How to Utilize the Unique Properties of Fluoroantimonic Acid?
JUN 20, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoroantimonic Acid Background and Objectives
Fluoroantimonic acid, a superacid composed of a mixture of hydrogen fluoride (HF) and antimony pentafluoride (SbF5), stands as one of the strongest known acids in the world. Its discovery and subsequent research have significantly advanced our understanding of superacidity and its potential applications in various fields of chemistry and industry.
The development of fluoroantimonic acid can be traced back to the early 20th century when researchers began exploring the properties of strong acids. However, it wasn't until the 1960s that the full potential of this superacid was realized. The groundbreaking work of George A. Olah in the field of superacids, including fluoroantimonic acid, earned him the Nobel Prize in Chemistry in 1994, highlighting the significance of this compound in the scientific community.
Fluoroantimonic acid's unique properties stem from its ability to protonate even very weak bases, making it an exceptionally powerful protonating agent. Its acidity is estimated to be more than a quadrillion times stronger than that of 100% sulfuric acid, placing it at the top of the acidity scale. This extreme acidity is attributed to the formation of the fluoroantimonite anion (SbF6-) in solution, which stabilizes the proton and enhances its acidity.
The technological evolution in the field of superacids has led to increased interest in harnessing the unique properties of fluoroantimonic acid. Current research aims to explore its potential in various applications, including catalysis, organic synthesis, and materials processing. The ability of fluoroantimonic acid to protonate hydrocarbons and initiate carbocation reactions opens up new possibilities in petrochemical processing and the development of novel synthetic pathways.
One of the primary objectives in the study of fluoroantimonic acid is to develop safer and more controlled methods for its handling and application. Due to its extreme reactivity and corrosive nature, conventional materials and containment methods are often inadequate. Researchers are exploring advanced materials and techniques to safely harness its power while minimizing risks associated with its use.
Another key goal is to expand the range of reactions and processes that can benefit from fluoroantimonic acid's unique properties. This includes investigating its potential in the activation of typically unreactive compounds, such as alkanes, and its use in the synthesis of novel materials with exceptional properties. The development of new catalytic systems based on fluoroantimonic acid is also a focus area, with potential applications in fine chemical synthesis and industrial-scale processes.
As we look to the future, the continued exploration of fluoroantimonic acid's properties and applications remains a priority in the field of superacid chemistry. The challenges and opportunities presented by this extraordinary compound drive ongoing research and innovation, promising to unlock new frontiers in chemical reactivity and materials science.
The development of fluoroantimonic acid can be traced back to the early 20th century when researchers began exploring the properties of strong acids. However, it wasn't until the 1960s that the full potential of this superacid was realized. The groundbreaking work of George A. Olah in the field of superacids, including fluoroantimonic acid, earned him the Nobel Prize in Chemistry in 1994, highlighting the significance of this compound in the scientific community.
Fluoroantimonic acid's unique properties stem from its ability to protonate even very weak bases, making it an exceptionally powerful protonating agent. Its acidity is estimated to be more than a quadrillion times stronger than that of 100% sulfuric acid, placing it at the top of the acidity scale. This extreme acidity is attributed to the formation of the fluoroantimonite anion (SbF6-) in solution, which stabilizes the proton and enhances its acidity.
The technological evolution in the field of superacids has led to increased interest in harnessing the unique properties of fluoroantimonic acid. Current research aims to explore its potential in various applications, including catalysis, organic synthesis, and materials processing. The ability of fluoroantimonic acid to protonate hydrocarbons and initiate carbocation reactions opens up new possibilities in petrochemical processing and the development of novel synthetic pathways.
One of the primary objectives in the study of fluoroantimonic acid is to develop safer and more controlled methods for its handling and application. Due to its extreme reactivity and corrosive nature, conventional materials and containment methods are often inadequate. Researchers are exploring advanced materials and techniques to safely harness its power while minimizing risks associated with its use.
Another key goal is to expand the range of reactions and processes that can benefit from fluoroantimonic acid's unique properties. This includes investigating its potential in the activation of typically unreactive compounds, such as alkanes, and its use in the synthesis of novel materials with exceptional properties. The development of new catalytic systems based on fluoroantimonic acid is also a focus area, with potential applications in fine chemical synthesis and industrial-scale processes.
As we look to the future, the continued exploration of fluoroantimonic acid's properties and applications remains a priority in the field of superacid chemistry. The challenges and opportunities presented by this extraordinary compound drive ongoing research and innovation, promising to unlock new frontiers in chemical reactivity and materials science.
Industrial Applications and Market Demand
Fluoroantimonic acid, known as the world's strongest superacid, has garnered significant attention in various industrial sectors due to its unique properties. The market demand for this powerful compound is driven by its exceptional ability to catalyze reactions and its potential applications in advanced materials processing.
In the petrochemical industry, fluoroantimonic acid has found a niche in the production of high-octane gasoline components. Its superacidic properties enable efficient isomerization of hydrocarbons, leading to improved fuel quality and performance. This application has seen steady growth as automotive manufacturers seek to meet increasingly stringent emissions standards and fuel efficiency requirements.
The electronics sector has also shown keen interest in fluoroantimonic acid for its potential in semiconductor manufacturing. Its ability to etch and clean surfaces at the nanoscale level makes it valuable in the production of advanced microchips and electronic components. As the demand for smaller, more powerful electronic devices continues to rise, the market for fluoroantimonic acid in this sector is expected to expand.
In the field of materials science, fluoroantimonic acid has opened new avenues for the synthesis of novel compounds and materials. Its extreme acidity allows for reactions that were previously impossible or impractical, leading to the development of advanced polymers, catalysts, and other high-performance materials. This has sparked interest from industries ranging from aerospace to renewable energy, where cutting-edge materials are crucial for innovation.
The pharmaceutical industry has begun exploring the use of fluoroantimonic acid in the synthesis of complex organic compounds. Its ability to facilitate certain reactions under milder conditions than traditional methods could potentially streamline drug manufacturing processes, reducing costs and improving efficiency.
However, the market demand for fluoroantimonic acid is tempered by significant challenges. Its extreme corrosiveness and reactivity necessitate specialized handling and storage equipment, which can be costly and limit its widespread adoption. Safety concerns and environmental regulations also play a crucial role in shaping market dynamics, as strict protocols must be followed in its use and disposal.
Despite these challenges, the unique properties of fluoroantimonic acid continue to drive research and development efforts across multiple industries. As new applications are discovered and existing processes are optimized, the market demand is expected to grow, albeit at a measured pace. The balance between the acid's extraordinary capabilities and the associated handling complexities will likely determine the trajectory of its industrial adoption and market expansion in the coming years.
In the petrochemical industry, fluoroantimonic acid has found a niche in the production of high-octane gasoline components. Its superacidic properties enable efficient isomerization of hydrocarbons, leading to improved fuel quality and performance. This application has seen steady growth as automotive manufacturers seek to meet increasingly stringent emissions standards and fuel efficiency requirements.
The electronics sector has also shown keen interest in fluoroantimonic acid for its potential in semiconductor manufacturing. Its ability to etch and clean surfaces at the nanoscale level makes it valuable in the production of advanced microchips and electronic components. As the demand for smaller, more powerful electronic devices continues to rise, the market for fluoroantimonic acid in this sector is expected to expand.
In the field of materials science, fluoroantimonic acid has opened new avenues for the synthesis of novel compounds and materials. Its extreme acidity allows for reactions that were previously impossible or impractical, leading to the development of advanced polymers, catalysts, and other high-performance materials. This has sparked interest from industries ranging from aerospace to renewable energy, where cutting-edge materials are crucial for innovation.
The pharmaceutical industry has begun exploring the use of fluoroantimonic acid in the synthesis of complex organic compounds. Its ability to facilitate certain reactions under milder conditions than traditional methods could potentially streamline drug manufacturing processes, reducing costs and improving efficiency.
However, the market demand for fluoroantimonic acid is tempered by significant challenges. Its extreme corrosiveness and reactivity necessitate specialized handling and storage equipment, which can be costly and limit its widespread adoption. Safety concerns and environmental regulations also play a crucial role in shaping market dynamics, as strict protocols must be followed in its use and disposal.
Despite these challenges, the unique properties of fluoroantimonic acid continue to drive research and development efforts across multiple industries. As new applications are discovered and existing processes are optimized, the market demand is expected to grow, albeit at a measured pace. The balance between the acid's extraordinary capabilities and the associated handling complexities will likely determine the trajectory of its industrial adoption and market expansion in the coming years.
Current Challenges in Handling and Utilization
The utilization of fluoroantimonic acid presents significant challenges due to its extreme reactivity and corrosive nature. As the strongest known superacid, it poses severe handling and storage difficulties. The acid's ability to protonate nearly all organic compounds makes it exceptionally dangerous to work with, requiring specialized equipment and rigorous safety protocols.
One of the primary challenges is containment. Fluoroantimonic acid reacts violently with water and most common materials, including glass and many metals. This necessitates the use of specially designed containers made from highly resistant materials such as Teflon or certain fluoropolymers. Even with these precautions, long-term storage remains problematic due to the acid's tendency to degrade even the most resistant materials over time.
The extreme reactivity of fluoroantimonic acid also complicates its transportation and on-site handling. Any exposure to moisture or organic matter can lead to explosive reactions, making it challenging to transfer the acid between containers or integrate it into industrial processes. This reactivity also limits its practical applications, as many potential uses are hindered by the difficulty of controlling its reactions in a production environment.
Another significant challenge lies in the precise measurement and dosing of fluoroantimonic acid. Its extreme acidity makes it difficult to accurately determine concentrations using conventional methods, and its reactivity can lead to rapid changes in composition. This unpredictability complicates its use in controlled chemical reactions and industrial processes where precise quantities are crucial.
The environmental and safety concerns associated with fluoroantimonic acid further complicate its utilization. Its potential for causing severe burns and tissue damage necessitates extensive personal protective equipment and specialized handling procedures. Moreover, the acid's environmental impact is severe, requiring stringent disposal protocols and presenting significant risks in case of accidental release.
Research into safer handling methods and more stable formulations of fluoroantimonic acid is ongoing, but progress is slow due to the inherent dangers involved. Some promising avenues include the development of novel containment materials and the exploration of less reactive analogues that retain some of the acid's unique properties. However, these solutions are still in early stages and have not yet overcome the fundamental challenges of working with this extraordinarily potent substance.
One of the primary challenges is containment. Fluoroantimonic acid reacts violently with water and most common materials, including glass and many metals. This necessitates the use of specially designed containers made from highly resistant materials such as Teflon or certain fluoropolymers. Even with these precautions, long-term storage remains problematic due to the acid's tendency to degrade even the most resistant materials over time.
The extreme reactivity of fluoroantimonic acid also complicates its transportation and on-site handling. Any exposure to moisture or organic matter can lead to explosive reactions, making it challenging to transfer the acid between containers or integrate it into industrial processes. This reactivity also limits its practical applications, as many potential uses are hindered by the difficulty of controlling its reactions in a production environment.
Another significant challenge lies in the precise measurement and dosing of fluoroantimonic acid. Its extreme acidity makes it difficult to accurately determine concentrations using conventional methods, and its reactivity can lead to rapid changes in composition. This unpredictability complicates its use in controlled chemical reactions and industrial processes where precise quantities are crucial.
The environmental and safety concerns associated with fluoroantimonic acid further complicate its utilization. Its potential for causing severe burns and tissue damage necessitates extensive personal protective equipment and specialized handling procedures. Moreover, the acid's environmental impact is severe, requiring stringent disposal protocols and presenting significant risks in case of accidental release.
Research into safer handling methods and more stable formulations of fluoroantimonic acid is ongoing, but progress is slow due to the inherent dangers involved. Some promising avenues include the development of novel containment materials and the exploration of less reactive analogues that retain some of the acid's unique properties. However, these solutions are still in early stages and have not yet overcome the fundamental challenges of working with this extraordinarily potent substance.
Existing Utilization Methods and Safety Protocols
01 Synthesis and production of fluoroantimonic acid
Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.- Synthesis and production of fluoroantimonic acid: Fluoroantimonic acid is synthesized through the reaction of hydrogen fluoride and antimony pentafluoride. The production process involves careful handling of highly reactive and corrosive materials under controlled conditions. Various methods and apparatus have been developed to optimize the synthesis and ensure the purity of the final product.
- Applications in organic synthesis and catalysis: Fluoroantimonic acid is utilized as a powerful superacid catalyst in various organic synthesis reactions. It facilitates alkylation, isomerization, and polymerization processes. The acid's extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acid catalysts, making it valuable in the production of certain chemicals and materials.
- Use in materials science and surface treatment: Fluoroantimonic acid finds applications in materials science, particularly in surface treatment and modification of various substrates. It can be used to etch or activate surfaces, create specialized coatings, or modify the properties of materials. The acid's unique properties make it suitable for generating highly reactive surfaces or initiating specific chemical transformations on material surfaces.
- Safety and handling considerations: Due to its extreme corrosiveness and reactivity, handling fluoroantimonic acid requires stringent safety measures. Specialized equipment, containment systems, and personal protective gear are necessary when working with this superacid. Proper storage, transportation, and disposal protocols must be followed to prevent accidents and environmental contamination.
- Analytical and characterization methods: Various analytical techniques have been developed to characterize fluoroantimonic acid and its reactions. These methods include spectroscopic analyses, electrochemical measurements, and specialized titration procedures. Advanced instrumentation and methodologies are employed to study the properties, composition, and behavior of this superacid in different chemical environments.
02 Applications in organic synthesis and catalysis
Fluoroantimonic acid is utilized as a powerful superacid catalyst in various organic synthesis reactions. It facilitates alkylation, isomerization, and polymerization processes. The acid's extreme acidity enables it to catalyze reactions that are difficult or impossible with conventional acids, making it valuable in the production of specialty chemicals and advanced materials.Expand Specific Solutions03 Use in materials science and surface treatment
Fluoroantimonic acid finds applications in materials science, particularly in surface treatment and modification of various substrates. It is used for etching, cleaning, and activating surfaces of metals, semiconductors, and other materials. The acid's unique properties allow for precise control of surface characteristics, enhancing material performance in specific applications.Expand Specific Solutions04 Safety and handling considerations
Due to its extreme corrosiveness and reactivity, handling fluoroantimonic acid requires stringent safety measures. Specialized equipment, containment systems, and personal protective gear are essential when working with this superacid. Proper storage, transportation, and disposal protocols have been developed to minimize risks associated with its use in industrial and research settings.Expand Specific Solutions05 Analytical and characterization techniques
Various analytical and characterization techniques have been developed to study fluoroantimonic acid and its reactions. These include spectroscopic methods, electrochemical analyses, and computational modeling. Such techniques are crucial for understanding the acid's behavior, optimizing its use in different applications, and developing new methodologies for its production and utilization.Expand Specific Solutions
Key Players in Superacid Research and Production
The utilization of fluoroantimonic acid's unique properties is an emerging field in advanced materials and chemical processing. The industry is in its early development stage, with a relatively small but growing market size. The technology's maturity is still evolving, with ongoing research and development efforts. Key players like DuPont de Nemours, Inc., Merck Sharp & Dohme Corp., and DAIKIN INDUSTRIES Ltd. are at the forefront of innovation. Academic institutions such as Central South University, University of California, and Emory University are contributing significantly to fundamental research. Government agencies and multinational corporations are also investing in exploring potential applications, indicating a competitive landscape with diverse stakeholders from academia, industry, and public sectors.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a proprietary process for the safe handling and application of fluoroantimonic acid in industrial settings. Their approach involves using specialized containment systems made from highly resistant materials such as fluoropolymers. They have also created a method for controlled dilution of the acid to reduce its corrosiveness while maintaining its superacid properties for specific chemical reactions. DuPont's technology allows for the use of fluoroantimonic acid in the production of high-performance polymers and as a catalyst in certain petrochemical processes.
Strengths: Expertise in handling superacids, advanced containment technology, and applications in polymer science. Weaknesses: High cost of implementation and strict safety requirements limit widespread use.
DAIKIN INDUSTRIES Ltd.
Technical Solution: DAIKIN has developed a novel approach to utilizing fluoroantimonic acid in the production of advanced fluoropolymers. Their method involves a controlled reaction environment where the superacid is used to initiate polymerization of fluorine-containing monomers. This process results in polymers with exceptional chemical resistance and thermal stability. DAIKIN has also engineered a recycling system for the acid, minimizing waste and environmental impact. Additionally, they have explored the use of fluoroantimonic acid as an etching agent for specialized glass and ceramic materials in the semiconductor industry.
Strengths: Innovative applications in fluoropolymer synthesis and semiconductor processing. Weaknesses: Limited to specific industrial applications, high operational costs.
Innovative Applications in Catalysis and Synthesis
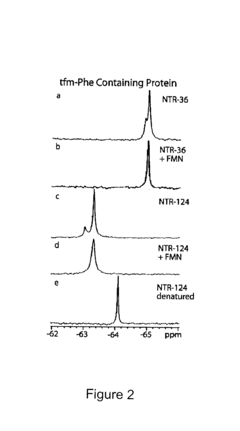
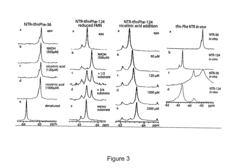
Site-specific incorporation of fluorinated amino acids into proteins
PatentActiveUS20070218483A1
Innovation
- Development of orthogonal pairs of aminoacyl-tRNA synthetase and tRNA that function independently of the endogenous translation system, allowing for the specific incorporation of fluorinated amino acids into proteins using selector codons, ensuring high efficiency and minimal structural disruption.
Diagnostic agents for amyloid beta imaging
PatentWO2011141515A1
Innovation
- Development of novel fluorine-18 labeled compounds, specifically styrylpyridine derivatives, which exhibit high brain uptake and rapid washout, enabling high contrast imaging of amyloid beta aggregates through optimized brain pharmacokinetics and specific binding.
Environmental Impact and Waste Management
Fluoroantimonic acid, known as the world's strongest superacid, poses significant environmental challenges due to its extreme reactivity and corrosive nature. The utilization of this powerful compound necessitates stringent environmental impact assessments and robust waste management strategies to mitigate potential risks to ecosystems and human health.
The environmental impact of fluoroantimonic acid primarily stems from its ability to react violently with water, producing highly toxic and corrosive hydrogen fluoride gas. This reaction can lead to severe air pollution, soil contamination, and water pollution if not properly contained. The acid's extreme acidity can cause rapid degradation of organic matter, disrupting soil ecosystems and potentially entering food chains.
To address these environmental concerns, specialized containment and handling protocols are essential. All processes involving fluoroantimonic acid must be conducted in sealed, moisture-free environments with multiple layers of containment to prevent accidental releases. Advanced air filtration systems and scrubbers are required to capture and neutralize any potential emissions or vapors.
Waste management for fluoroantimonic acid requires a comprehensive approach. Neutralization is the primary method for treating waste, typically involving careful dilution with large volumes of cold water or ice, followed by neutralization with bases such as sodium hydroxide or calcium oxide. This process must be conducted in specially designed facilities equipped to handle the heat and gases generated during neutralization.
Recycling and recovery strategies for fluoroantimonic acid and its byproducts are limited due to the compound's extreme reactivity. However, research into advanced separation techniques and catalytic processes may offer potential avenues for recovering valuable components, such as antimony and fluorine, from waste streams.
Long-term storage and disposal of neutralized waste products require careful consideration. Specialized landfills with multiple protective layers and ongoing monitoring systems are necessary to prevent any potential leaching or environmental contamination. Additionally, the development of innovative solidification and stabilization techniques can enhance the safety of long-term storage.
Environmental monitoring programs are crucial for assessing the potential impact of fluoroantimonic acid use on surrounding ecosystems. These programs should include regular soil, water, and air quality testing, as well as biomonitoring of local flora and fauna to detect any signs of contamination or ecological disturbance.
Research into alternative compounds or processes that can achieve similar results with reduced environmental impact is ongoing. This includes exploring less hazardous superacids or developing novel catalytic systems that can replicate the unique properties of fluoroantimonic acid without its associated environmental risks.
The environmental impact of fluoroantimonic acid primarily stems from its ability to react violently with water, producing highly toxic and corrosive hydrogen fluoride gas. This reaction can lead to severe air pollution, soil contamination, and water pollution if not properly contained. The acid's extreme acidity can cause rapid degradation of organic matter, disrupting soil ecosystems and potentially entering food chains.
To address these environmental concerns, specialized containment and handling protocols are essential. All processes involving fluoroantimonic acid must be conducted in sealed, moisture-free environments with multiple layers of containment to prevent accidental releases. Advanced air filtration systems and scrubbers are required to capture and neutralize any potential emissions or vapors.
Waste management for fluoroantimonic acid requires a comprehensive approach. Neutralization is the primary method for treating waste, typically involving careful dilution with large volumes of cold water or ice, followed by neutralization with bases such as sodium hydroxide or calcium oxide. This process must be conducted in specially designed facilities equipped to handle the heat and gases generated during neutralization.
Recycling and recovery strategies for fluoroantimonic acid and its byproducts are limited due to the compound's extreme reactivity. However, research into advanced separation techniques and catalytic processes may offer potential avenues for recovering valuable components, such as antimony and fluorine, from waste streams.
Long-term storage and disposal of neutralized waste products require careful consideration. Specialized landfills with multiple protective layers and ongoing monitoring systems are necessary to prevent any potential leaching or environmental contamination. Additionally, the development of innovative solidification and stabilization techniques can enhance the safety of long-term storage.
Environmental monitoring programs are crucial for assessing the potential impact of fluoroantimonic acid use on surrounding ecosystems. These programs should include regular soil, water, and air quality testing, as well as biomonitoring of local flora and fauna to detect any signs of contamination or ecological disturbance.
Research into alternative compounds or processes that can achieve similar results with reduced environmental impact is ongoing. This includes exploring less hazardous superacids or developing novel catalytic systems that can replicate the unique properties of fluoroantimonic acid without its associated environmental risks.
Regulatory Framework for Superacid Usage
The regulatory framework for superacid usage, particularly concerning fluoroantimonic acid, is a complex and critical aspect of its utilization. Given the extreme corrosiveness and reactivity of this superacid, stringent regulations are in place to ensure safe handling, storage, and disposal. The Occupational Safety and Health Administration (OSHA) in the United States has established specific guidelines for working with highly hazardous chemicals, including fluoroantimonic acid. These regulations mandate the implementation of process safety management programs, which include detailed operating procedures, employee training, and emergency response plans.
Environmental protection agencies worldwide have also imposed strict controls on the use and disposal of superacids. The Environmental Protection Agency (EPA) in the U.S. classifies fluoroantimonic acid as a hazardous substance under the Resource Conservation and Recovery Act (RCRA). This classification necessitates special handling and disposal procedures to prevent environmental contamination. Companies using fluoroantimonic acid must comply with rigorous waste management protocols and obtain necessary permits for its storage and transportation.
International agreements, such as the Rotterdam Convention on the Prior Informed Consent Procedure for Certain Hazardous Chemicals and Pesticides in International Trade, further regulate the global movement of superacids. These agreements ensure that countries are informed about the potential risks associated with importing such substances and can make informed decisions regarding their use within their borders.
In the European Union, the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation governs the use of fluoroantimonic acid and other hazardous substances. REACH requires manufacturers and importers to register chemicals and provide detailed safety information, including potential risks and appropriate risk management measures. This regulatory framework aims to protect human health and the environment while promoting innovation in the chemical industry.
Research institutions and industrial facilities working with fluoroantimonic acid must adhere to strict laboratory safety standards. These standards often include the use of specialized containment systems, personal protective equipment, and rigorous safety protocols. Regular safety audits and inspections are typically required to ensure ongoing compliance with these regulations.
The transportation of fluoroantimonic acid is subject to international regulations such as the United Nations Recommendations on the Transport of Dangerous Goods. These guidelines specify packaging requirements, labeling standards, and documentation procedures for the safe movement of hazardous materials across borders. Compliance with these regulations is essential for companies involved in the production, distribution, or use of fluoroantimonic acid in global supply chains.
Environmental protection agencies worldwide have also imposed strict controls on the use and disposal of superacids. The Environmental Protection Agency (EPA) in the U.S. classifies fluoroantimonic acid as a hazardous substance under the Resource Conservation and Recovery Act (RCRA). This classification necessitates special handling and disposal procedures to prevent environmental contamination. Companies using fluoroantimonic acid must comply with rigorous waste management protocols and obtain necessary permits for its storage and transportation.
International agreements, such as the Rotterdam Convention on the Prior Informed Consent Procedure for Certain Hazardous Chemicals and Pesticides in International Trade, further regulate the global movement of superacids. These agreements ensure that countries are informed about the potential risks associated with importing such substances and can make informed decisions regarding their use within their borders.
In the European Union, the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation governs the use of fluoroantimonic acid and other hazardous substances. REACH requires manufacturers and importers to register chemicals and provide detailed safety information, including potential risks and appropriate risk management measures. This regulatory framework aims to protect human health and the environment while promoting innovation in the chemical industry.
Research institutions and industrial facilities working with fluoroantimonic acid must adhere to strict laboratory safety standards. These standards often include the use of specialized containment systems, personal protective equipment, and rigorous safety protocols. Regular safety audits and inspections are typically required to ensure ongoing compliance with these regulations.
The transportation of fluoroantimonic acid is subject to international regulations such as the United Nations Recommendations on the Transport of Dangerous Goods. These guidelines specify packaging requirements, labeling standards, and documentation procedures for the safe movement of hazardous materials across borders. Compliance with these regulations is essential for companies involved in the production, distribution, or use of fluoroantimonic acid in global supply chains.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!