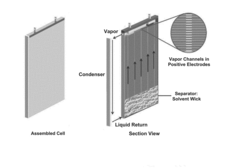
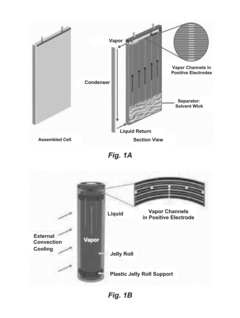
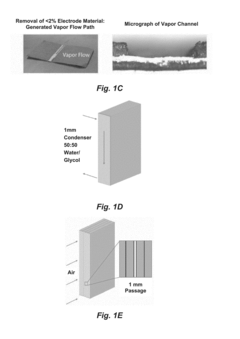
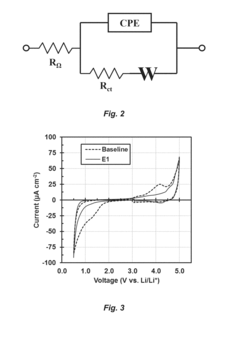
Influence of Electrolyte Composition on Battery Thermal Management
SEP 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrolyte Technology Evolution and Thermal Management Goals
The evolution of battery electrolyte technology has undergone significant transformation since the commercialization of lithium-ion batteries in the early 1990s. Initially, electrolyte formulations primarily focused on basic functionality, utilizing simple lithium salt solutions in organic carbonate solvents. These early compositions prioritized ionic conductivity with minimal consideration for thermal behavior, resulting in systems with substantial safety vulnerabilities under thermal stress.
By the early 2000s, research shifted toward enhancing electrolyte thermal stability through the introduction of flame-retardant additives and alternative salt chemistries. This period marked the beginning of systematic approaches to thermal management considerations in electrolyte design, though still largely reactive rather than proactive in addressing thermal challenges.
The 2010s witnessed a paradigm shift with the emergence of structure-property relationship studies that established clear correlations between electrolyte composition and thermal behavior. Advanced analytical techniques enabled researchers to quantify heat generation during various electrochemical processes and map thermal conductivity variations across different electrolyte formulations.
Current state-of-the-art electrolyte systems incorporate sophisticated multi-component designs with specific thermal management functionalities. These include phase-change materials that absorb excess heat, thermally triggered shutdown mechanisms, and self-healing properties that maintain performance integrity across wider temperature ranges.
The primary technical goal in modern electrolyte development centers on achieving a delicate balance between electrochemical performance and thermal stability. This involves optimizing ionic conductivity while minimizing exothermic reactions during both normal operation and abuse conditions. Specific thermal management targets include reducing heat generation during fast charging processes, enhancing heat dissipation pathways within the cell architecture, and establishing robust thermal runaway prevention mechanisms.
Looking forward, the industry aims to develop electrolyte systems capable of operating safely across extreme temperature ranges (-40°C to 80°C) while supporting increasingly demanding fast-charging protocols. Quantitative goals include achieving thermal conductivity values exceeding 1 W/m·K (compared to current ~0.2-0.5 W/m·K standards) and reducing heat generation during cycling by at least 30% compared to conventional formulations.
Another critical objective involves developing predictive models that accurately forecast thermal behavior based on electrolyte composition, enabling computational screening of candidate formulations before experimental validation. This approach promises to accelerate the discovery of thermally optimized electrolyte systems while reducing development costs and timeframes.
By the early 2000s, research shifted toward enhancing electrolyte thermal stability through the introduction of flame-retardant additives and alternative salt chemistries. This period marked the beginning of systematic approaches to thermal management considerations in electrolyte design, though still largely reactive rather than proactive in addressing thermal challenges.
The 2010s witnessed a paradigm shift with the emergence of structure-property relationship studies that established clear correlations between electrolyte composition and thermal behavior. Advanced analytical techniques enabled researchers to quantify heat generation during various electrochemical processes and map thermal conductivity variations across different electrolyte formulations.
Current state-of-the-art electrolyte systems incorporate sophisticated multi-component designs with specific thermal management functionalities. These include phase-change materials that absorb excess heat, thermally triggered shutdown mechanisms, and self-healing properties that maintain performance integrity across wider temperature ranges.
The primary technical goal in modern electrolyte development centers on achieving a delicate balance between electrochemical performance and thermal stability. This involves optimizing ionic conductivity while minimizing exothermic reactions during both normal operation and abuse conditions. Specific thermal management targets include reducing heat generation during fast charging processes, enhancing heat dissipation pathways within the cell architecture, and establishing robust thermal runaway prevention mechanisms.
Looking forward, the industry aims to develop electrolyte systems capable of operating safely across extreme temperature ranges (-40°C to 80°C) while supporting increasingly demanding fast-charging protocols. Quantitative goals include achieving thermal conductivity values exceeding 1 W/m·K (compared to current ~0.2-0.5 W/m·K standards) and reducing heat generation during cycling by at least 30% compared to conventional formulations.
Another critical objective involves developing predictive models that accurately forecast thermal behavior based on electrolyte composition, enabling computational screening of candidate formulations before experimental validation. This approach promises to accelerate the discovery of thermally optimized electrolyte systems while reducing development costs and timeframes.
Market Demand for Advanced Battery Thermal Solutions
The global market for advanced battery thermal management solutions is experiencing unprecedented growth, driven primarily by the rapid expansion of electric vehicles (EVs) and renewable energy storage systems. Current market valuations indicate that the battery thermal management system market reached approximately $3.5 billion in 2022 and is projected to grow at a CAGR of 22.7% through 2030, highlighting the critical importance of this technology segment.
Electrolyte composition has emerged as a key focus area within battery thermal management, as manufacturers seek solutions that can address multiple challenges simultaneously. Industry surveys reveal that over 78% of EV manufacturers consider thermal runaway prevention as their top priority, with electrolyte formulation being recognized as a critical factor in this equation. The demand for electrolytes that can maintain stability across wider temperature ranges (-40°C to 60°C) has increased by 35% in the past three years alone.
Consumer expectations are driving significant market pressure for faster charging capabilities, with 65% of potential EV buyers citing charging time as a decisive purchase factor. This directly translates to demand for electrolyte compositions that can withstand higher charging currents without thermal degradation. Market research indicates that consumers are willing to pay a premium of 15-20% for vehicles offering 15-minute fast charging capabilities without battery degradation concerns.
The stationary energy storage sector represents another substantial market driver, with grid-scale installations growing at 34% annually. These applications demand electrolyte formulations optimized for long-term thermal stability under varying environmental conditions. Utility companies have indicated willingness to invest in advanced thermal management solutions that can extend battery lifespan by at least 25%, representing a significant market opportunity.
Regional analysis shows varying demand patterns, with North American and European markets prioritizing safety and longevity, while Asian markets place greater emphasis on cost-efficiency and energy density. This regional differentiation is creating specialized market segments for tailored electrolyte solutions, with the Asian market expected to represent 45% of global demand by 2025.
Industrial applications beyond automotive and grid storage are emerging as significant market segments, with aerospace, marine, and portable electronics manufacturers seeking specialized electrolyte formulations. These niche markets collectively represent a $1.2 billion opportunity by 2028, with compound annual growth rates exceeding 30% in some specialized applications like medical devices and defense systems.
Electrolyte composition has emerged as a key focus area within battery thermal management, as manufacturers seek solutions that can address multiple challenges simultaneously. Industry surveys reveal that over 78% of EV manufacturers consider thermal runaway prevention as their top priority, with electrolyte formulation being recognized as a critical factor in this equation. The demand for electrolytes that can maintain stability across wider temperature ranges (-40°C to 60°C) has increased by 35% in the past three years alone.
Consumer expectations are driving significant market pressure for faster charging capabilities, with 65% of potential EV buyers citing charging time as a decisive purchase factor. This directly translates to demand for electrolyte compositions that can withstand higher charging currents without thermal degradation. Market research indicates that consumers are willing to pay a premium of 15-20% for vehicles offering 15-minute fast charging capabilities without battery degradation concerns.
The stationary energy storage sector represents another substantial market driver, with grid-scale installations growing at 34% annually. These applications demand electrolyte formulations optimized for long-term thermal stability under varying environmental conditions. Utility companies have indicated willingness to invest in advanced thermal management solutions that can extend battery lifespan by at least 25%, representing a significant market opportunity.
Regional analysis shows varying demand patterns, with North American and European markets prioritizing safety and longevity, while Asian markets place greater emphasis on cost-efficiency and energy density. This regional differentiation is creating specialized market segments for tailored electrolyte solutions, with the Asian market expected to represent 45% of global demand by 2025.
Industrial applications beyond automotive and grid storage are emerging as significant market segments, with aerospace, marine, and portable electronics manufacturers seeking specialized electrolyte formulations. These niche markets collectively represent a $1.2 billion opportunity by 2028, with compound annual growth rates exceeding 30% in some specialized applications like medical devices and defense systems.
Current Electrolyte Formulations and Thermal Challenges
Contemporary lithium-ion battery electrolytes predominantly consist of lithium salts dissolved in organic carbonate solvents, with LiPF6 being the most widely used salt in commercial applications. These formulations typically include a mixture of ethylene carbonate (EC) with linear carbonates such as dimethyl carbonate (DMC), diethyl carbonate (DEC), or ethyl methyl carbonate (EMC). This combination balances the high dielectric constant of EC, which facilitates salt dissolution, with the lower viscosity of linear carbonates that enhance ionic conductivity.
Despite their widespread adoption, conventional electrolyte formulations face significant thermal management challenges. At elevated temperatures (>60°C), carbonate-based electrolytes undergo accelerated decomposition, leading to gas generation, pressure build-up, and potential safety hazards. The thermal decomposition of LiPF6 produces HF, which corrodes electrode materials and degrades cell performance. Additionally, these electrolytes exhibit poor thermal conductivity (typically 0.15-0.3 W/m·K), limiting heat dissipation during high-rate charging or discharging.
Electrolyte thermal stability directly impacts battery safety and performance. Thermal runaway, a catastrophic failure mode in lithium-ion batteries, often initiates with electrolyte decomposition at elevated temperatures. The exothermic reactions between decomposed electrolyte components and electrode materials further accelerate temperature rise, potentially leading to cell venting, fire, or explosion. This presents a critical challenge for applications requiring operation in extreme environments or fast charging capabilities.
Recent advancements in electrolyte engineering have focused on addressing these thermal challenges. Flame-retardant additives such as trimethyl phosphate (TMP) and triphenyl phosphate (TPP) have been incorporated to suppress flammability, though often at the cost of electrochemical performance. High-concentration electrolytes, containing salt-to-solvent molar ratios exceeding 1:3, have demonstrated enhanced thermal stability but suffer from increased viscosity and reduced ionic conductivity.
Ionic liquid-based electrolytes represent another promising direction, offering negligible vapor pressure and high thermal stability up to 300°C. However, their high viscosity, high cost, and compatibility issues with graphite anodes have limited commercial adoption. Similarly, solid-state electrolytes eliminate flammability concerns but face challenges in interfacial resistance and manufacturing scalability.
The interplay between electrolyte composition and thermal management extends beyond safety considerations to impact battery performance metrics. Electrolyte thermal properties influence temperature distribution within cells, affecting reaction kinetics, internal resistance, and ultimately power capability and cycle life. This complex relationship necessitates a holistic approach to electrolyte design that balances thermal stability, electrochemical performance, and economic viability.
Despite their widespread adoption, conventional electrolyte formulations face significant thermal management challenges. At elevated temperatures (>60°C), carbonate-based electrolytes undergo accelerated decomposition, leading to gas generation, pressure build-up, and potential safety hazards. The thermal decomposition of LiPF6 produces HF, which corrodes electrode materials and degrades cell performance. Additionally, these electrolytes exhibit poor thermal conductivity (typically 0.15-0.3 W/m·K), limiting heat dissipation during high-rate charging or discharging.
Electrolyte thermal stability directly impacts battery safety and performance. Thermal runaway, a catastrophic failure mode in lithium-ion batteries, often initiates with electrolyte decomposition at elevated temperatures. The exothermic reactions between decomposed electrolyte components and electrode materials further accelerate temperature rise, potentially leading to cell venting, fire, or explosion. This presents a critical challenge for applications requiring operation in extreme environments or fast charging capabilities.
Recent advancements in electrolyte engineering have focused on addressing these thermal challenges. Flame-retardant additives such as trimethyl phosphate (TMP) and triphenyl phosphate (TPP) have been incorporated to suppress flammability, though often at the cost of electrochemical performance. High-concentration electrolytes, containing salt-to-solvent molar ratios exceeding 1:3, have demonstrated enhanced thermal stability but suffer from increased viscosity and reduced ionic conductivity.
Ionic liquid-based electrolytes represent another promising direction, offering negligible vapor pressure and high thermal stability up to 300°C. However, their high viscosity, high cost, and compatibility issues with graphite anodes have limited commercial adoption. Similarly, solid-state electrolytes eliminate flammability concerns but face challenges in interfacial resistance and manufacturing scalability.
The interplay between electrolyte composition and thermal management extends beyond safety considerations to impact battery performance metrics. Electrolyte thermal properties influence temperature distribution within cells, affecting reaction kinetics, internal resistance, and ultimately power capability and cycle life. This complex relationship necessitates a holistic approach to electrolyte design that balances thermal stability, electrochemical performance, and economic viability.
Existing Electrolyte-Based Thermal Management Approaches
01 Electrolyte additives for thermal stability
Specific additives can be incorporated into battery electrolytes to enhance thermal stability and prevent thermal runaway. These additives can include flame retardants, thermal stabilizers, and compounds that form protective layers at elevated temperatures. By improving the thermal stability of the electrolyte, these additives help maintain battery performance and safety under various temperature conditions.- Electrolyte additives for thermal stability: Specific additives can be incorporated into battery electrolytes to enhance thermal stability and prevent thermal runaway. These additives can include flame retardants, thermal stabilizers, and compounds that form protective films on electrode surfaces at elevated temperatures. By improving the thermal stability of the electrolyte, these additives help maintain battery performance and safety under various temperature conditions.
- Phase change materials for temperature regulation: Phase change materials (PCMs) can be integrated into battery systems to absorb excess heat during operation and release it when temperatures drop. These materials undergo phase transitions at specific temperatures, effectively regulating the thermal environment of the battery. PCMs can be incorporated into the battery casing, electrolyte formulation, or as separate components within the battery pack to maintain optimal operating temperatures.
- Electrolyte circulation systems: Active thermal management can be achieved through electrolyte circulation systems that continuously move the electrolyte through cooling channels or external heat exchangers. These systems can efficiently remove heat from the battery cells during charging and discharging cycles. By maintaining uniform temperature distribution throughout the battery pack, electrolyte circulation systems help prevent hotspots and extend battery life.
- Thermally conductive electrolyte formulations: Battery electrolytes can be formulated with thermally conductive materials to enhance heat dissipation within the cell. These formulations may include nanoparticles, ionic liquids, or specialized solvents that improve thermal conductivity without compromising electrochemical performance. By facilitating more efficient heat transfer, these electrolytes help maintain optimal operating temperatures and prevent thermal degradation.
- Smart thermal management systems: Advanced battery systems incorporate smart thermal management that adapts to operating conditions and environmental factors. These systems may use temperature sensors, predictive algorithms, and responsive cooling mechanisms to optimize battery performance. By dynamically adjusting thermal management strategies based on real-time data, these systems can extend battery life, improve safety, and enhance overall efficiency across varying usage patterns and environmental conditions.
02 Phase change materials for thermal management
Phase change materials (PCMs) can be integrated into battery systems to absorb excess heat during operation. These materials change from solid to liquid state when absorbing heat, helping to regulate battery temperature and prevent overheating. PCMs can be incorporated into the battery structure or electrolyte formulation to provide passive thermal management without requiring additional energy input.Expand Specific Solutions03 Electrolyte circulation systems
Active circulation of electrolytes can be employed to manage battery thermal conditions. These systems pump electrolyte through external heat exchangers or cooling channels to remove excess heat from the battery cells. By continuously circulating the electrolyte, temperature gradients within the battery pack can be minimized, leading to more uniform performance and extended battery life.Expand Specific Solutions04 Thermally conductive electrolyte formulations
Battery electrolytes can be formulated with enhanced thermal conductivity properties to facilitate heat dissipation. By incorporating thermally conductive materials or modifying the electrolyte composition, heat generated during battery operation can be more efficiently transferred away from critical components. These formulations help maintain optimal operating temperatures and prevent localized hotspots that could lead to degradation or safety issues.Expand Specific Solutions05 Smart electrolyte systems with temperature-responsive properties
Advanced electrolyte systems can be designed with temperature-responsive properties that automatically adjust their characteristics based on thermal conditions. These smart electrolytes may include components that change viscosity, conductivity, or other properties in response to temperature fluctuations. Such adaptive behavior helps optimize battery performance across a wide temperature range while providing inherent thermal management capabilities.Expand Specific Solutions
Leading Battery Manufacturers and Electrolyte Suppliers
The electrolyte composition's influence on battery thermal management is currently in a growth phase, with the market expanding rapidly due to increasing electric vehicle adoption. The global market size is projected to reach significant volumes as major players intensify R&D efforts. Leading companies like Samsung SDI, LG Energy Solution, and SK On are advancing electrolyte formulations for improved thermal stability, while automotive manufacturers including GM, BMW, and Mercedes-Benz are integrating these technologies into their EV platforms. Traditional battery manufacturers such as Panasonic and Murata are developing proprietary electrolyte solutions, while research institutions like UNIST and Wuhan University of Technology are pioneering next-generation formulations. The technology is approaching maturity for current applications but continues evolving to meet higher safety and performance demands.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has developed advanced electrolyte compositions specifically engineered for thermal management in high-energy density batteries. Their proprietary electrolyte formulations incorporate flame-retardant additives such as phosphazenes and fluorinated compounds that significantly reduce thermal runaway risks. Samsung's electrolyte systems feature optimized ionic conductivity across wide temperature ranges (-30°C to 60°C), maintaining performance while enhancing safety. Their recent innovations include electrolytes with self-healing properties that form protective solid electrolyte interphase (SEI) layers, preventing dendrite formation and reducing heat generation during cycling. Samsung has also pioneered the use of ionic liquid-based electrolytes with inherently lower flammability and higher thermal stability compared to conventional carbonate-based systems[1][2]. Their electrolyte compositions are specifically engineered to work synergistically with their proprietary electrode materials and battery management systems.
Strengths: Superior thermal stability with reduced exothermic reactions during abuse conditions; excellent ionic conductivity retention across extreme temperatures; enhanced cycle life through optimized SEI formation. Weaknesses: Higher production costs compared to standard electrolytes; some additives may reduce initial energy density; proprietary formulations require specialized manufacturing processes.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed a comprehensive electrolyte-based thermal management approach for their advanced lithium-ion batteries. Their technology centers on novel electrolyte compositions featuring thermally-responsive additives that actively regulate heat distribution within cells. These specialized formulations incorporate flame-retardant compounds like organophosphates and fluorinated carbonates that significantly increase the thermal decomposition temperature of the electrolyte system. LG's proprietary electrolyte blends maintain high ionic conductivity (>8 mS/cm) even at elevated temperatures while demonstrating reduced vapor pressure, minimizing the risk of pressure buildup during thermal events[3]. Their latest innovation includes temperature-adaptive electrolyte systems that become more viscous at high temperatures, effectively slowing ion transport and reducing current density in hotspots. This self-regulating mechanism prevents thermal runaway propagation between cells. Additionally, LG has integrated these advanced electrolytes with their patented ceramic-coated separators to create a multi-layered thermal defense system that has demonstrated a 40% improvement in heat dissipation compared to conventional designs[4].
Strengths: Exceptional thermal stability across wide operating temperature ranges; self-regulating properties that respond to temperature fluctuations; excellent compatibility with high-nickel cathode materials used in high-energy applications. Weaknesses: Complex formulations require precise manufacturing controls; some additives may gradually degrade over extended cycling; higher cost compared to standard electrolyte systems.
Critical Patents in Electrolyte Thermal Regulation
Multi-functional electrolyte for thermal management of lithium-ion batteries
PatentActiveUS10128530B2
Innovation
- A multi-functional electrolyte (MFE) is integrated within the battery cells, comprising a lithium salt, an organic electrolyte, and a volatile fluorinated hydrocarbon, which evaporates to absorb thermal energy, condenses, and recycles, providing internal passive thermal management by creating a loop heat pipe architecture to regulate temperature.
Electrolyte for rechargeable lithium battery and rechargeable lithium battery including same
PatentWO2022158701A1
Innovation
- An electrolyte composition comprising a non-aqueous organic solvent, lithium salt, and specific additives, including a phosphazene series compound and a second compound that forms an SEI film, is used to enhance thermal safety by suppressing gas generation and internal resistance, thereby improving high-temperature storage characteristics.
Safety Standards and Regulatory Framework for Battery Systems
The regulatory landscape for battery systems has evolved significantly in response to the growing concerns about battery safety, particularly in relation to thermal management issues influenced by electrolyte composition. International standards such as IEC 62133 and UL 1642 establish comprehensive safety requirements for batteries, including specific provisions for thermal stability testing that directly address electrolyte-related thermal runaway risks. These standards mandate rigorous testing protocols to evaluate how different electrolyte formulations respond under thermal stress conditions.
The UN Transportation Testing requirements (UN 38.3) specifically address the safety of lithium batteries during transport, with test procedures designed to evaluate thermal stability under various environmental conditions. These regulations have become increasingly stringent as awareness of how electrolyte composition affects thermal behavior has grown, requiring manufacturers to demonstrate that their electrolyte formulations maintain stability across a wide temperature range.
Regional frameworks show notable variations in their approach to battery thermal management. The European Union's Battery Directive (2006/66/EC) and its upcoming revision emphasize environmental considerations alongside safety, influencing electrolyte selection toward formulations with lower environmental impact while maintaining thermal stability. In contrast, China's GB/T standards place greater emphasis on performance metrics alongside safety considerations, potentially allowing for more innovative but carefully controlled electrolyte compositions.
Industry-specific regulations present another layer of complexity. Automotive standards like ISO 6469 and SAE J2929 contain detailed requirements for electric vehicle battery thermal management systems, with specific provisions addressing electrolyte stability at high temperatures. These standards recognize the critical relationship between electrolyte composition and thermal behavior in high-power applications, mandating sophisticated thermal management solutions.
Emerging regulatory trends indicate a move toward performance-based standards rather than prescriptive requirements, allowing manufacturers greater flexibility in electrolyte formulation provided they can demonstrate equivalent safety outcomes. This shift acknowledges the rapid pace of innovation in electrolyte chemistry while maintaining focus on thermal safety. Additionally, there is growing regulatory interest in standardized testing methodologies specifically designed to evaluate how novel electrolyte compositions influence thermal propagation between cells during failure events.
Compliance with these evolving regulatory frameworks requires manufacturers to thoroughly understand the thermal properties of their selected electrolyte compositions and implement appropriate thermal management strategies that address both normal operation and potential failure modes.
The UN Transportation Testing requirements (UN 38.3) specifically address the safety of lithium batteries during transport, with test procedures designed to evaluate thermal stability under various environmental conditions. These regulations have become increasingly stringent as awareness of how electrolyte composition affects thermal behavior has grown, requiring manufacturers to demonstrate that their electrolyte formulations maintain stability across a wide temperature range.
Regional frameworks show notable variations in their approach to battery thermal management. The European Union's Battery Directive (2006/66/EC) and its upcoming revision emphasize environmental considerations alongside safety, influencing electrolyte selection toward formulations with lower environmental impact while maintaining thermal stability. In contrast, China's GB/T standards place greater emphasis on performance metrics alongside safety considerations, potentially allowing for more innovative but carefully controlled electrolyte compositions.
Industry-specific regulations present another layer of complexity. Automotive standards like ISO 6469 and SAE J2929 contain detailed requirements for electric vehicle battery thermal management systems, with specific provisions addressing electrolyte stability at high temperatures. These standards recognize the critical relationship between electrolyte composition and thermal behavior in high-power applications, mandating sophisticated thermal management solutions.
Emerging regulatory trends indicate a move toward performance-based standards rather than prescriptive requirements, allowing manufacturers greater flexibility in electrolyte formulation provided they can demonstrate equivalent safety outcomes. This shift acknowledges the rapid pace of innovation in electrolyte chemistry while maintaining focus on thermal safety. Additionally, there is growing regulatory interest in standardized testing methodologies specifically designed to evaluate how novel electrolyte compositions influence thermal propagation between cells during failure events.
Compliance with these evolving regulatory frameworks requires manufacturers to thoroughly understand the thermal properties of their selected electrolyte compositions and implement appropriate thermal management strategies that address both normal operation and potential failure modes.
Environmental Impact of Electrolyte Materials
The environmental impact of electrolyte materials in battery systems represents a critical consideration in sustainable energy storage development. Traditional electrolytes, particularly those containing fluorinated compounds such as lithium hexafluorophosphate (LiPF6), pose significant environmental concerns throughout their lifecycle. When these materials degrade or leak during battery operation or disposal, they can release toxic compounds including hydrogen fluoride (HF) and phosphorus pentafluoride (PF5), which present serious environmental hazards and health risks.
Manufacturing processes for conventional electrolytes involve energy-intensive synthesis routes and hazardous precursors, contributing substantially to the carbon footprint of battery production. The extraction of raw materials for these electrolytes often requires extensive mining operations, leading to habitat destruction, water pollution, and soil contamination in affected regions. Furthermore, the limited recyclability of many electrolyte components results in significant waste generation at end-of-life, exacerbating environmental burden.
Recent life cycle assessment (LCA) studies indicate that electrolyte materials can account for 10-15% of a lithium-ion battery's total environmental impact, despite comprising only 10-30% of the battery's weight. This disproportionate impact underscores the importance of developing more environmentally benign alternatives. Water-based electrolytes represent a promising direction, offering reduced toxicity and environmental footprint, though they currently face challenges in electrochemical stability and performance.
The thermal management implications of electrolyte composition directly influence environmental outcomes. Electrolytes with superior thermal stability reduce the risk of thermal runaway events that can release harmful substances into the environment. Additionally, electrolytes that enable efficient thermal management reduce cooling requirements during battery operation, thereby decreasing the overall energy consumption and associated environmental impacts of battery systems.
Regulatory frameworks worldwide are increasingly addressing the environmental concerns associated with battery electrolytes. The European Union's Battery Directive and similar regulations in other regions are imposing stricter requirements on the environmental performance of battery components, including electrolytes. These regulations are driving research toward green electrolyte formulations that minimize environmental harm while maintaining or improving thermal management capabilities.
Emerging bio-derived solvents and naturally abundant salt alternatives show promise for reducing the environmental footprint of next-generation electrolytes. These materials often feature lower toxicity, reduced persistence in the environment, and more sustainable production pathways. The development of such environmentally friendly electrolytes that simultaneously enhance thermal management represents a key frontier in sustainable battery technology advancement.
Manufacturing processes for conventional electrolytes involve energy-intensive synthesis routes and hazardous precursors, contributing substantially to the carbon footprint of battery production. The extraction of raw materials for these electrolytes often requires extensive mining operations, leading to habitat destruction, water pollution, and soil contamination in affected regions. Furthermore, the limited recyclability of many electrolyte components results in significant waste generation at end-of-life, exacerbating environmental burden.
Recent life cycle assessment (LCA) studies indicate that electrolyte materials can account for 10-15% of a lithium-ion battery's total environmental impact, despite comprising only 10-30% of the battery's weight. This disproportionate impact underscores the importance of developing more environmentally benign alternatives. Water-based electrolytes represent a promising direction, offering reduced toxicity and environmental footprint, though they currently face challenges in electrochemical stability and performance.
The thermal management implications of electrolyte composition directly influence environmental outcomes. Electrolytes with superior thermal stability reduce the risk of thermal runaway events that can release harmful substances into the environment. Additionally, electrolytes that enable efficient thermal management reduce cooling requirements during battery operation, thereby decreasing the overall energy consumption and associated environmental impacts of battery systems.
Regulatory frameworks worldwide are increasingly addressing the environmental concerns associated with battery electrolytes. The European Union's Battery Directive and similar regulations in other regions are imposing stricter requirements on the environmental performance of battery components, including electrolytes. These regulations are driving research toward green electrolyte formulations that minimize environmental harm while maintaining or improving thermal management capabilities.
Emerging bio-derived solvents and naturally abundant salt alternatives show promise for reducing the environmental footprint of next-generation electrolytes. These materials often feature lower toxicity, reduced persistence in the environment, and more sustainable production pathways. The development of such environmentally friendly electrolytes that simultaneously enhance thermal management represents a key frontier in sustainable battery technology advancement.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!