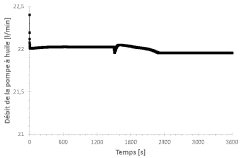
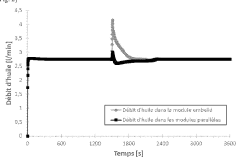
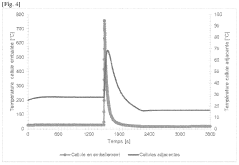
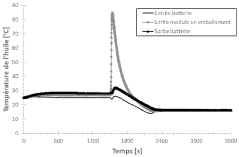
Research on Battery Thermal Management in Pharmaceuticals
SEP 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Battery Thermal Management Background and Objectives
Battery thermal management systems (BTMS) have evolved significantly over the past decades, transitioning from simple cooling mechanisms to sophisticated integrated systems. Initially developed for consumer electronics and automotive applications, these systems have recently gained attention in pharmaceutical contexts due to the increasing reliance on temperature-sensitive battery-powered medical devices and cold chain logistics. The evolution of BTMS technology has been driven by the need for greater efficiency, reliability, and safety in battery operations across various temperature ranges.
The pharmaceutical industry presents unique challenges for battery thermal management, as medications and biological samples often require precise temperature control during storage, transportation, and administration. Traditional BTMS approaches from other industries cannot be directly applied due to the stringent regulatory requirements, safety considerations, and precision demands specific to pharmaceutical applications.
Current technological trends in this field include the development of phase change materials (PCMs) for passive thermal regulation, advanced thermal interface materials, intelligent thermal management algorithms, and hybrid cooling systems that combine active and passive approaches. These innovations aim to address the critical need for maintaining pharmaceutical efficacy while ensuring battery performance and longevity in various environmental conditions.
The primary objectives of battery thermal management research in pharmaceuticals include developing systems that can maintain optimal temperature ranges for both batteries and pharmaceutical products simultaneously, creating energy-efficient solutions that extend battery life without compromising thermal performance, and designing compact systems suitable for portable medical devices and transportation containers.
Additionally, researchers aim to enhance the predictability and reliability of thermal management systems through advanced modeling and simulation techniques, allowing for better design optimization before physical prototyping. This includes computational fluid dynamics (CFD) analysis, thermal network modeling, and machine learning approaches for predictive temperature control.
Another key objective is to develop adaptive thermal management systems capable of responding to changing environmental conditions and varying thermal loads, particularly important for pharmaceutical applications where products may be exposed to diverse climates during global distribution. These systems must balance the sometimes competing requirements of battery performance optimization and pharmaceutical product preservation.
The ultimate goal of this research is to establish standardized approaches to battery thermal management specifically tailored to pharmaceutical applications, addressing industry-specific challenges while leveraging technological advances from adjacent fields such as automotive and consumer electronics.
The pharmaceutical industry presents unique challenges for battery thermal management, as medications and biological samples often require precise temperature control during storage, transportation, and administration. Traditional BTMS approaches from other industries cannot be directly applied due to the stringent regulatory requirements, safety considerations, and precision demands specific to pharmaceutical applications.
Current technological trends in this field include the development of phase change materials (PCMs) for passive thermal regulation, advanced thermal interface materials, intelligent thermal management algorithms, and hybrid cooling systems that combine active and passive approaches. These innovations aim to address the critical need for maintaining pharmaceutical efficacy while ensuring battery performance and longevity in various environmental conditions.
The primary objectives of battery thermal management research in pharmaceuticals include developing systems that can maintain optimal temperature ranges for both batteries and pharmaceutical products simultaneously, creating energy-efficient solutions that extend battery life without compromising thermal performance, and designing compact systems suitable for portable medical devices and transportation containers.
Additionally, researchers aim to enhance the predictability and reliability of thermal management systems through advanced modeling and simulation techniques, allowing for better design optimization before physical prototyping. This includes computational fluid dynamics (CFD) analysis, thermal network modeling, and machine learning approaches for predictive temperature control.
Another key objective is to develop adaptive thermal management systems capable of responding to changing environmental conditions and varying thermal loads, particularly important for pharmaceutical applications where products may be exposed to diverse climates during global distribution. These systems must balance the sometimes competing requirements of battery performance optimization and pharmaceutical product preservation.
The ultimate goal of this research is to establish standardized approaches to battery thermal management specifically tailored to pharmaceutical applications, addressing industry-specific challenges while leveraging technological advances from adjacent fields such as automotive and consumer electronics.
Pharmaceutical Industry Demand Analysis
The pharmaceutical industry is experiencing a significant transformation in its manufacturing and logistics processes, with battery-powered systems becoming increasingly prevalent. Market research indicates that the global pharmaceutical cold chain logistics market, which heavily relies on battery-powered temperature control systems, was valued at approximately $16.3 billion in 2022 and is projected to grow at a CAGR of 8.1% through 2030. This growth is primarily driven by the increasing demand for temperature-sensitive biopharmaceuticals and vaccines, which require precise thermal management throughout their lifecycle.
The COVID-19 pandemic has substantially accelerated this trend, with unprecedented demand for vaccine distribution highlighting critical gaps in existing cold chain infrastructure. According to industry reports, nearly 25% of vaccines reach their destination degraded due to breaks in the cold chain, representing billions in annual losses. This has created urgent market demand for more reliable battery thermal management systems specifically designed for pharmaceutical applications.
Regulatory requirements are another significant market driver. The FDA, EMA, and WHO have all strengthened their guidelines regarding temperature control in pharmaceutical handling, with GDP (Good Distribution Practice) and GMP (Good Manufacturing Practice) regulations becoming increasingly stringent. These regulations mandate continuous temperature monitoring and control, creating demand for advanced battery systems with sophisticated thermal management capabilities.
The rise of personalized medicine and cell and gene therapies has further intensified market needs. These products often require ultra-cold storage conditions (-70°C or lower) and have extremely short shelf lives, making efficient and reliable battery thermal management systems essential. Industry analysts project that the market for ultra-cold chain solutions will grow at nearly 12% annually through 2028, outpacing the broader pharmaceutical logistics market.
From a geographical perspective, emerging markets present significant growth opportunities. Countries across Asia-Pacific and Africa are expanding their healthcare infrastructure and pharmaceutical manufacturing capabilities, creating new demand for reliable cold chain solutions. However, these regions often face challenges related to unreliable power grids, making battery-powered systems with efficient thermal management particularly valuable.
Cost considerations remain paramount for industry stakeholders. Current battery thermal management systems for pharmaceutical applications can represent up to 30% of total cold chain logistics costs. This has created strong market demand for more energy-efficient solutions that can extend battery life while maintaining precise temperature control, ultimately reducing operational expenses and environmental impact.
The COVID-19 pandemic has substantially accelerated this trend, with unprecedented demand for vaccine distribution highlighting critical gaps in existing cold chain infrastructure. According to industry reports, nearly 25% of vaccines reach their destination degraded due to breaks in the cold chain, representing billions in annual losses. This has created urgent market demand for more reliable battery thermal management systems specifically designed for pharmaceutical applications.
Regulatory requirements are another significant market driver. The FDA, EMA, and WHO have all strengthened their guidelines regarding temperature control in pharmaceutical handling, with GDP (Good Distribution Practice) and GMP (Good Manufacturing Practice) regulations becoming increasingly stringent. These regulations mandate continuous temperature monitoring and control, creating demand for advanced battery systems with sophisticated thermal management capabilities.
The rise of personalized medicine and cell and gene therapies has further intensified market needs. These products often require ultra-cold storage conditions (-70°C or lower) and have extremely short shelf lives, making efficient and reliable battery thermal management systems essential. Industry analysts project that the market for ultra-cold chain solutions will grow at nearly 12% annually through 2028, outpacing the broader pharmaceutical logistics market.
From a geographical perspective, emerging markets present significant growth opportunities. Countries across Asia-Pacific and Africa are expanding their healthcare infrastructure and pharmaceutical manufacturing capabilities, creating new demand for reliable cold chain solutions. However, these regions often face challenges related to unreliable power grids, making battery-powered systems with efficient thermal management particularly valuable.
Cost considerations remain paramount for industry stakeholders. Current battery thermal management systems for pharmaceutical applications can represent up to 30% of total cold chain logistics costs. This has created strong market demand for more energy-efficient solutions that can extend battery life while maintaining precise temperature control, ultimately reducing operational expenses and environmental impact.
Current Challenges in Battery Thermal Control
Battery thermal management in pharmaceutical applications faces significant challenges that require innovative solutions. The primary issue is temperature control precision, as pharmaceutical batteries often operate in environments requiring strict temperature ranges (2-8°C for cold chain or controlled room temperature 20-25°C). Conventional thermal management systems struggle to maintain these narrow bands consistently, especially when ambient conditions fluctuate dramatically.
Energy efficiency presents another major challenge. Pharmaceutical applications frequently require portable or remote deployment where power sources are limited. Current thermal management systems consume substantial energy, reducing overall battery life and operational efficiency. This is particularly problematic in field applications or during transportation of temperature-sensitive pharmaceuticals.
Size and weight constraints further complicate thermal management design. Pharmaceutical devices increasingly trend toward miniaturization and portability, leaving minimal space for thermal control components. Engineers must balance effective thermal regulation against space limitations without compromising pharmaceutical integrity or device functionality.
Material compatibility poses unique challenges in pharmaceutical contexts. Thermal management components must not only perform efficiently but also comply with strict biocompatibility and regulatory requirements. Materials that might be optimal for thermal conductivity may present contamination risks or fail to meet pharmaceutical-grade standards.
Reliability concerns are heightened in pharmaceutical applications where thermal management failure could compromise medication efficacy or patient safety. Current systems often lack redundancy or fail-safe mechanisms appropriate for critical pharmaceutical applications, creating significant reliability gaps.
Thermal response time represents another critical challenge. Many pharmaceutical processes require rapid temperature adjustments or immediate response to environmental changes. Existing thermal management technologies frequently exhibit lag in response time, potentially compromising sensitive pharmaceutical products during temperature excursions.
Cost considerations remain a persistent obstacle. Advanced thermal management solutions with pharmaceutical-grade specifications typically carry premium costs that limit widespread adoption. The industry struggles to balance performance requirements against economic constraints, particularly for mass-produced pharmaceutical products.
Integration complexity with existing pharmaceutical manufacturing and distribution systems presents additional challenges. Thermal management solutions must seamlessly interface with established pharmaceutical processes without disrupting validated workflows or requiring extensive revalidation procedures.
Energy efficiency presents another major challenge. Pharmaceutical applications frequently require portable or remote deployment where power sources are limited. Current thermal management systems consume substantial energy, reducing overall battery life and operational efficiency. This is particularly problematic in field applications or during transportation of temperature-sensitive pharmaceuticals.
Size and weight constraints further complicate thermal management design. Pharmaceutical devices increasingly trend toward miniaturization and portability, leaving minimal space for thermal control components. Engineers must balance effective thermal regulation against space limitations without compromising pharmaceutical integrity or device functionality.
Material compatibility poses unique challenges in pharmaceutical contexts. Thermal management components must not only perform efficiently but also comply with strict biocompatibility and regulatory requirements. Materials that might be optimal for thermal conductivity may present contamination risks or fail to meet pharmaceutical-grade standards.
Reliability concerns are heightened in pharmaceutical applications where thermal management failure could compromise medication efficacy or patient safety. Current systems often lack redundancy or fail-safe mechanisms appropriate for critical pharmaceutical applications, creating significant reliability gaps.
Thermal response time represents another critical challenge. Many pharmaceutical processes require rapid temperature adjustments or immediate response to environmental changes. Existing thermal management technologies frequently exhibit lag in response time, potentially compromising sensitive pharmaceutical products during temperature excursions.
Cost considerations remain a persistent obstacle. Advanced thermal management solutions with pharmaceutical-grade specifications typically carry premium costs that limit widespread adoption. The industry struggles to balance performance requirements against economic constraints, particularly for mass-produced pharmaceutical products.
Integration complexity with existing pharmaceutical manufacturing and distribution systems presents additional challenges. Thermal management solutions must seamlessly interface with established pharmaceutical processes without disrupting validated workflows or requiring extensive revalidation procedures.
Current Battery Cooling Solutions
01 Liquid cooling systems for battery thermal management
Liquid cooling systems are employed in battery thermal management to efficiently regulate temperature. These systems use coolant circulation through channels or plates in direct contact with battery cells to absorb and dissipate heat. The liquid medium provides superior heat transfer capabilities compared to air cooling, allowing for more precise temperature control and uniform cooling across battery packs, which is crucial for high-performance electric vehicles and energy storage systems.- Liquid cooling systems for battery thermal management: Liquid cooling systems are effective for battery thermal management, utilizing coolant circulation through channels or plates in direct contact with battery cells. These systems provide efficient heat transfer and temperature uniformity across battery packs. Advanced designs incorporate optimized flow paths, specialized coolants, and integrated heat exchangers to maintain batteries within optimal temperature ranges during charging and discharging cycles.
- Phase change materials for thermal regulation: Phase change materials (PCMs) are incorporated into battery thermal management systems to absorb and release thermal energy during phase transitions. These materials provide passive temperature control by absorbing excess heat during high-load operations and releasing it when temperatures drop. PCM-based solutions offer advantages in weight reduction, space efficiency, and operation without external power, making them suitable for various battery applications from portable electronics to electric vehicles.
- Air cooling and ventilation techniques: Air cooling systems utilize forced or natural convection to manage battery temperature. These systems incorporate strategically placed fans, air channels, and heat sinks to direct airflow across battery modules. Advanced designs feature variable speed fans, temperature sensors for adaptive control, and optimized air path geometries to enhance cooling efficiency while minimizing power consumption and noise. Air cooling solutions are particularly valuable for applications where weight, cost, and simplicity are prioritized.
- Thermal management control systems and algorithms: Sophisticated control systems and algorithms optimize battery thermal management by continuously monitoring temperature sensors and adjusting cooling or heating parameters accordingly. These intelligent systems predict thermal behavior based on usage patterns, ambient conditions, and battery state of charge. Advanced algorithms implement predictive control strategies to preemptively manage thermal conditions before critical thresholds are reached, extending battery life and enhancing safety while maximizing performance and efficiency.
- Integrated heating solutions for cold environments: Heating systems are crucial for battery operation in cold environments, as low temperatures significantly reduce battery performance and charging capabilities. These solutions include resistive heating elements, positive temperature coefficient (PTC) heaters, and waste heat recovery systems. Advanced designs integrate heating elements directly into battery modules or cooling plates for efficient thermal transfer. Smart heating control strategies optimize power consumption by selectively heating critical components and utilizing predictive algorithms based on environmental conditions and planned usage.
02 Phase change materials for thermal regulation
Phase change materials (PCMs) are integrated into battery thermal management systems to absorb excess heat during operation and release it when temperatures drop. These materials utilize their latent heat properties during phase transitions to maintain battery temperature within optimal ranges. PCMs can be incorporated into battery modules as dedicated layers or components, providing passive thermal regulation that reduces the energy requirements of active cooling systems and improves overall thermal stability.Expand Specific Solutions03 Integrated thermal management control systems
Advanced control systems are implemented to optimize battery thermal management by continuously monitoring temperature sensors and adjusting cooling or heating mechanisms accordingly. These intelligent systems use predictive algorithms to anticipate thermal needs based on operating conditions, battery state of charge, and environmental factors. The integration of thermal management with battery management systems enables coordinated control strategies that maximize battery performance, efficiency, and lifespan while preventing thermal runaway events.Expand Specific Solutions04 Air cooling and ventilation designs
Air cooling systems utilize strategic airflow patterns to remove heat from battery packs through natural or forced convection. These designs incorporate optimized air channels, heat sinks, and fan configurations to enhance heat dissipation efficiency. While less effective than liquid cooling for high-power applications, air cooling systems offer advantages in terms of simplicity, lower cost, reduced maintenance requirements, and elimination of potential coolant leakage issues, making them suitable for certain electric vehicle and stationary storage applications.Expand Specific Solutions05 Thermal insulation and heat distribution technologies
Specialized thermal insulation materials and heat distribution technologies are employed to manage temperature gradients within battery packs. These solutions include thermally conductive materials that spread heat evenly across cells, insulating barriers that prevent external temperature influences, and composite structures that combine both properties. By minimizing temperature differences between cells and protecting batteries from extreme ambient conditions, these technologies improve performance consistency, prevent localized hotspots, and extend overall battery system lifespan.Expand Specific Solutions
Key Industry Players and Competitors
The battery thermal management landscape in pharmaceuticals is evolving rapidly, currently in a growth phase with increasing market adoption. The sector is characterized by significant technological development as pharmaceutical applications require precise temperature control for sensitive products. Leading players include Samsung SDI and CATL, who are leveraging their extensive battery expertise to develop specialized pharmaceutical solutions. Gentherm has emerged as a key innovator with thermal management technologies specifically adapted for pharmaceutical applications. Traditional automotive thermal management leaders like BYD and NIO are expanding into this space, bringing crossover technologies. Research institutions including Guangdong University of Technology and Illinois Institute of Technology are advancing fundamental research, creating a competitive environment where commercial applications are beginning to mature from experimental phases to practical implementation.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has developed advanced battery thermal management systems specifically designed for pharmaceutical cold chain applications. Their solution integrates phase change materials (PCMs) with intelligent thermal control algorithms to maintain precise temperature ranges required for sensitive pharmaceutical products. The system employs a network of micro-sensors throughout the battery pack that continuously monitor temperature variations and trigger adaptive cooling or heating mechanisms as needed. Samsung's pharmaceutical BTMS utilizes proprietary cell-to-cell thermal balancing technology that prevents hotspots and ensures uniform temperature distribution across all cells, critical for maintaining pharmaceutical product integrity during transportation and storage. Their system can maintain temperatures within ±0.5°C accuracy even in extreme ambient conditions, making it suitable for vaccines, biologics, and temperature-sensitive medications.
Strengths: Superior temperature precision control (±0.5°C) ideal for strict pharmaceutical requirements; advanced predictive thermal management algorithms; extensive experience in battery technology. Weaknesses: Higher implementation costs compared to conventional systems; requires specialized training for maintenance personnel.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed a comprehensive battery thermal management system tailored for pharmaceutical applications that combines liquid cooling with intelligent thermal control algorithms. Their pharmaceutical BTMS utilizes a multi-layer approach with specialized cooling plates positioned between battery cells to ensure uniform temperature distribution. The system employs environmentally friendly refrigerants with low global warming potential, addressing sustainability concerns in pharmaceutical logistics. CATL's solution features adaptive thermal management that automatically adjusts cooling/heating intensity based on real-time temperature monitoring and predictive modeling. Their system incorporates redundant temperature sensors and control units to ensure fail-safe operation, critical for maintaining the efficacy of temperature-sensitive pharmaceuticals during power fluctuations or system component failures.
Strengths: Industry-leading energy density while maintaining thermal stability; extensive manufacturing capacity enabling economies of scale; advanced battery management systems with pharmaceutical-specific algorithms. Weaknesses: Relatively newer entrant to pharmaceutical-specific applications; higher complexity in system integration.
Critical Patents in Pharmaceutical Battery Management
Thermal management and battery safety process and device for its implementation.
PatentPendingFR3138243A1
Innovation
- A method and device for thermal management and safety that involves detecting a fault in a cell, modulating the flow rate of a cooling fluid by redirecting it primarily to the faulty cell, using valves to control the flow rate, and employing a cooling circuit with sensors and an air conditioning circuit to manage temperature and pressure.
Safety Compliance and Regulations
Battery thermal management in pharmaceutical applications is subject to stringent regulatory frameworks that ensure safety, efficacy, and reliability. The pharmaceutical industry operates under comprehensive regulations from multiple authorities, including the FDA (Food and Drug Administration), EMA (European Medicines Agency), and ICH (International Council for Harmonisation). These bodies have established specific guidelines addressing battery-powered medical devices and temperature-controlled pharmaceutical storage systems.
FDA regulations, particularly 21 CFR Part 820 for medical devices and 21 CFR Part 211 for pharmaceutical manufacturing, mandate rigorous validation of thermal management systems. These regulations require extensive documentation of design controls, risk management procedures, and performance verification protocols for battery systems used in pharmaceutical applications.
The IEC 60601 series of standards specifically addresses the safety requirements for medical electrical equipment, including detailed specifications for battery thermal management. Compliance with these standards necessitates thorough testing of thermal runaway prevention mechanisms, temperature monitoring systems, and fail-safe protocols.
For pharmaceutical cold chain applications, WHO Technical Report Series No. 961 provides guidelines for temperature-controlled pharmaceutical products. These guidelines emphasize the importance of reliable battery thermal management systems in maintaining product integrity during storage and transportation.
Risk classification frameworks, such as ISO 14971, require manufacturers to identify, evaluate, and mitigate thermal risks associated with battery systems. This includes comprehensive hazard analysis for potential thermal events like overheating, thermal runaway, and temperature fluctuations that could compromise pharmaceutical product quality.
Emerging regulations are increasingly focusing on environmental sustainability aspects of battery thermal management systems. The EU Battery Directive (2006/66/EC) and its recent amendments impose requirements for battery recycling, hazardous material limitations, and energy efficiency standards that directly impact thermal management system design.
Compliance documentation requirements have become more stringent, with regulatory bodies demanding detailed thermal validation studies, stability data, and continuous monitoring records. This includes temperature mapping studies, thermal stress testing results, and documented evidence of thermal management system performance under various environmental conditions.
Regulatory trends indicate a movement toward harmonized global standards for battery thermal management in pharmaceutical applications, with increasing emphasis on real-time monitoring capabilities, predictive thermal management technologies, and enhanced safety features to prevent thermal-related incidents.
FDA regulations, particularly 21 CFR Part 820 for medical devices and 21 CFR Part 211 for pharmaceutical manufacturing, mandate rigorous validation of thermal management systems. These regulations require extensive documentation of design controls, risk management procedures, and performance verification protocols for battery systems used in pharmaceutical applications.
The IEC 60601 series of standards specifically addresses the safety requirements for medical electrical equipment, including detailed specifications for battery thermal management. Compliance with these standards necessitates thorough testing of thermal runaway prevention mechanisms, temperature monitoring systems, and fail-safe protocols.
For pharmaceutical cold chain applications, WHO Technical Report Series No. 961 provides guidelines for temperature-controlled pharmaceutical products. These guidelines emphasize the importance of reliable battery thermal management systems in maintaining product integrity during storage and transportation.
Risk classification frameworks, such as ISO 14971, require manufacturers to identify, evaluate, and mitigate thermal risks associated with battery systems. This includes comprehensive hazard analysis for potential thermal events like overheating, thermal runaway, and temperature fluctuations that could compromise pharmaceutical product quality.
Emerging regulations are increasingly focusing on environmental sustainability aspects of battery thermal management systems. The EU Battery Directive (2006/66/EC) and its recent amendments impose requirements for battery recycling, hazardous material limitations, and energy efficiency standards that directly impact thermal management system design.
Compliance documentation requirements have become more stringent, with regulatory bodies demanding detailed thermal validation studies, stability data, and continuous monitoring records. This includes temperature mapping studies, thermal stress testing results, and documented evidence of thermal management system performance under various environmental conditions.
Regulatory trends indicate a movement toward harmonized global standards for battery thermal management in pharmaceutical applications, with increasing emphasis on real-time monitoring capabilities, predictive thermal management technologies, and enhanced safety features to prevent thermal-related incidents.
Environmental Impact Assessment
The environmental impact of battery thermal management systems in pharmaceutical applications extends beyond their immediate operational efficiency. These systems, while critical for maintaining medication stability, contribute to the pharmaceutical industry's overall environmental footprint through energy consumption, material usage, and waste generation.
Energy consumption represents a significant environmental concern in battery thermal management. Traditional cooling systems often rely on continuous power supply, contributing to greenhouse gas emissions when powered by non-renewable sources. Recent assessments indicate that pharmaceutical cold chain operations account for approximately 3-4% of the industry's total carbon footprint, with thermal management systems being substantial contributors.
Material sustainability presents another environmental challenge. Conventional thermal management solutions frequently incorporate materials with high environmental impact, including certain refrigerants with significant global warming potential and non-biodegradable insulation components. The lifecycle assessment of these materials reveals concerning environmental burdens, particularly in disposal phases.
Waste generation from battery thermal management systems primarily stems from component replacement and system obsolescence. Batteries themselves contain potentially hazardous materials that require specialized disposal procedures. Studies indicate that improper disposal of lithium-ion batteries used in pharmaceutical thermal management can lead to soil contamination and water pollution through leaching of heavy metals and electrolytes.
Regulatory frameworks increasingly address these environmental concerns. The EU's F-Gas Regulation and similar global initiatives are progressively restricting high-GWP refrigerants commonly used in pharmaceutical cooling systems. Meanwhile, extended producer responsibility regulations are shifting end-of-life management responsibilities to manufacturers, encouraging more sustainable design approaches.
Emerging sustainable alternatives show promising environmental benefits. Phase-change materials derived from bio-based sources demonstrate reduced environmental impact while maintaining thermal performance. Additionally, passive cooling technologies that minimize or eliminate energy requirements during operation significantly reduce lifetime carbon emissions compared to active cooling systems.
Water consumption represents an often-overlooked environmental aspect of thermal management systems. Water-cooled systems, while energy-efficient, can place significant pressure on local water resources, particularly in water-stressed regions where many pharmaceutical manufacturing facilities operate.
Energy consumption represents a significant environmental concern in battery thermal management. Traditional cooling systems often rely on continuous power supply, contributing to greenhouse gas emissions when powered by non-renewable sources. Recent assessments indicate that pharmaceutical cold chain operations account for approximately 3-4% of the industry's total carbon footprint, with thermal management systems being substantial contributors.
Material sustainability presents another environmental challenge. Conventional thermal management solutions frequently incorporate materials with high environmental impact, including certain refrigerants with significant global warming potential and non-biodegradable insulation components. The lifecycle assessment of these materials reveals concerning environmental burdens, particularly in disposal phases.
Waste generation from battery thermal management systems primarily stems from component replacement and system obsolescence. Batteries themselves contain potentially hazardous materials that require specialized disposal procedures. Studies indicate that improper disposal of lithium-ion batteries used in pharmaceutical thermal management can lead to soil contamination and water pollution through leaching of heavy metals and electrolytes.
Regulatory frameworks increasingly address these environmental concerns. The EU's F-Gas Regulation and similar global initiatives are progressively restricting high-GWP refrigerants commonly used in pharmaceutical cooling systems. Meanwhile, extended producer responsibility regulations are shifting end-of-life management responsibilities to manufacturers, encouraging more sustainable design approaches.
Emerging sustainable alternatives show promising environmental benefits. Phase-change materials derived from bio-based sources demonstrate reduced environmental impact while maintaining thermal performance. Additionally, passive cooling technologies that minimize or eliminate energy requirements during operation significantly reduce lifetime carbon emissions compared to active cooling systems.
Water consumption represents an often-overlooked environmental aspect of thermal management systems. Water-cooled systems, while energy-efficient, can place significant pressure on local water resources, particularly in water-stressed regions where many pharmaceutical manufacturing facilities operate.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!