Investigating the Phase Dynamics of Nitinol in Biological Systems
AUG 6, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nitinol Phase Dynamics in Biosystems: Background and Objectives
Nitinol, an equiatomic alloy of nickel and titanium, has emerged as a revolutionary material in biomedical applications due to its unique shape memory and superelastic properties. The investigation of Nitinol's phase dynamics in biological systems represents a critical area of research, with far-reaching implications for medical device design and implant technology.
The development of Nitinol can be traced back to 1962 when William J. Buehler and Frederick Wang at the Naval Ordnance Laboratory discovered its extraordinary properties. Since then, the material has undergone extensive research and development, leading to its widespread adoption in various biomedical applications, including stents, orthodontic wires, and surgical instruments.
The phase dynamics of Nitinol are central to its functionality in biological systems. At its core, Nitinol exhibits two distinct crystal structures: austenite (high-temperature phase) and martensite (low-temperature phase). The transition between these phases, known as the martensitic transformation, is responsible for the material's shape memory and superelastic behavior.
In biological systems, Nitinol's phase dynamics are influenced by various factors, including temperature, stress, and the surrounding physiological environment. Understanding these dynamics is crucial for predicting and optimizing the material's performance in vivo. The interaction between Nitinol and biological tissues, fluids, and cellular components can significantly impact its phase behavior and, consequently, its mechanical properties and biocompatibility.
The primary objective of investigating Nitinol's phase dynamics in biological systems is to enhance its efficacy and safety in medical applications. This research aims to elucidate the complex interplay between the material's microstructure, phase transformations, and the biological environment. By gaining a deeper understanding of these interactions, researchers and engineers can develop more advanced Nitinol-based medical devices with improved functionality, durability, and biocompatibility.
Key areas of focus in this investigation include the effects of cyclic loading on Nitinol's phase stability in physiological conditions, the impact of surface treatments on phase transformation temperatures, and the role of Nitinol's phase dynamics in its corrosion resistance and fatigue behavior within biological systems. Additionally, researchers are exploring how Nitinol's phase dynamics can be tailored to specific medical applications, such as optimizing stent deployment or enhancing the material's ability to promote tissue healing.
As the field of biomedical engineering continues to advance, the study of Nitinol's phase dynamics in biological systems remains at the forefront of materials science and medical technology. This research not only promises to unlock new possibilities for Nitinol-based medical devices but also contributes to our broader understanding of smart materials in biological environments, paving the way for innovative therapeutic approaches and improved patient outcomes.
The development of Nitinol can be traced back to 1962 when William J. Buehler and Frederick Wang at the Naval Ordnance Laboratory discovered its extraordinary properties. Since then, the material has undergone extensive research and development, leading to its widespread adoption in various biomedical applications, including stents, orthodontic wires, and surgical instruments.
The phase dynamics of Nitinol are central to its functionality in biological systems. At its core, Nitinol exhibits two distinct crystal structures: austenite (high-temperature phase) and martensite (low-temperature phase). The transition between these phases, known as the martensitic transformation, is responsible for the material's shape memory and superelastic behavior.
In biological systems, Nitinol's phase dynamics are influenced by various factors, including temperature, stress, and the surrounding physiological environment. Understanding these dynamics is crucial for predicting and optimizing the material's performance in vivo. The interaction between Nitinol and biological tissues, fluids, and cellular components can significantly impact its phase behavior and, consequently, its mechanical properties and biocompatibility.
The primary objective of investigating Nitinol's phase dynamics in biological systems is to enhance its efficacy and safety in medical applications. This research aims to elucidate the complex interplay between the material's microstructure, phase transformations, and the biological environment. By gaining a deeper understanding of these interactions, researchers and engineers can develop more advanced Nitinol-based medical devices with improved functionality, durability, and biocompatibility.
Key areas of focus in this investigation include the effects of cyclic loading on Nitinol's phase stability in physiological conditions, the impact of surface treatments on phase transformation temperatures, and the role of Nitinol's phase dynamics in its corrosion resistance and fatigue behavior within biological systems. Additionally, researchers are exploring how Nitinol's phase dynamics can be tailored to specific medical applications, such as optimizing stent deployment or enhancing the material's ability to promote tissue healing.
As the field of biomedical engineering continues to advance, the study of Nitinol's phase dynamics in biological systems remains at the forefront of materials science and medical technology. This research not only promises to unlock new possibilities for Nitinol-based medical devices but also contributes to our broader understanding of smart materials in biological environments, paving the way for innovative therapeutic approaches and improved patient outcomes.
Market Analysis for Nitinol in Biomedical Applications
The market for Nitinol in biomedical applications has experienced significant growth in recent years, driven by the material's unique properties and expanding use cases in medical devices. Nitinol, an alloy of nickel and titanium, exhibits shape memory and superelasticity, making it ideal for various medical implants and instruments.
The global Nitinol medical devices market was valued at approximately $12 billion in 2020 and is projected to reach $23 billion by 2027, growing at a CAGR of around 8.5% during the forecast period. This growth is primarily attributed to the increasing prevalence of cardiovascular diseases, rising demand for minimally invasive surgical procedures, and technological advancements in Nitinol-based medical devices.
Cardiovascular applications dominate the Nitinol market, accounting for over 50% of the total market share. Stents, guidewires, and catheters are the primary products driving this segment. The orthopedic segment is also witnessing rapid growth, with Nitinol being used in spinal implants, bone staples, and arthroscopic instruments.
Geographically, North America holds the largest market share, followed by Europe and Asia-Pacific. The United States, in particular, is a key market due to its advanced healthcare infrastructure and high adoption rate of innovative medical technologies. However, emerging economies in Asia-Pacific, such as China and India, are expected to exhibit the highest growth rates in the coming years.
Key market players include Medtronic, Abbott Laboratories, Boston Scientific Corporation, and B. Braun Melsungen AG. These companies are investing heavily in research and development to expand their product portfolios and maintain their competitive edge. Collaborations between medical device manufacturers and research institutions are also on the rise, focusing on developing novel Nitinol-based solutions for unmet medical needs.
The market is witnessing several trends that are shaping its future. There is a growing focus on developing Nitinol-based devices for neurovascular applications, such as aneurysm treatment. Additionally, the integration of Nitinol with other advanced materials and technologies, like drug-eluting coatings and 3D printing, is opening up new possibilities for customized medical solutions.
Despite the positive outlook, the market faces challenges such as the high cost of Nitinol devices, stringent regulatory requirements, and concerns regarding nickel allergies in some patients. However, ongoing research into improving Nitinol's biocompatibility and reducing production costs is expected to address these issues and further drive market growth.
The global Nitinol medical devices market was valued at approximately $12 billion in 2020 and is projected to reach $23 billion by 2027, growing at a CAGR of around 8.5% during the forecast period. This growth is primarily attributed to the increasing prevalence of cardiovascular diseases, rising demand for minimally invasive surgical procedures, and technological advancements in Nitinol-based medical devices.
Cardiovascular applications dominate the Nitinol market, accounting for over 50% of the total market share. Stents, guidewires, and catheters are the primary products driving this segment. The orthopedic segment is also witnessing rapid growth, with Nitinol being used in spinal implants, bone staples, and arthroscopic instruments.
Geographically, North America holds the largest market share, followed by Europe and Asia-Pacific. The United States, in particular, is a key market due to its advanced healthcare infrastructure and high adoption rate of innovative medical technologies. However, emerging economies in Asia-Pacific, such as China and India, are expected to exhibit the highest growth rates in the coming years.
Key market players include Medtronic, Abbott Laboratories, Boston Scientific Corporation, and B. Braun Melsungen AG. These companies are investing heavily in research and development to expand their product portfolios and maintain their competitive edge. Collaborations between medical device manufacturers and research institutions are also on the rise, focusing on developing novel Nitinol-based solutions for unmet medical needs.
The market is witnessing several trends that are shaping its future. There is a growing focus on developing Nitinol-based devices for neurovascular applications, such as aneurysm treatment. Additionally, the integration of Nitinol with other advanced materials and technologies, like drug-eluting coatings and 3D printing, is opening up new possibilities for customized medical solutions.
Despite the positive outlook, the market faces challenges such as the high cost of Nitinol devices, stringent regulatory requirements, and concerns regarding nickel allergies in some patients. However, ongoing research into improving Nitinol's biocompatibility and reducing production costs is expected to address these issues and further drive market growth.
Current Challenges in Nitinol Phase Behavior Research
The investigation of Nitinol phase dynamics in biological systems presents several significant challenges that researchers are currently grappling with. One of the primary obstacles is the complexity of the biological environment, which can significantly influence the phase behavior of Nitinol. The presence of various proteins, enzymes, and other biomolecules can interact with the Nitinol surface, potentially altering its phase transformation characteristics.
Another challenge lies in the real-time monitoring of phase changes within living organisms. Traditional methods for studying Nitinol phase dynamics often involve techniques that are not compatible with in vivo conditions. This necessitates the development of novel, non-invasive imaging and sensing technologies capable of detecting subtle phase transitions in biological settings without disrupting the surrounding tissues.
The variability of physiological conditions among different organisms and even within the same organism poses another hurdle. Factors such as temperature fluctuations, pH changes, and mechanical stresses in biological systems can all affect Nitinol's phase behavior. Researchers must account for these dynamic variables when designing experiments and interpreting results, making it challenging to establish standardized protocols for studying Nitinol in diverse biological contexts.
Furthermore, the long-term stability and biocompatibility of Nitinol in biological systems remain areas of concern. While Nitinol is generally considered biocompatible, the potential for corrosion or degradation over extended periods could alter its phase dynamics and impact surrounding tissues. Understanding these long-term effects requires prolonged studies, which are often difficult to conduct and interpret.
The miniaturization of Nitinol-based devices for biological applications introduces additional complexities. As the size of Nitinol structures decreases to the nanoscale, their phase transformation behaviors can deviate significantly from bulk properties. This size-dependent behavior necessitates new theoretical frameworks and experimental approaches to accurately predict and control phase dynamics in nano-sized Nitinol structures within biological systems.
Lastly, the integration of Nitinol with other materials in biomedical devices presents challenges in understanding the composite phase behavior. The interaction between Nitinol and other materials, such as polymers or ceramics, can lead to unexpected phase transformations or mechanical responses. Elucidating these complex interactions and their effects on the overall performance of biomedical devices remains a significant research challenge.
Another challenge lies in the real-time monitoring of phase changes within living organisms. Traditional methods for studying Nitinol phase dynamics often involve techniques that are not compatible with in vivo conditions. This necessitates the development of novel, non-invasive imaging and sensing technologies capable of detecting subtle phase transitions in biological settings without disrupting the surrounding tissues.
The variability of physiological conditions among different organisms and even within the same organism poses another hurdle. Factors such as temperature fluctuations, pH changes, and mechanical stresses in biological systems can all affect Nitinol's phase behavior. Researchers must account for these dynamic variables when designing experiments and interpreting results, making it challenging to establish standardized protocols for studying Nitinol in diverse biological contexts.
Furthermore, the long-term stability and biocompatibility of Nitinol in biological systems remain areas of concern. While Nitinol is generally considered biocompatible, the potential for corrosion or degradation over extended periods could alter its phase dynamics and impact surrounding tissues. Understanding these long-term effects requires prolonged studies, which are often difficult to conduct and interpret.
The miniaturization of Nitinol-based devices for biological applications introduces additional complexities. As the size of Nitinol structures decreases to the nanoscale, their phase transformation behaviors can deviate significantly from bulk properties. This size-dependent behavior necessitates new theoretical frameworks and experimental approaches to accurately predict and control phase dynamics in nano-sized Nitinol structures within biological systems.
Lastly, the integration of Nitinol with other materials in biomedical devices presents challenges in understanding the composite phase behavior. The interaction between Nitinol and other materials, such as polymers or ceramics, can lead to unexpected phase transformations or mechanical responses. Elucidating these complex interactions and their effects on the overall performance of biomedical devices remains a significant research challenge.
Existing Methodologies for Studying Nitinol Phase Dynamics
01 Phase transformation characteristics of Nitinol
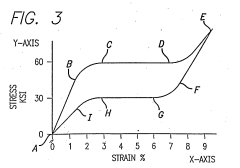
Nitinol exhibits unique phase transformation properties, transitioning between austenite and martensite phases. This transformation is responsible for its shape memory and superelastic behavior, which are crucial for various applications. The dynamics of these phase changes are influenced by temperature, stress, and composition, affecting the material's mechanical properties and performance.- Phase transformation characteristics of Nitinol: Nitinol exhibits unique phase transformation properties, transitioning between austenite and martensite phases. This transformation is responsible for its shape memory and superelastic behavior, which are crucial for various applications. The dynamics of these phase changes are influenced by temperature and stress, affecting the material's mechanical properties.
- Temperature-induced phase changes in Nitinol: The phase dynamics of Nitinol are highly temperature-dependent. As temperature increases, the material transforms from martensite to austenite, and vice versa when cooled. This temperature-induced phase change is the basis for Nitinol's shape memory effect, allowing it to return to a predetermined shape when heated above its transformation temperature.
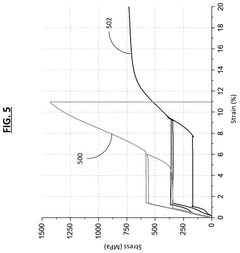
- Stress-induced phase transformation in Nitinol: Nitinol can undergo stress-induced phase transformation, where applied stress causes a shift from austenite to martensite. This property is the foundation of Nitinol's superelasticity, allowing it to undergo large deformations and return to its original shape upon stress removal. The stress-induced phase dynamics are critical in applications requiring high flexibility and recovery.
- Fatigue behavior and cyclic loading effects on Nitinol phase dynamics: The phase dynamics of Nitinol are affected by cyclic loading and fatigue. Repeated stress-induced phase transformations can lead to changes in transformation temperatures and mechanical properties. Understanding these effects is crucial for designing Nitinol components with long-term stability and reliability, especially in applications involving frequent shape changes or stress cycles.
- Microstructural aspects of Nitinol phase dynamics: The phase dynamics of Nitinol are closely related to its microstructure. Factors such as grain size, texture, and precipitates can significantly influence the phase transformation behavior. Heat treatment and processing methods can be used to modify the microstructure and, consequently, the phase dynamics, allowing for tailored properties in specific applications.
02 Temperature-induced phase changes in Nitinol
Temperature plays a significant role in Nitinol's phase dynamics. The material undergoes a reversible transformation between its low-temperature martensite phase and high-temperature austenite phase. This temperature-dependent behavior is utilized in various applications, including actuators, sensors, and medical devices. Understanding and controlling these temperature-induced phase changes are crucial for optimizing Nitinol's performance in different environments.Expand Specific Solutions03 Stress-induced phase transformation in Nitinol
Nitinol can undergo phase transformation not only due to temperature changes but also in response to applied stress. This stress-induced transformation is the basis for its superelastic behavior, allowing the material to recover large strains. The dynamics of this stress-induced phase change are important in applications where Nitinol is subjected to mechanical loading, such as in medical stents or orthodontic wires.Expand Specific Solutions04 Composition effects on Nitinol phase dynamics
The composition of Nitinol, particularly the ratio of nickel to titanium, significantly influences its phase transformation characteristics. Small variations in composition can alter transformation temperatures, hysteresis, and mechanical properties. Understanding and controlling these compositional effects are crucial for tailoring Nitinol's behavior for specific applications and ensuring consistent performance across different batches of the material.Expand Specific Solutions05 Characterization and modeling of Nitinol phase dynamics
Advanced characterization techniques and modeling approaches are employed to study and predict Nitinol's phase dynamics. These methods include differential scanning calorimetry, in-situ X-ray diffraction, and computational models that simulate phase transformations. Such tools are essential for understanding the complex behavior of Nitinol under various conditions and for designing optimal Nitinol-based devices and structures.Expand Specific Solutions
Key Players in Nitinol-Based Biomedical Devices
The investigation into the phase dynamics of Nitinol in biological systems is at an early stage of development, with the market still emerging. The technology's potential applications in medical devices and bioengineering are driving interest, but the market size remains relatively small. Research institutions like The Salk Institute for Biological Studies and universities such as Washington University in St. Louis are leading the charge in fundamental research. Meanwhile, companies like Janssen Pharmaceutica NV and Merck Sharp & Dohme Corp. are exploring potential commercial applications. The technology's maturity is still low, with significant challenges in understanding Nitinol's behavior in complex biological environments. Collaborative efforts between academia and industry are crucial for advancing this field.
Institute of Metal Research Chinese Academy of Sciences
Technical Solution: The Institute of Metal Research (IMR) has been at the forefront of Nitinol research in biological systems. They have developed advanced techniques for controlling the phase dynamics of Nitinol, including precise heat treatment protocols and surface modification methods. Their research focuses on optimizing the superelastic and shape memory properties of Nitinol for biomedical applications. IMR has successfully engineered Nitinol-based stents with improved biocompatibility and reduced risk of restenosis[1]. They have also investigated the use of Nitinol in orthopedic implants, demonstrating enhanced osseointegration and mechanical compatibility with bone tissue[2]. Recent studies have explored the potential of Nitinol nanoparticles for targeted drug delivery in cancer treatment, leveraging the material's phase transformation behavior to trigger drug release[3].
Strengths: Extensive experience in Nitinol metallurgy, advanced characterization facilities, and strong collaboration with medical institutions. Weaknesses: Potential challenges in translating laboratory findings to clinical applications and regulatory hurdles for novel Nitinol-based medical devices.
Cornell University
Technical Solution: Cornell University has made significant contributions to understanding the phase dynamics of Nitinol in biological systems. Their research team has developed innovative in situ characterization techniques to study Nitinol's behavior under physiological conditions. They have pioneered the use of synchrotron-based X-ray diffraction to observe real-time phase transformations in Nitinol implants[4]. Cornell researchers have also investigated the impact of cyclic loading on Nitinol's phase stability in cardiovascular applications, leading to improved fatigue resistance in Nitinol-based devices[5]. Additionally, they have explored the potential of Nitinol as a substrate for cell culture, demonstrating that its phase transitions can influence cell adhesion and differentiation[6]. Recent work has focused on developing Nitinol-based actuators for minimally invasive surgical tools, utilizing the material's unique phase dynamics for precise control and manipulation.
Strengths: Cutting-edge characterization techniques, interdisciplinary approach combining materials science and bioengineering. Weaknesses: May face challenges in scaling up laboratory discoveries for commercial applications.
Breakthrough Technologies in Nitinol Phase Analysis
Wires of superelastic nickel-titanium alloy and methods of forming the same
PatentPendingUS20240336997A1
Innovation
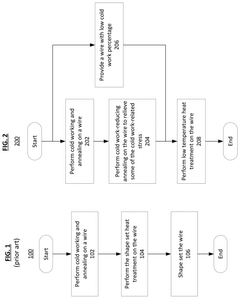
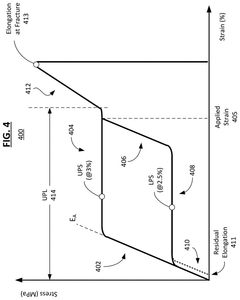
- The development of nickel-titanium alloys with specific grain sizes between 0.2 and 10 microns, combined with shape set heat treatments at temperatures between 225° C. and 350° C. for 20-240 minutes, and partial annealing at 550° C. to 700° C. for up to 60 minutes, to achieve increased recoverable strain, upper plateau length, and elongation at fracture.
Nitinol alloy for with good mechanical stability and a good superelastic operating window
PatentWO2006081011A2
Innovation
- A nickel-titanium alloy with a ternary element such as platinum or palladium is used to enhance radiopacity while maintaining superelastic properties, allowing for a thinner strut design that maintains flexibility and mechanical stability.
Biocompatibility and Safety Considerations
The biocompatibility and safety considerations of Nitinol in biological systems are paramount when investigating its phase dynamics. Nitinol, an alloy of nickel and titanium, exhibits unique shape memory and superelastic properties, making it attractive for various biomedical applications. However, its use in biological environments necessitates a thorough evaluation of potential risks and safety concerns.
One of the primary considerations is the potential release of nickel ions from Nitinol implants or devices. Nickel is known to cause allergic reactions in some individuals and may have toxic effects at high concentrations. Studies have shown that the release of nickel ions from Nitinol is generally low and within acceptable limits, but long-term exposure effects must be carefully assessed. The formation of a stable titanium oxide layer on the surface of Nitinol plays a crucial role in minimizing nickel release and enhancing biocompatibility.
The mechanical properties of Nitinol, particularly its superelasticity, can impact tissue interaction and potential adverse effects. While the material's ability to conform to anatomical structures is beneficial, it may also lead to localized stress concentrations in surrounding tissues. Careful design and optimization of Nitinol-based devices are essential to minimize these effects and ensure proper integration with biological systems.
Surface modifications and coatings have been explored to further enhance the biocompatibility of Nitinol. Techniques such as plasma treatment, chemical etching, and the application of biocompatible coatings can improve surface properties, reduce nickel release, and promote favorable cellular responses. These modifications can also influence the material's corrosion resistance, which is crucial for long-term implant stability and safety.
The phase transformation behavior of Nitinol in biological environments must be thoroughly understood to ensure safe and predictable performance. Factors such as temperature fluctuations, mechanical stresses, and chemical interactions within the body can influence the material's phase dynamics. It is essential to characterize these behaviors under physiological conditions to prevent unexpected shape changes or mechanical responses that could compromise device function or patient safety.
Biocompatibility testing protocols for Nitinol-based devices typically include in vitro cytotoxicity assays, genotoxicity studies, and in vivo implantation tests. These evaluations assess the material's impact on cell viability, potential DNA damage, and tissue response over time. Additionally, hemocompatibility testing is crucial for devices in contact with blood, ensuring that Nitinol does not induce thrombosis or hemolysis.
Regulatory considerations play a significant role in the development and approval of Nitinol-based medical devices. Compliance with standards such as ISO 10993 for biological evaluation of medical devices is essential. Manufacturers must provide comprehensive data on material characterization, biocompatibility testing, and risk assessment to regulatory bodies to demonstrate the safety and efficacy of their Nitinol-based products.
One of the primary considerations is the potential release of nickel ions from Nitinol implants or devices. Nickel is known to cause allergic reactions in some individuals and may have toxic effects at high concentrations. Studies have shown that the release of nickel ions from Nitinol is generally low and within acceptable limits, but long-term exposure effects must be carefully assessed. The formation of a stable titanium oxide layer on the surface of Nitinol plays a crucial role in minimizing nickel release and enhancing biocompatibility.
The mechanical properties of Nitinol, particularly its superelasticity, can impact tissue interaction and potential adverse effects. While the material's ability to conform to anatomical structures is beneficial, it may also lead to localized stress concentrations in surrounding tissues. Careful design and optimization of Nitinol-based devices are essential to minimize these effects and ensure proper integration with biological systems.
Surface modifications and coatings have been explored to further enhance the biocompatibility of Nitinol. Techniques such as plasma treatment, chemical etching, and the application of biocompatible coatings can improve surface properties, reduce nickel release, and promote favorable cellular responses. These modifications can also influence the material's corrosion resistance, which is crucial for long-term implant stability and safety.
The phase transformation behavior of Nitinol in biological environments must be thoroughly understood to ensure safe and predictable performance. Factors such as temperature fluctuations, mechanical stresses, and chemical interactions within the body can influence the material's phase dynamics. It is essential to characterize these behaviors under physiological conditions to prevent unexpected shape changes or mechanical responses that could compromise device function or patient safety.
Biocompatibility testing protocols for Nitinol-based devices typically include in vitro cytotoxicity assays, genotoxicity studies, and in vivo implantation tests. These evaluations assess the material's impact on cell viability, potential DNA damage, and tissue response over time. Additionally, hemocompatibility testing is crucial for devices in contact with blood, ensuring that Nitinol does not induce thrombosis or hemolysis.
Regulatory considerations play a significant role in the development and approval of Nitinol-based medical devices. Compliance with standards such as ISO 10993 for biological evaluation of medical devices is essential. Manufacturers must provide comprehensive data on material characterization, biocompatibility testing, and risk assessment to regulatory bodies to demonstrate the safety and efficacy of their Nitinol-based products.
Regulatory Framework for Nitinol Medical Devices
The regulatory framework for Nitinol medical devices is a complex and evolving landscape that reflects the unique properties and applications of this shape memory alloy in biological systems. As Nitinol continues to gain prominence in medical applications, regulatory bodies worldwide have developed specific guidelines and standards to ensure the safety and efficacy of devices incorporating this material.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating Nitinol-based medical devices. The FDA has established a comprehensive set of requirements for manufacturers, including rigorous testing protocols to assess the biocompatibility, mechanical properties, and long-term stability of Nitinol implants. These guidelines are particularly stringent due to the material's phase transformation behavior and its potential interactions with biological tissues.
The European Union, through its Medical Device Regulation (MDR), has also implemented specific provisions for Nitinol devices. The MDR emphasizes the need for thorough risk assessment and post-market surveillance, recognizing the unique challenges posed by Nitinol's superelastic and shape memory properties in vivo. Manufacturers must demonstrate compliance with these regulations to obtain CE marking for their Nitinol-based products.
International standards, such as those developed by ASTM International and the International Organization for Standardization (ISO), provide crucial guidance for the testing and characterization of Nitinol medical devices. These standards address various aspects, including fatigue resistance, corrosion behavior, and surface finish requirements, which are critical for ensuring the long-term performance and safety of Nitinol implants in biological environments.
Regulatory bodies are increasingly focusing on the potential for nickel release from Nitinol devices, given the allergenic properties of nickel. As a result, manufacturers are required to conduct extensive leaching studies and implement surface treatments to minimize nickel release. This aspect of regulation is particularly relevant when investigating the phase dynamics of Nitinol in biological systems, as the material's phase transformations can influence its corrosion behavior and ion release characteristics.
The regulatory framework also addresses the unique manufacturing processes associated with Nitinol devices. Quality control measures, such as those outlined in ISO 13485 for medical device quality management systems, are essential for ensuring consistency in the production of Nitinol components with precise transformation temperatures and mechanical properties. These regulations extend to the entire supply chain, from raw material sourcing to final device assembly.
As research continues to uncover new insights into the behavior of Nitinol in biological systems, regulatory requirements are expected to evolve. Emerging areas of focus include the long-term effects of cyclic loading on Nitinol's phase stability, the potential for fretting corrosion in modular implants, and the impact of sterilization processes on the material's properties. Manufacturers and researchers must stay abreast of these regulatory developments to ensure compliance and drive innovation in Nitinol-based medical technologies.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating Nitinol-based medical devices. The FDA has established a comprehensive set of requirements for manufacturers, including rigorous testing protocols to assess the biocompatibility, mechanical properties, and long-term stability of Nitinol implants. These guidelines are particularly stringent due to the material's phase transformation behavior and its potential interactions with biological tissues.
The European Union, through its Medical Device Regulation (MDR), has also implemented specific provisions for Nitinol devices. The MDR emphasizes the need for thorough risk assessment and post-market surveillance, recognizing the unique challenges posed by Nitinol's superelastic and shape memory properties in vivo. Manufacturers must demonstrate compliance with these regulations to obtain CE marking for their Nitinol-based products.
International standards, such as those developed by ASTM International and the International Organization for Standardization (ISO), provide crucial guidance for the testing and characterization of Nitinol medical devices. These standards address various aspects, including fatigue resistance, corrosion behavior, and surface finish requirements, which are critical for ensuring the long-term performance and safety of Nitinol implants in biological environments.
Regulatory bodies are increasingly focusing on the potential for nickel release from Nitinol devices, given the allergenic properties of nickel. As a result, manufacturers are required to conduct extensive leaching studies and implement surface treatments to minimize nickel release. This aspect of regulation is particularly relevant when investigating the phase dynamics of Nitinol in biological systems, as the material's phase transformations can influence its corrosion behavior and ion release characteristics.
The regulatory framework also addresses the unique manufacturing processes associated with Nitinol devices. Quality control measures, such as those outlined in ISO 13485 for medical device quality management systems, are essential for ensuring consistency in the production of Nitinol components with precise transformation temperatures and mechanical properties. These regulations extend to the entire supply chain, from raw material sourcing to final device assembly.
As research continues to uncover new insights into the behavior of Nitinol in biological systems, regulatory requirements are expected to evolve. Emerging areas of focus include the long-term effects of cyclic loading on Nitinol's phase stability, the potential for fretting corrosion in modular implants, and the impact of sterilization processes on the material's properties. Manufacturers and researchers must stay abreast of these regulatory developments to ensure compliance and drive innovation in Nitinol-based medical technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!