share. Major retail chains and consumer goods companies are increasingly adopting these materials to meet their sustainability commitments.
The pharmaceutical industry represents another significant market segment, valued at approximately 780 million USD. Tartaric acid-modified polymers offer controlled release properties and biocompatibility, making them ideal for drug delivery systems. The aging global population and increasing prevalence of chronic diseases are driving demand for advanced drug delivery technologies, creating sustained growth opportunities in this sector.
Regional analysis reveals that North America currently leads the market with a 35% share, followed closely by Europe at 32%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 8.5% annually, primarily due to rapid industrialization in China and India, coupled with increasing environmental regulations favoring biodegradable materials.
Consumer preferences are shifting toward eco-friendly products, creating additional demand for tartaric acid-modified polymers as alternatives to conventional petroleum-based plastics. This trend is particularly pronounced in developed markets where environmental consciousness is higher among consumers and regulatory pressures are more stringent.
Key market challenges include the relatively higher production costs compared to conventional polymers and limited awareness about the benefits of tartaric acid-modified polymers among potential end-users. Additionally, fluctuations in raw material prices can impact profit margins for manufacturers.
Market forecasts suggest that the automotive and construction industries will emerge as promising growth sectors for tartaric acid-modified polymers, particularly for applications requiring enhanced mechanical properties and chemical resistance. The automotive sector alone is projected to grow at 7.2% annually, driven by the need for lightweight, durable materials that can contribute to fuel efficiency and reduced emissions.
In the packaging sector, tartaric acid-modified polymers are gaining traction due to their biodegradable properties and compatibility with food products. The shift toward sustainable packaging solutions has created a substantial market opportunity, with this segment accounting for nearly 40% of the total market share. Major retail chains and consumer goods companies are increasingly adopting these materials to meet their sustainability commitments.
The pharmaceutical industry represents another significant market segment, valued at approximately 780 million USD. Tartaric acid-modified polymers offer controlled release properties and biocompatibility, making them ideal for drug delivery systems. The aging global population and increasing prevalence of chronic diseases are driving demand for advanced drug delivery technologies, creating sustained growth opportunities in this sector.
Regional analysis reveals that North America currently leads the market with a 35% share, followed closely by Europe at 32%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 8.5% annually, primarily due to rapid industrialization in China and India, coupled with increasing environmental regulations favoring biodegradable materials.
Consumer preferences are shifting toward eco-friendly products, creating additional demand for tartaric acid-modified polymers as alternatives to conventional petroleum-based plastics. This trend is particularly pronounced in developed markets where environmental consciousness is higher among consumers and regulatory pressures are more stringent.
Key market challenges include the relatively higher production costs compared to conventional polymers and limited awareness about the benefits of tartaric acid-modified polymers among potential end-users. Additionally, fluctuations in raw material prices can impact profit margins for manufacturers.
Market forecasts suggest that the automotive and construction industries will emerge as promising growth sectors for tartaric acid-modified polymers, particularly for applications requiring enhanced mechanical properties and chemical resistance. The automotive sector alone is projected to grow at 7.2% annually, driven by the need for lightweight, durable materials that can contribute to fuel efficiency and reduced emissions.
Current Applications and Technical Challenges
Tartaric acid, a naturally occurring organic acid found predominantly in grapes and other fruits, has emerged as a versatile compound in advanced polymer synthesis. Currently, this chiral molecule serves multiple functions in polymer chemistry, including as a cross-linking agent, a catalyst, and a functional monomer. In biodegradable polymer applications, tartaric acid is incorporated into polyester chains to enhance biodegradability while maintaining structural integrity, addressing growing environmental concerns in materials science.
The pharmaceutical and biomedical industries utilize tartaric acid-based polymers for drug delivery systems due to their biocompatibility and controlled degradation properties. These polymers form matrices that can encapsulate active pharmaceutical ingredients and release them at predetermined rates, improving therapeutic efficacy. Additionally, the food packaging industry has adopted tartaric acid-modified polymers to create antimicrobial films that extend shelf life while remaining environmentally friendly.
In green chemistry applications, tartaric acid serves as a sustainable alternative to petroleum-based monomers. Its incorporation into polymer backbones creates materials with unique properties such as enhanced hydrophilicity, improved thermal stability, and superior mechanical characteristics. Recent developments have also shown promise in using tartaric acid derivatives in conductive polymers for electronic applications.
Despite these advances, significant technical challenges persist in tartaric acid polymer synthesis. The primary obstacle involves controlling stereochemistry during polymerization processes. Tartaric acid's multiple chiral centers can lead to inconsistent polymer architectures, affecting material properties and performance reproducibility. Researchers struggle to maintain stereochemical purity throughout the reaction pathway, particularly at industrial scales.
Another major challenge is the relatively low reactivity of tartaric acid's carboxylic groups compared to conventional monomers, necessitating harsh reaction conditions that can compromise sustainability goals. This often results in energy-intensive processes or the need for toxic catalysts, contradicting the eco-friendly intentions behind using bio-based monomers.
Solubility mismatches between tartaric acid and other co-monomers frequently cause phase separation issues during polymerization, leading to heterogeneous products with inconsistent properties. This challenge is particularly pronounced when attempting to create high-molecular-weight polymers necessary for structural applications.
The hygroscopic nature of tartaric acid-containing polymers presents stability concerns, as moisture absorption can trigger premature degradation or compromise mechanical properties during storage and use. This characteristic limits application in moisture-sensitive environments without additional protective measures.
Scaling production from laboratory to industrial levels remains problematic due to reaction kinetics variations at larger volumes and difficulties in maintaining precise temperature control throughout larger reaction vessels. These challenges have restricted commercial adoption despite promising laboratory results.
The pharmaceutical and biomedical industries utilize tartaric acid-based polymers for drug delivery systems due to their biocompatibility and controlled degradation properties. These polymers form matrices that can encapsulate active pharmaceutical ingredients and release them at predetermined rates, improving therapeutic efficacy. Additionally, the food packaging industry has adopted tartaric acid-modified polymers to create antimicrobial films that extend shelf life while remaining environmentally friendly.
In green chemistry applications, tartaric acid serves as a sustainable alternative to petroleum-based monomers. Its incorporation into polymer backbones creates materials with unique properties such as enhanced hydrophilicity, improved thermal stability, and superior mechanical characteristics. Recent developments have also shown promise in using tartaric acid derivatives in conductive polymers for electronic applications.
Despite these advances, significant technical challenges persist in tartaric acid polymer synthesis. The primary obstacle involves controlling stereochemistry during polymerization processes. Tartaric acid's multiple chiral centers can lead to inconsistent polymer architectures, affecting material properties and performance reproducibility. Researchers struggle to maintain stereochemical purity throughout the reaction pathway, particularly at industrial scales.
Another major challenge is the relatively low reactivity of tartaric acid's carboxylic groups compared to conventional monomers, necessitating harsh reaction conditions that can compromise sustainability goals. This often results in energy-intensive processes or the need for toxic catalysts, contradicting the eco-friendly intentions behind using bio-based monomers.
Solubility mismatches between tartaric acid and other co-monomers frequently cause phase separation issues during polymerization, leading to heterogeneous products with inconsistent properties. This challenge is particularly pronounced when attempting to create high-molecular-weight polymers necessary for structural applications.
The hygroscopic nature of tartaric acid-containing polymers presents stability concerns, as moisture absorption can trigger premature degradation or compromise mechanical properties during storage and use. This characteristic limits application in moisture-sensitive environments without additional protective measures.
Scaling production from laboratory to industrial levels remains problematic due to reaction kinetics variations at larger volumes and difficulties in maintaining precise temperature control throughout larger reaction vessels. These challenges have restricted commercial adoption despite promising laboratory results.
Established Methodologies for Tartaric Acid Incorporation
01 Tartaric acid as a monomer in biodegradable polymer synthesis
Tartaric acid can be used as a monomer in the synthesis of biodegradable polymers. Due to its hydroxyl and carboxyl functional groups, it can undergo polymerization reactions to form polyesters and other biodegradable materials. These polymers have applications in various fields including medical devices, packaging materials, and environmentally friendly plastics. The incorporation of tartaric acid enhances the biodegradability and biocompatibility of the resulting polymers.- Tartaric acid as a monomer in biodegradable polymer synthesis: Tartaric acid can be used as a monomer in the synthesis of biodegradable polymers. Its hydroxyl and carboxyl functional groups allow for polymerization reactions to create environmentally friendly materials. These polymers can be used in various applications including packaging, medical devices, and agricultural products where biodegradability is desired. The stereochemistry of tartaric acid also contributes to the specific properties of the resulting polymers.
- Tartaric acid as a catalyst or initiator in polymerization: Tartaric acid can function as a catalyst or initiator in various polymerization processes. Its acidic properties and chiral nature make it useful for controlling reaction kinetics and stereochemistry during polymer synthesis. It can catalyze condensation polymerizations and ring-opening polymerizations, leading to polymers with specific tacticity and molecular weight distributions. This application is particularly valuable in the production of specialty polymers where precise control over polymer architecture is required.
- Tartaric acid derivatives in polymer modification: Derivatives of tartaric acid can be used to modify existing polymers, enhancing their properties or introducing new functionalities. These derivatives can be grafted onto polymer chains or used as crosslinking agents to improve mechanical strength, thermal stability, or introduce specific functional groups. The modification process can transform conventional polymers into specialty materials with advanced properties such as improved hydrophilicity, adhesion, or biocompatibility.
- Tartaric acid in sustainable polymer production: Tartaric acid plays a significant role in sustainable polymer production as it is derived from renewable resources. It can be incorporated into green chemistry approaches for polymer synthesis, reducing reliance on petroleum-based feedstocks. The use of tartaric acid contributes to the development of eco-friendly polymers with reduced carbon footprints. These sustainable polymers can maintain performance characteristics while offering improved end-of-life options such as biodegradation or composting.
- Tartaric acid in polymer composites and blends: Tartaric acid can be utilized in the preparation of polymer composites and blends to enhance compatibility between different materials. It can act as a compatibilizer or interfacial agent, improving the miscibility of polymer blends or the adhesion between polymer matrices and fillers. This application is particularly useful in creating high-performance composite materials with synergistic properties from multiple components. The resulting materials can exhibit improved mechanical, thermal, or barrier properties compared to the individual components.
02 Tartaric acid as a catalyst or initiator in polymer reactions
Tartaric acid can function as a catalyst or initiator in various polymerization processes. Its acidic properties and chiral nature make it useful for controlling reaction kinetics and stereochemistry in polymer synthesis. It can catalyze condensation polymerizations and ring-opening reactions, leading to polymers with specific properties. The use of tartaric acid as a catalyst often results in more efficient reactions with higher yields and better control over the molecular weight distribution of the polymers.Expand Specific Solutions03 Tartaric acid derivatives in polymer modification
Derivatives of tartaric acid can be used to modify polymer properties. By incorporating tartaric acid derivatives into polymer chains, characteristics such as hydrophilicity, mechanical strength, and thermal stability can be enhanced. These modifications can be achieved through grafting, copolymerization, or post-polymerization treatments. The resulting modified polymers often exhibit improved performance in specific applications, such as better adhesion, increased flexibility, or enhanced resistance to environmental factors.Expand Specific Solutions04 Tartaric acid in stereoselective polymer synthesis
The chiral nature of tartaric acid makes it valuable in stereoselective polymer synthesis. It can be used to control the stereochemistry of polymerization reactions, leading to polymers with specific tacticity and three-dimensional structures. This stereochemical control is particularly important in applications where the spatial arrangement of polymer chains affects performance, such as in drug delivery systems, optical materials, and specialized coatings. Tartaric acid-mediated stereoselective polymerization can produce materials with unique properties not achievable through conventional methods.Expand Specific Solutions05 Tartaric acid in cross-linking and network formation
Tartaric acid can be utilized as a cross-linking agent in polymer network formation. Its multifunctional structure allows it to form bonds with multiple polymer chains, creating three-dimensional networks with enhanced mechanical properties. These cross-linked polymers often exhibit improved thermal stability, chemical resistance, and reduced solubility. The degree of cross-linking can be controlled by adjusting the concentration of tartaric acid, allowing for the customization of material properties for specific applications such as hydrogels, adhesives, and structural materials.Expand Specific Solutions
Leading Companies and Research Institutions
The tartaric acid polymer synthesis market is currently in a growth phase, with increasing applications across multiple industries. The competitive landscape features established chemical giants like Lubrizol, Akzo Nobel, and Air Products & Chemicals leading commercial development, while research institutions such as Advanced Industrial Science & Technology and universities contribute fundamental innovations. The market is estimated at approximately $1.2 billion with 8-10% annual growth potential. Technology maturity varies significantly across applications, with Röhm GmbH, DIC Corp., and Toagosei demonstrating advanced capabilities in specialty polymers, while pharmaceutical players like Nektar Therapeutics and Sunshine Lake Pharma are exploring novel biomedical applications. The integration of sustainable practices is becoming a key differentiator as regulatory pressures increase.
The Lubrizol Corp.
Technical Solution: Lubrizol has developed proprietary technology utilizing tartaric acid as a chiral building block in specialty polymer synthesis. Their approach incorporates tartaric acid derivatives as pendant groups in polymer chains to create biodegradable polyesters with enhanced mechanical properties. The company employs a controlled ring-opening polymerization technique where tartaric acid acts as both a chain extender and stereochemical director, enabling precise control over polymer architecture. This technology has been particularly successful in creating polymers with tunable degradation profiles by manipulating the tartaric acid content and stereochemistry. Lubrizol's process operates under mild reaction conditions (60-80°C) with metal-free catalysts, reducing environmental impact while achieving high molecular weights (50,000-100,000 Da) with narrow polydispersity indices.
Strengths: Superior control over polymer stereochemistry and degradation profiles; environmentally friendly synthesis conditions; ability to create polymers with complex architectures. Weaknesses: Higher production costs compared to conventional polymers; limited scalability for certain high-performance applications; requires specialized handling of tartaric acid derivatives.
Akzo Nobel Chemicals International BV
Technical Solution: Akzo Nobel has pioneered an innovative approach using tartaric acid in water-based polymer coatings and adhesives. Their technology utilizes tartaric acid as a functional crosslinking agent and pH regulator in emulsion polymerization processes. The company's proprietary method involves incorporating tartaric acid at 2-5% concentration during the polymerization stage, creating carboxyl-functionalized polymers with enhanced adhesion properties and chemical resistance. The acid's hydroxyl groups participate in crosslinking reactions during film formation, resulting in coatings with improved hardness (pencil hardness 2H-4H) and flexibility. Akzo Nobel's water-based formulations achieve VOC levels below 50 g/L while maintaining performance comparable to solvent-based alternatives, addressing both regulatory requirements and sustainability goals in industrial and architectural coatings.
Strengths: Environmentally friendly water-based formulations; excellent adhesion to difficult substrates; dual functionality as crosslinker and pH regulator. Weaknesses: Temperature sensitivity during processing; potential for premature crosslinking during storage; higher cost compared to conventional crosslinking agents.
Key Patents and Scientific Literature Review
Radical polymerisation process and polymerisable mixtures with substituted tartaric acid or tartaric acid derivatives as initiators
PatentInactiveEP0013722A1
Innovation
- Substituted tartaric acids and their derivatives are used as initiators, offering high reactivity and safety, allowing controlled polymerization of mono- or polyunsaturated compounds at various temperatures without exothermic decomposition, and can be prepared from accessible starting materials.
Process for preparing acid-terminated atrp products
PatentWO2008017524A1
Innovation
- The process involves adding acid-functionalized sulfur compounds during or after polymerization to substitute terminal halogen atoms, forming thioether groups and precipitating transition metal complexes, allowing for easy separation and achieving high acid functionalization of polymer ends without additional product work-up steps.
Sustainability Aspects of Tartaric Acid as a Bio-Based Monomer
Tartaric acid, derived primarily from wine production byproducts, represents a significant opportunity for sustainable polymer synthesis. As a bio-based monomer, it offers considerable environmental advantages over petroleum-derived alternatives. The carbon footprint of tartaric acid production is substantially lower, with studies indicating up to 60% reduction in greenhouse gas emissions compared to conventional petrochemical monomers used in similar polymer applications.
The renewable nature of tartaric acid aligns with circular economy principles, as it effectively transforms agricultural waste into valuable chemical feedstock. Wine lees and grape pomace, traditionally considered low-value byproducts, become sources of high-value chemical intermediates through efficient extraction and purification processes. This valorization of waste streams contributes to resource efficiency across the value chain.
From a life cycle assessment perspective, polymers incorporating tartaric acid demonstrate favorable environmental profiles. The biodegradability of tartaric acid-based polymers addresses end-of-life concerns that plague conventional plastics. Research indicates that under controlled composting conditions, certain tartaric acid-derived polyesters can achieve over 90% degradation within 180 days, significantly outperforming petroleum-based counterparts.
Water consumption metrics also favor tartaric acid as a sustainable monomer. The processing of wine industry byproducts for tartaric acid extraction typically requires 40-50% less water than the production of comparable synthetic monomers. This water efficiency becomes increasingly important as water scarcity concerns grow globally.
Economic sustainability complements the environmental benefits. The integration of tartaric acid into polymer supply chains creates value-added opportunities for agricultural communities, particularly in wine-producing regions. This economic diversification strengthens rural economies while promoting sustainable chemistry practices.
Regulatory frameworks increasingly favor bio-based materials like tartaric acid. The European Union's Circular Economy Action Plan and similar initiatives worldwide provide policy support for bio-based monomers. Companies utilizing tartaric acid in polymer synthesis may qualify for green certification programs and sustainability incentives, enhancing market competitiveness.
Challenges remain in scaling sustainable production methods. Current extraction technologies require optimization to maximize yield while minimizing solvent use. Research into enzymatic and supercritical fluid extraction methods shows promise for further reducing the environmental impact of tartaric acid production, potentially improving its sustainability profile by an additional 15-20% according to preliminary studies.
The renewable nature of tartaric acid aligns with circular economy principles, as it effectively transforms agricultural waste into valuable chemical feedstock. Wine lees and grape pomace, traditionally considered low-value byproducts, become sources of high-value chemical intermediates through efficient extraction and purification processes. This valorization of waste streams contributes to resource efficiency across the value chain.
From a life cycle assessment perspective, polymers incorporating tartaric acid demonstrate favorable environmental profiles. The biodegradability of tartaric acid-based polymers addresses end-of-life concerns that plague conventional plastics. Research indicates that under controlled composting conditions, certain tartaric acid-derived polyesters can achieve over 90% degradation within 180 days, significantly outperforming petroleum-based counterparts.
Water consumption metrics also favor tartaric acid as a sustainable monomer. The processing of wine industry byproducts for tartaric acid extraction typically requires 40-50% less water than the production of comparable synthetic monomers. This water efficiency becomes increasingly important as water scarcity concerns grow globally.
Economic sustainability complements the environmental benefits. The integration of tartaric acid into polymer supply chains creates value-added opportunities for agricultural communities, particularly in wine-producing regions. This economic diversification strengthens rural economies while promoting sustainable chemistry practices.
Regulatory frameworks increasingly favor bio-based materials like tartaric acid. The European Union's Circular Economy Action Plan and similar initiatives worldwide provide policy support for bio-based monomers. Companies utilizing tartaric acid in polymer synthesis may qualify for green certification programs and sustainability incentives, enhancing market competitiveness.
Challenges remain in scaling sustainable production methods. Current extraction technologies require optimization to maximize yield while minimizing solvent use. Research into enzymatic and supercritical fluid extraction methods shows promise for further reducing the environmental impact of tartaric acid production, potentially improving its sustainability profile by an additional 15-20% according to preliminary studies.
Scalability and Industrial Production Considerations
The scalability of tartaric acid-based polymer synthesis processes represents a critical consideration for industrial implementation. Current laboratory-scale procedures utilizing tartaric acid as a chiral building block or catalyst often employ batch processing methods that face significant challenges when scaled to commercial production volumes. These challenges include maintaining reaction homogeneity, controlling heat transfer in larger vessels, and ensuring consistent product quality across batches.
Equipment modification requirements present another key consideration. Conventional polymer production equipment may require substantial adaptation to accommodate tartaric acid-based synthesis routes. Materials compatibility issues arise due to the acidic nature of tartaric acid, potentially necessitating specialized reactor linings or construction materials resistant to corrosion, which increases capital investment requirements.
Process economics significantly impact industrial viability. While tartaric acid offers advantages as a renewable, bio-derived feedstock, its current market price remains higher than many petroleum-derived alternatives. Economic analyses indicate that process optimization could potentially reduce production costs by 15-25% through improved catalyst recovery systems and reaction efficiency enhancements, bringing tartaric acid-based polymers closer to price parity with conventional materials.
Continuous flow processing presents a promising approach to overcome batch scaling limitations. Recent pilot studies demonstrate that microreactor and flow chemistry techniques can improve reaction control and product consistency while reducing solvent usage by up to 40%. These systems allow precise temperature control and mixing parameters critical for stereoselective reactions involving tartaric acid derivatives.
Purification and downstream processing constitute significant cost factors in industrial production. Traditional polymer purification methods may require modification when applied to tartaric acid-incorporated polymers due to their unique solubility profiles and functional group interactions. Membrane filtration technologies and advanced chromatographic techniques show promise for more efficient separation of tartaric acid-based polymers from reaction mixtures.
Regulatory considerations also impact industrial implementation. As a food-derived substance, tartaric acid offers potential advantages in regulatory approval pathways for certain applications. However, novel polymers incorporating tartaric acid may still require extensive toxicological and environmental impact assessments before large-scale production authorization, particularly for medical or food-contact applications.
Equipment modification requirements present another key consideration. Conventional polymer production equipment may require substantial adaptation to accommodate tartaric acid-based synthesis routes. Materials compatibility issues arise due to the acidic nature of tartaric acid, potentially necessitating specialized reactor linings or construction materials resistant to corrosion, which increases capital investment requirements.
Process economics significantly impact industrial viability. While tartaric acid offers advantages as a renewable, bio-derived feedstock, its current market price remains higher than many petroleum-derived alternatives. Economic analyses indicate that process optimization could potentially reduce production costs by 15-25% through improved catalyst recovery systems and reaction efficiency enhancements, bringing tartaric acid-based polymers closer to price parity with conventional materials.
Continuous flow processing presents a promising approach to overcome batch scaling limitations. Recent pilot studies demonstrate that microreactor and flow chemistry techniques can improve reaction control and product consistency while reducing solvent usage by up to 40%. These systems allow precise temperature control and mixing parameters critical for stereoselective reactions involving tartaric acid derivatives.
Purification and downstream processing constitute significant cost factors in industrial production. Traditional polymer purification methods may require modification when applied to tartaric acid-incorporated polymers due to their unique solubility profiles and functional group interactions. Membrane filtration technologies and advanced chromatographic techniques show promise for more efficient separation of tartaric acid-based polymers from reaction mixtures.
Regulatory considerations also impact industrial implementation. As a food-derived substance, tartaric acid offers potential advantages in regulatory approval pathways for certain applications. However, novel polymers incorporating tartaric acid may still require extensive toxicological and environmental impact assessments before large-scale production authorization, particularly for medical or food-contact applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!