How to Verify Carbon Tetrachloride's Chemical Contributions?
JUL 3, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Analysis Background and Objectives
Carbon tetrachloride (CCl4) has been a subject of significant scientific and environmental interest for decades. This compound, once widely used in various industrial and consumer applications, has become a focal point for research due to its potential environmental impacts and chemical properties. The evolution of CCl4 analysis techniques has been driven by the need to understand its behavior in different environments and its role in atmospheric chemistry.
The primary objective of this technical research is to explore and evaluate methods for verifying carbon tetrachloride's chemical contributions in various contexts. This encompasses not only the detection and quantification of CCl4 but also the assessment of its chemical interactions and transformations in different environmental matrices. Understanding these aspects is crucial for developing effective strategies to mitigate its environmental impact and for advancing our knowledge of atmospheric chemistry.
The development of CCl4 analysis techniques has progressed significantly over the years, moving from basic chemical tests to sophisticated spectroscopic and chromatographic methods. Early detection methods relied on simple chemical reactions, but these lacked the sensitivity and specificity required for trace analysis in complex environmental samples. The advent of gas chromatography coupled with mass spectrometry (GC-MS) marked a significant milestone, enabling highly sensitive and specific detection of CCl4 in various matrices.
Recent technological advancements have further expanded our capabilities in CCl4 analysis. High-resolution mass spectrometry, for instance, has allowed for the differentiation of CCl4 from other similar compounds and the detection of its degradation products. Additionally, remote sensing technologies have been developed to monitor CCl4 concentrations in the atmosphere on a global scale, providing valuable data for climate models and environmental assessments.
The ongoing research in this field aims to address several key challenges. These include improving the sensitivity and accuracy of CCl4 detection in complex environmental samples, developing real-time monitoring systems for atmospheric CCl4, and enhancing our understanding of CCl4's chemical fate in different environmental compartments. There is also a growing focus on developing methods to trace the sources of CCl4 emissions, which is crucial for enforcing regulations and developing mitigation strategies.
As we look towards the future, the goals of CCl4 analysis are expanding beyond mere detection and quantification. There is an increasing emphasis on understanding the chemical pathways through which CCl4 interacts with other atmospheric constituents, its role in stratospheric ozone depletion, and its potential impacts on global climate. This broader perspective necessitates the development of more sophisticated analytical techniques and modeling approaches to capture the full complexity of CCl4's environmental behavior.
The primary objective of this technical research is to explore and evaluate methods for verifying carbon tetrachloride's chemical contributions in various contexts. This encompasses not only the detection and quantification of CCl4 but also the assessment of its chemical interactions and transformations in different environmental matrices. Understanding these aspects is crucial for developing effective strategies to mitigate its environmental impact and for advancing our knowledge of atmospheric chemistry.
The development of CCl4 analysis techniques has progressed significantly over the years, moving from basic chemical tests to sophisticated spectroscopic and chromatographic methods. Early detection methods relied on simple chemical reactions, but these lacked the sensitivity and specificity required for trace analysis in complex environmental samples. The advent of gas chromatography coupled with mass spectrometry (GC-MS) marked a significant milestone, enabling highly sensitive and specific detection of CCl4 in various matrices.
Recent technological advancements have further expanded our capabilities in CCl4 analysis. High-resolution mass spectrometry, for instance, has allowed for the differentiation of CCl4 from other similar compounds and the detection of its degradation products. Additionally, remote sensing technologies have been developed to monitor CCl4 concentrations in the atmosphere on a global scale, providing valuable data for climate models and environmental assessments.
The ongoing research in this field aims to address several key challenges. These include improving the sensitivity and accuracy of CCl4 detection in complex environmental samples, developing real-time monitoring systems for atmospheric CCl4, and enhancing our understanding of CCl4's chemical fate in different environmental compartments. There is also a growing focus on developing methods to trace the sources of CCl4 emissions, which is crucial for enforcing regulations and developing mitigation strategies.
As we look towards the future, the goals of CCl4 analysis are expanding beyond mere detection and quantification. There is an increasing emphasis on understanding the chemical pathways through which CCl4 interacts with other atmospheric constituents, its role in stratospheric ozone depletion, and its potential impacts on global climate. This broader perspective necessitates the development of more sophisticated analytical techniques and modeling approaches to capture the full complexity of CCl4's environmental behavior.
Market Demand for CCl4 Verification
The market demand for Carbon Tetrachloride (CCl4) verification has been steadily growing due to increasing environmental concerns and regulatory pressures. CCl4, once widely used in various industrial applications, has been identified as a potent ozone-depleting substance and a potential carcinogen. This has led to strict regulations on its production, use, and disposal, creating a significant need for accurate verification methods.
The primary drivers of this market demand are environmental protection agencies, chemical manufacturers, and industries that historically used CCl4. These stakeholders require reliable methods to detect and quantify CCl4 in various matrices, including air, water, soil, and industrial products. The verification process is crucial for ensuring compliance with international agreements such as the Montreal Protocol, which aims to phase out ozone-depleting substances.
In the industrial sector, there is a growing need for CCl4 verification in quality control processes. Companies that have transitioned away from CCl4 use need to confirm the absence of this compound in their products and manufacturing processes. This is particularly important in industries such as dry cleaning, fire extinguishers, and certain chemical synthesis processes where CCl4 was previously common.
Environmental monitoring agencies represent another significant market segment. These organizations require advanced verification techniques to assess CCl4 levels in the atmosphere, groundwater, and soil. The data collected is essential for tracking the effectiveness of CCl4 phase-out programs and identifying potential sources of illegal production or use.
The research and development sector also contributes to the market demand for CCl4 verification. Scientists studying atmospheric chemistry, climate change, and environmental toxicology need precise methods to measure CCl4 concentrations in various environmental samples. This research is crucial for understanding the long-term impacts of CCl4 on the ozone layer and global climate systems.
Furthermore, the remediation industry has emerged as a key player in driving demand for CCl4 verification technologies. As efforts to clean up contaminated sites intensify, there is a growing need for accurate methods to assess CCl4 levels before, during, and after remediation processes. This ensures the effectiveness of cleanup efforts and compliance with environmental standards.
The global nature of CCl4 regulations has also expanded the geographical scope of the verification market. Developing countries, in particular, are seeing an increased demand for CCl4 verification as they align their environmental policies with international standards. This has created opportunities for technology transfer and capacity building in CCl4 detection and quantification methods.
The primary drivers of this market demand are environmental protection agencies, chemical manufacturers, and industries that historically used CCl4. These stakeholders require reliable methods to detect and quantify CCl4 in various matrices, including air, water, soil, and industrial products. The verification process is crucial for ensuring compliance with international agreements such as the Montreal Protocol, which aims to phase out ozone-depleting substances.
In the industrial sector, there is a growing need for CCl4 verification in quality control processes. Companies that have transitioned away from CCl4 use need to confirm the absence of this compound in their products and manufacturing processes. This is particularly important in industries such as dry cleaning, fire extinguishers, and certain chemical synthesis processes where CCl4 was previously common.
Environmental monitoring agencies represent another significant market segment. These organizations require advanced verification techniques to assess CCl4 levels in the atmosphere, groundwater, and soil. The data collected is essential for tracking the effectiveness of CCl4 phase-out programs and identifying potential sources of illegal production or use.
The research and development sector also contributes to the market demand for CCl4 verification. Scientists studying atmospheric chemistry, climate change, and environmental toxicology need precise methods to measure CCl4 concentrations in various environmental samples. This research is crucial for understanding the long-term impacts of CCl4 on the ozone layer and global climate systems.
Furthermore, the remediation industry has emerged as a key player in driving demand for CCl4 verification technologies. As efforts to clean up contaminated sites intensify, there is a growing need for accurate methods to assess CCl4 levels before, during, and after remediation processes. This ensures the effectiveness of cleanup efforts and compliance with environmental standards.
The global nature of CCl4 regulations has also expanded the geographical scope of the verification market. Developing countries, in particular, are seeing an increased demand for CCl4 verification as they align their environmental policies with international standards. This has created opportunities for technology transfer and capacity building in CCl4 detection and quantification methods.
Current Challenges in CCl4 Detection
The detection of carbon tetrachloride (CCl4) presents several significant challenges in contemporary analytical chemistry. One of the primary difficulties lies in the compound's volatility and low concentration in environmental samples. CCl4 readily evaporates at room temperature, making it challenging to capture and analyze accurately, especially in field conditions.
Another major hurdle is the potential for interference from other halogenated compounds. Many environmental samples contain a complex mixture of chlorinated hydrocarbons, which can have similar chemical properties to CCl4. This similarity often leads to false positives or inaccurate quantification, necessitating highly selective detection methods.
The stability of CCl4 under various environmental conditions also poses a challenge. In certain matrices, such as soil or water, CCl4 can undergo degradation or transformation, altering its chemical signature. This instability complicates long-term monitoring efforts and can lead to underestimation of CCl4 levels in historical samples.
Sensitivity is another critical issue in CCl4 detection. Environmental and health regulations often require the measurement of CCl4 at very low concentrations, sometimes in the parts per billion (ppb) range. Achieving such high sensitivity while maintaining accuracy and precision demands sophisticated analytical techniques and instrumentation.
The need for rapid, on-site detection methods further compounds these challenges. Traditional laboratory-based analyses, while accurate, are often time-consuming and not suitable for immediate decision-making in environmental monitoring or industrial safety applications. Developing portable, reliable, and user-friendly detection systems that can provide real-time data remains an ongoing challenge.
Additionally, the matrix effect in complex samples can significantly impact CCl4 detection. Soil, water, and air samples often contain numerous compounds that can interfere with the analysis, either by masking the CCl4 signal or by causing matrix-induced ionization suppression in mass spectrometry-based methods.
Lastly, the calibration and standardization of CCl4 detection methods across different laboratories and analytical platforms present ongoing challenges. Ensuring consistency and comparability of results is crucial for regulatory compliance and scientific research, yet it remains difficult due to variations in sample preparation, instrumental parameters, and data interpretation methodologies.
Another major hurdle is the potential for interference from other halogenated compounds. Many environmental samples contain a complex mixture of chlorinated hydrocarbons, which can have similar chemical properties to CCl4. This similarity often leads to false positives or inaccurate quantification, necessitating highly selective detection methods.
The stability of CCl4 under various environmental conditions also poses a challenge. In certain matrices, such as soil or water, CCl4 can undergo degradation or transformation, altering its chemical signature. This instability complicates long-term monitoring efforts and can lead to underestimation of CCl4 levels in historical samples.
Sensitivity is another critical issue in CCl4 detection. Environmental and health regulations often require the measurement of CCl4 at very low concentrations, sometimes in the parts per billion (ppb) range. Achieving such high sensitivity while maintaining accuracy and precision demands sophisticated analytical techniques and instrumentation.
The need for rapid, on-site detection methods further compounds these challenges. Traditional laboratory-based analyses, while accurate, are often time-consuming and not suitable for immediate decision-making in environmental monitoring or industrial safety applications. Developing portable, reliable, and user-friendly detection systems that can provide real-time data remains an ongoing challenge.
Additionally, the matrix effect in complex samples can significantly impact CCl4 detection. Soil, water, and air samples often contain numerous compounds that can interfere with the analysis, either by masking the CCl4 signal or by causing matrix-induced ionization suppression in mass spectrometry-based methods.
Lastly, the calibration and standardization of CCl4 detection methods across different laboratories and analytical platforms present ongoing challenges. Ensuring consistency and comparability of results is crucial for regulatory compliance and scientific research, yet it remains difficult due to variations in sample preparation, instrumental parameters, and data interpretation methodologies.
Existing CCl4 Verification Methods
01 Synthesis and production methods
Carbon tetrachloride is synthesized through various chemical processes, including chlorination of methane or carbon disulfide. These methods involve specific reaction conditions and catalysts to optimize yield and purity. Improvements in production techniques focus on increasing efficiency and reducing environmental impact.- Synthesis and production methods: Carbon tetrachloride is synthesized through various chemical processes, including chlorination of methane or carbon disulfide. These methods involve specific reaction conditions and catalysts to optimize yield and purity. Improvements in production techniques have led to more efficient and environmentally friendly manufacturing processes.
- Applications in chemical industry: Carbon tetrachloride has been widely used in the chemical industry as a solvent, cleaning agent, and raw material for the production of other chemicals. It has played a significant role in various industrial processes, including the manufacture of refrigerants, pesticides, and pharmaceuticals.
- Environmental impact and regulations: Due to its ozone-depleting properties and potential health hazards, the use of carbon tetrachloride has been heavily regulated and phased out in many applications. Research has focused on developing alternatives and mitigating its environmental impact, leading to the implementation of strict control measures and disposal protocols.
- Analytical and detection methods: Various analytical techniques have been developed to detect and quantify carbon tetrachloride in environmental samples and industrial products. These methods include gas chromatography, mass spectrometry, and spectrophotometric analysis, which have improved the accuracy and sensitivity of carbon tetrachloride detection.
- Remediation and treatment technologies: Efforts have been made to develop technologies for the remediation of carbon tetrachloride contamination in soil and groundwater. These include chemical reduction, bioremediation, and advanced oxidation processes. Research has focused on improving the efficiency and cost-effectiveness of these treatment methods.
02 Applications in chemical industry
Carbon tetrachloride serves as a versatile solvent and reagent in numerous chemical processes. It is used in the production of chlorofluorocarbons, as a cleaning agent for industrial equipment, and as a raw material for various organic syntheses. Its unique properties make it valuable in specific industrial applications.Expand Specific Solutions03 Environmental impact and regulations
Due to its ozone-depleting properties and potential health hazards, the use of carbon tetrachloride has been heavily regulated. Research focuses on developing alternatives and mitigating its environmental impact. Efforts are made to improve detection methods and reduce emissions in industrial processes where its use is still necessary.Expand Specific Solutions04 Analytical and detection methods
Advancements in analytical techniques for detecting and quantifying carbon tetrachloride in various matrices have been developed. These methods include improved spectroscopic techniques, chromatography, and sensor technologies. Such developments are crucial for environmental monitoring and ensuring compliance with regulations.Expand Specific Solutions05 Remediation and treatment technologies
Research on remediation techniques for carbon tetrachloride contamination in soil and groundwater has led to innovative treatment methods. These include advanced oxidation processes, bioremediation strategies, and novel adsorbent materials. Such technologies aim to efficiently remove or degrade carbon tetrachloride in contaminated sites.Expand Specific Solutions
Key Players in Chemical Analysis Industry
The carbon tetrachloride market is in a mature phase, with a relatively stable global demand. The market size is estimated to be in the range of several hundred million dollars annually. Technologically, carbon tetrachloride production is well-established, with key players like Occidental Chemical Corp., DuPont de Nemours, Inc., and Tokuyama Corp. having significant expertise. However, due to environmental concerns and regulations, there's a shift towards developing alternatives and more sustainable production methods. Research institutions like Central South University and the University of Copenhagen are likely contributing to these efforts, focusing on reducing environmental impact and improving efficiency in carbon tetrachloride's chemical applications.
DuPont de Nemours, Inc.
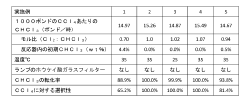
Technical Solution: DuPont employs advanced spectroscopic techniques, including Fourier Transform Infrared (FTIR) spectroscopy and Gas Chromatography-Mass Spectrometry (GC-MS), to verify carbon tetrachloride's chemical contributions. Their method involves precise sample preparation and analysis under controlled conditions. The FTIR analysis identifies characteristic absorption bands of CCl4 at around 790 cm^-1 and 460 cm^-1 [1], while GC-MS provides detailed molecular fragmentation patterns. DuPont's approach also incorporates isotope ratio mass spectrometry (IRMS) to distinguish between natural and synthetic sources of carbon tetrachloride, enhancing the accuracy of source attribution in environmental samples [2].
Strengths: High precision and reliability in identifying CCl4, capability to distinguish between sources. Weaknesses: Requires sophisticated equipment and expertise, potentially time-consuming for large-scale analyses.
Shimadzu Corp.
Technical Solution: Shimadzu has developed a comprehensive analytical system for verifying carbon tetrachloride's chemical contributions using their advanced GCMS-TQ8050 NX triple quadrupole gas chromatograph mass spectrometer. This system employs multiple reaction monitoring (MRM) for highly selective and sensitive detection of CCl4 in complex matrices. The method includes a specialized sample introduction technique that minimizes degradation of the analyte. Shimadzu's approach can detect CCl4 at ultra-trace levels (parts per trillion) in various environmental and industrial samples [3]. The company also integrates their LabSolutions software for data analysis, which includes advanced algorithms for peak deconvolution and quantification, ensuring accurate results even in the presence of interfering compounds [4].
Strengths: Extremely high sensitivity and selectivity, capable of ultra-trace analysis. Weaknesses: High initial investment cost, requires specialized training for operation and data interpretation.
Innovative CCl4 Detection Technologies
Method for testing carbon tetrachloride-like toxicity that chemical substance has
PatentInactiveJP2010252684A
Innovation
- A method utilizing specific genes with registered nucleotide sequences, such as those listed in GenBank, to measure and compare expression levels in mammalian specimens exposed to chemical substances, allowing for the evaluation of carbon tetrachloride-like toxicity by measuring the difference in gene expression levels.
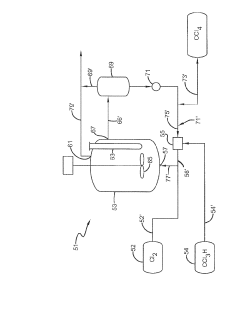
Producing carbon tetrachloride by photochlorination of chloroform
PatentActiveJP2024069268A
Innovation
- A method involving the photochlorination of chloroform with chlorine in the presence of electromagnetic radiation, maintaining a low concentration of chloroform and a stoichiometric concentration of chlorine, to produce carbon tetrachloride with high selectivity and minimize the formation of hexachloroethane.
Environmental Impact of CCl4
Carbon tetrachloride (CCl4) has been recognized as a significant environmental pollutant with far-reaching impacts on the Earth's ecosystems. This compound, once widely used in various industrial applications, has been phased out in many countries due to its detrimental effects on the environment, particularly the ozone layer.
The primary environmental concern associated with CCl4 is its role in ozone depletion. When released into the atmosphere, CCl4 molecules can reach the stratosphere, where they are broken down by ultraviolet radiation. This process releases chlorine atoms, which catalyze the destruction of ozone molecules. The ozone layer plays a crucial role in protecting life on Earth from harmful ultraviolet radiation, and its depletion can lead to increased risks of skin cancer, cataracts, and damage to marine ecosystems.
In addition to its impact on the ozone layer, CCl4 contributes to global warming. It is a potent greenhouse gas with a global warming potential approximately 1,400 times that of carbon dioxide over a 100-year period. This means that even small emissions of CCl4 can have a disproportionate effect on climate change compared to more abundant greenhouse gases.
CCl4 also poses risks to terrestrial and aquatic ecosystems. When released into soil or water, it can persist for long periods due to its chemical stability. This persistence allows CCl4 to accumulate in the environment and potentially enter food chains. In aquatic systems, CCl4 can be toxic to various organisms, including fish and invertebrates, potentially disrupting ecosystem balance.
The compound's impact on human health is another critical aspect of its environmental footprint. Exposure to CCl4 through contaminated air, water, or soil can lead to liver and kidney damage, and in high concentrations, it can be fatal. While direct exposure has been significantly reduced due to regulatory actions, the long-term environmental persistence of CCl4 means that historical contamination may continue to pose risks.
Efforts to mitigate the environmental impact of CCl4 have included international agreements such as the Montreal Protocol, which has successfully reduced global emissions. However, unexpected levels of CCl4 in the atmosphere suggest that there may be ongoing unreported emissions or natural sources that require further investigation. Continued monitoring and research are essential to fully understand and address the long-term environmental consequences of CCl4 use and to develop effective strategies for remediation and prevention of future impacts.
The primary environmental concern associated with CCl4 is its role in ozone depletion. When released into the atmosphere, CCl4 molecules can reach the stratosphere, where they are broken down by ultraviolet radiation. This process releases chlorine atoms, which catalyze the destruction of ozone molecules. The ozone layer plays a crucial role in protecting life on Earth from harmful ultraviolet radiation, and its depletion can lead to increased risks of skin cancer, cataracts, and damage to marine ecosystems.
In addition to its impact on the ozone layer, CCl4 contributes to global warming. It is a potent greenhouse gas with a global warming potential approximately 1,400 times that of carbon dioxide over a 100-year period. This means that even small emissions of CCl4 can have a disproportionate effect on climate change compared to more abundant greenhouse gases.
CCl4 also poses risks to terrestrial and aquatic ecosystems. When released into soil or water, it can persist for long periods due to its chemical stability. This persistence allows CCl4 to accumulate in the environment and potentially enter food chains. In aquatic systems, CCl4 can be toxic to various organisms, including fish and invertebrates, potentially disrupting ecosystem balance.
The compound's impact on human health is another critical aspect of its environmental footprint. Exposure to CCl4 through contaminated air, water, or soil can lead to liver and kidney damage, and in high concentrations, it can be fatal. While direct exposure has been significantly reduced due to regulatory actions, the long-term environmental persistence of CCl4 means that historical contamination may continue to pose risks.
Efforts to mitigate the environmental impact of CCl4 have included international agreements such as the Montreal Protocol, which has successfully reduced global emissions. However, unexpected levels of CCl4 in the atmosphere suggest that there may be ongoing unreported emissions or natural sources that require further investigation. Continued monitoring and research are essential to fully understand and address the long-term environmental consequences of CCl4 use and to develop effective strategies for remediation and prevention of future impacts.
Regulatory Framework for CCl4 Use
The regulatory framework for carbon tetrachloride (CCl4) use has evolved significantly over the past few decades, reflecting growing concerns about its environmental and health impacts. At the international level, the Montreal Protocol on Substances that Deplete the Ozone Layer, adopted in 1987, has been instrumental in phasing out the production and consumption of CCl4 along with other ozone-depleting substances.
Under the Montreal Protocol, developed countries were required to cease production and consumption of CCl4 by 1996, while developing countries were given until 2010. This global agreement has been highly successful in reducing CCl4 emissions, with global production dropping from over 1 million tonnes per year in the 1980s to less than 100,000 tonnes in recent years.
In the United States, the Environmental Protection Agency (EPA) regulates CCl4 under various statutes. The Clean Air Act classifies CCl4 as a hazardous air pollutant and sets emission standards for industrial sources. The Safe Drinking Water Act establishes a maximum contaminant level for CCl4 in public water systems. Additionally, the Toxic Substances Control Act gives the EPA authority to restrict the manufacture, processing, and distribution of CCl4.
The European Union has implemented stringent regulations on CCl4 through the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation. REACH requires manufacturers and importers to register CCl4 with the European Chemicals Agency and provide safety data. The use of CCl4 is severely restricted, with exemptions only for specific laboratory and analytical purposes.
In China, which was historically a major producer of CCl4, regulations have been tightened in recent years. The Ministry of Ecology and Environment has implemented strict controls on CCl4 production and use, aligning with international commitments under the Montreal Protocol.
Despite these regulatory efforts, challenges remain in verifying CCl4's chemical contributions. Atmospheric measurements have revealed discrepancies between reported emissions and observed concentrations, suggesting potential unreported sources. This has led to increased focus on improving monitoring and verification methods, including the use of advanced atmospheric modeling techniques and satellite observations.
To address these challenges, regulatory frameworks are evolving to incorporate more sophisticated verification mechanisms. These include enhanced reporting requirements for industrial users, improved tracking of CCl4 in chemical supply chains, and the development of more sensitive detection methods for environmental monitoring. International cooperation in data sharing and joint research initiatives is also being strengthened to improve global oversight of CCl4 emissions and use.
Under the Montreal Protocol, developed countries were required to cease production and consumption of CCl4 by 1996, while developing countries were given until 2010. This global agreement has been highly successful in reducing CCl4 emissions, with global production dropping from over 1 million tonnes per year in the 1980s to less than 100,000 tonnes in recent years.
In the United States, the Environmental Protection Agency (EPA) regulates CCl4 under various statutes. The Clean Air Act classifies CCl4 as a hazardous air pollutant and sets emission standards for industrial sources. The Safe Drinking Water Act establishes a maximum contaminant level for CCl4 in public water systems. Additionally, the Toxic Substances Control Act gives the EPA authority to restrict the manufacture, processing, and distribution of CCl4.
The European Union has implemented stringent regulations on CCl4 through the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation. REACH requires manufacturers and importers to register CCl4 with the European Chemicals Agency and provide safety data. The use of CCl4 is severely restricted, with exemptions only for specific laboratory and analytical purposes.
In China, which was historically a major producer of CCl4, regulations have been tightened in recent years. The Ministry of Ecology and Environment has implemented strict controls on CCl4 production and use, aligning with international commitments under the Montreal Protocol.
Despite these regulatory efforts, challenges remain in verifying CCl4's chemical contributions. Atmospheric measurements have revealed discrepancies between reported emissions and observed concentrations, suggesting potential unreported sources. This has led to increased focus on improving monitoring and verification methods, including the use of advanced atmospheric modeling techniques and satellite observations.
To address these challenges, regulatory frameworks are evolving to incorporate more sophisticated verification mechanisms. These include enhanced reporting requirements for industrial users, improved tracking of CCl4 in chemical supply chains, and the development of more sensitive detection methods for environmental monitoring. International cooperation in data sharing and joint research initiatives is also being strengthened to improve global oversight of CCl4 emissions and use.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!