Nitinol's Part in Improving Ergonomics of Assistive Devices
AUG 6, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nitinol in Assistive Devices: Background and Objectives
Nitinol, a unique alloy of nickel and titanium, has emerged as a revolutionary material in the field of assistive devices, offering unprecedented opportunities for improving ergonomics and user experience. This shape memory alloy, discovered in the 1960s, has been steadily gaining traction in various industries due to its exceptional properties, including superelasticity and shape memory effect.
The evolution of Nitinol technology in assistive devices can be traced back to the early 2000s when researchers began exploring its potential in medical applications. As the understanding of its properties deepened, engineers and designers started incorporating Nitinol into assistive devices, recognizing its ability to enhance functionality and comfort for users with disabilities or mobility challenges.
The primary objective of integrating Nitinol into assistive devices is to overcome the limitations of traditional materials, such as rigid metals or plastics, which often fail to provide the necessary flexibility and adaptability required for optimal ergonomics. By leveraging Nitinol's unique characteristics, developers aim to create devices that can dynamically adjust to users' needs, providing personalized support and improving overall quality of life.
One of the key goals in this technological pursuit is to develop assistive devices that can seamlessly transition between different shapes or levels of rigidity, adapting to various user activities and environmental conditions. This adaptability is crucial for enhancing user comfort, reducing fatigue, and preventing secondary complications often associated with prolonged use of conventional assistive devices.
Another significant objective is to miniaturize and lightweight assistive devices without compromising their functionality or durability. Nitinol's high strength-to-weight ratio and its ability to recover from large strains make it an ideal candidate for achieving this goal, potentially leading to more discreet and user-friendly designs.
The integration of Nitinol in assistive devices also aims to address the challenge of customization. Traditional assistive devices often require extensive customization processes, which can be time-consuming and costly. By incorporating Nitinol components, manufacturers seek to develop devices that can be easily adjusted or programmed to suit individual user needs, potentially reducing the need for multiple fittings and adjustments.
As research in this field progresses, there is a growing focus on combining Nitinol with smart technologies, such as sensors and actuators, to create intelligent assistive devices. These advanced systems could potentially anticipate user needs, automatically adjust to changing conditions, and provide real-time feedback, further enhancing the ergonomic benefits of Nitinol-based assistive devices.
In conclusion, the integration of Nitinol in assistive devices represents a significant technological advancement with the potential to revolutionize the field of ergonomics in assistive technology. By addressing key challenges and leveraging the unique properties of this remarkable alloy, researchers and developers are paving the way for a new generation of adaptive, comfortable, and highly effective assistive devices.
The evolution of Nitinol technology in assistive devices can be traced back to the early 2000s when researchers began exploring its potential in medical applications. As the understanding of its properties deepened, engineers and designers started incorporating Nitinol into assistive devices, recognizing its ability to enhance functionality and comfort for users with disabilities or mobility challenges.
The primary objective of integrating Nitinol into assistive devices is to overcome the limitations of traditional materials, such as rigid metals or plastics, which often fail to provide the necessary flexibility and adaptability required for optimal ergonomics. By leveraging Nitinol's unique characteristics, developers aim to create devices that can dynamically adjust to users' needs, providing personalized support and improving overall quality of life.
One of the key goals in this technological pursuit is to develop assistive devices that can seamlessly transition between different shapes or levels of rigidity, adapting to various user activities and environmental conditions. This adaptability is crucial for enhancing user comfort, reducing fatigue, and preventing secondary complications often associated with prolonged use of conventional assistive devices.
Another significant objective is to miniaturize and lightweight assistive devices without compromising their functionality or durability. Nitinol's high strength-to-weight ratio and its ability to recover from large strains make it an ideal candidate for achieving this goal, potentially leading to more discreet and user-friendly designs.
The integration of Nitinol in assistive devices also aims to address the challenge of customization. Traditional assistive devices often require extensive customization processes, which can be time-consuming and costly. By incorporating Nitinol components, manufacturers seek to develop devices that can be easily adjusted or programmed to suit individual user needs, potentially reducing the need for multiple fittings and adjustments.
As research in this field progresses, there is a growing focus on combining Nitinol with smart technologies, such as sensors and actuators, to create intelligent assistive devices. These advanced systems could potentially anticipate user needs, automatically adjust to changing conditions, and provide real-time feedback, further enhancing the ergonomic benefits of Nitinol-based assistive devices.
In conclusion, the integration of Nitinol in assistive devices represents a significant technological advancement with the potential to revolutionize the field of ergonomics in assistive technology. By addressing key challenges and leveraging the unique properties of this remarkable alloy, researchers and developers are paving the way for a new generation of adaptive, comfortable, and highly effective assistive devices.
Market Analysis for Ergonomic Assistive Devices
The market for ergonomic assistive devices has been experiencing significant growth in recent years, driven by an aging population, increased awareness of workplace ergonomics, and advancements in materials science. The global assistive devices market, which includes ergonomic solutions, is projected to reach substantial value in the coming years, with a compound annual growth rate outpacing many other healthcare sectors.
Demographic trends play a crucial role in market demand. As the population ages, there is a growing need for devices that can enhance mobility, reduce strain, and improve quality of life for seniors and individuals with disabilities. This demographic shift is particularly pronounced in developed countries, where the proportion of elderly citizens is rapidly increasing.
In the workplace, there is a rising emphasis on ergonomics to prevent injuries and improve productivity. This has led to increased demand for ergonomic assistive devices in office environments, manufacturing facilities, and healthcare settings. Employers are recognizing the long-term benefits of investing in ergonomic solutions, including reduced worker compensation claims and improved employee satisfaction.
The healthcare sector represents a significant market for ergonomic assistive devices. Hospitals, rehabilitation centers, and home care services are key consumers of these products. The trend towards home healthcare is particularly noteworthy, as it drives demand for portable and adaptable ergonomic devices that can be used in diverse settings.
Technological advancements, particularly in materials like Nitinol, are reshaping the market landscape. These innovations allow for the development of more sophisticated, lightweight, and user-friendly devices. The integration of smart technologies, such as sensors and connectivity features, is also opening new market segments and enhancing the functionality of traditional assistive devices.
Geographically, North America and Europe currently dominate the market for ergonomic assistive devices, owing to their advanced healthcare systems and higher awareness of ergonomic principles. However, rapid growth is expected in Asia-Pacific regions, particularly in countries like Japan and China, where aging populations and increasing healthcare expenditures are driving market expansion.
Consumer preferences are shifting towards more personalized and aesthetically pleasing assistive devices. This trend is encouraging manufacturers to focus on design aspects alongside functionality, potentially broadening the appeal of these products beyond traditional medical contexts.
The market is characterized by a mix of established medical device companies and innovative startups. Competition is intensifying as new players enter the market with novel technologies and designs. This competitive landscape is likely to drive further innovation and potentially lead to more affordable solutions for consumers.
Demographic trends play a crucial role in market demand. As the population ages, there is a growing need for devices that can enhance mobility, reduce strain, and improve quality of life for seniors and individuals with disabilities. This demographic shift is particularly pronounced in developed countries, where the proportion of elderly citizens is rapidly increasing.
In the workplace, there is a rising emphasis on ergonomics to prevent injuries and improve productivity. This has led to increased demand for ergonomic assistive devices in office environments, manufacturing facilities, and healthcare settings. Employers are recognizing the long-term benefits of investing in ergonomic solutions, including reduced worker compensation claims and improved employee satisfaction.
The healthcare sector represents a significant market for ergonomic assistive devices. Hospitals, rehabilitation centers, and home care services are key consumers of these products. The trend towards home healthcare is particularly noteworthy, as it drives demand for portable and adaptable ergonomic devices that can be used in diverse settings.
Technological advancements, particularly in materials like Nitinol, are reshaping the market landscape. These innovations allow for the development of more sophisticated, lightweight, and user-friendly devices. The integration of smart technologies, such as sensors and connectivity features, is also opening new market segments and enhancing the functionality of traditional assistive devices.
Geographically, North America and Europe currently dominate the market for ergonomic assistive devices, owing to their advanced healthcare systems and higher awareness of ergonomic principles. However, rapid growth is expected in Asia-Pacific regions, particularly in countries like Japan and China, where aging populations and increasing healthcare expenditures are driving market expansion.
Consumer preferences are shifting towards more personalized and aesthetically pleasing assistive devices. This trend is encouraging manufacturers to focus on design aspects alongside functionality, potentially broadening the appeal of these products beyond traditional medical contexts.
The market is characterized by a mix of established medical device companies and innovative startups. Competition is intensifying as new players enter the market with novel technologies and designs. This competitive landscape is likely to drive further innovation and potentially lead to more affordable solutions for consumers.
Nitinol Technology: Current State and Challenges
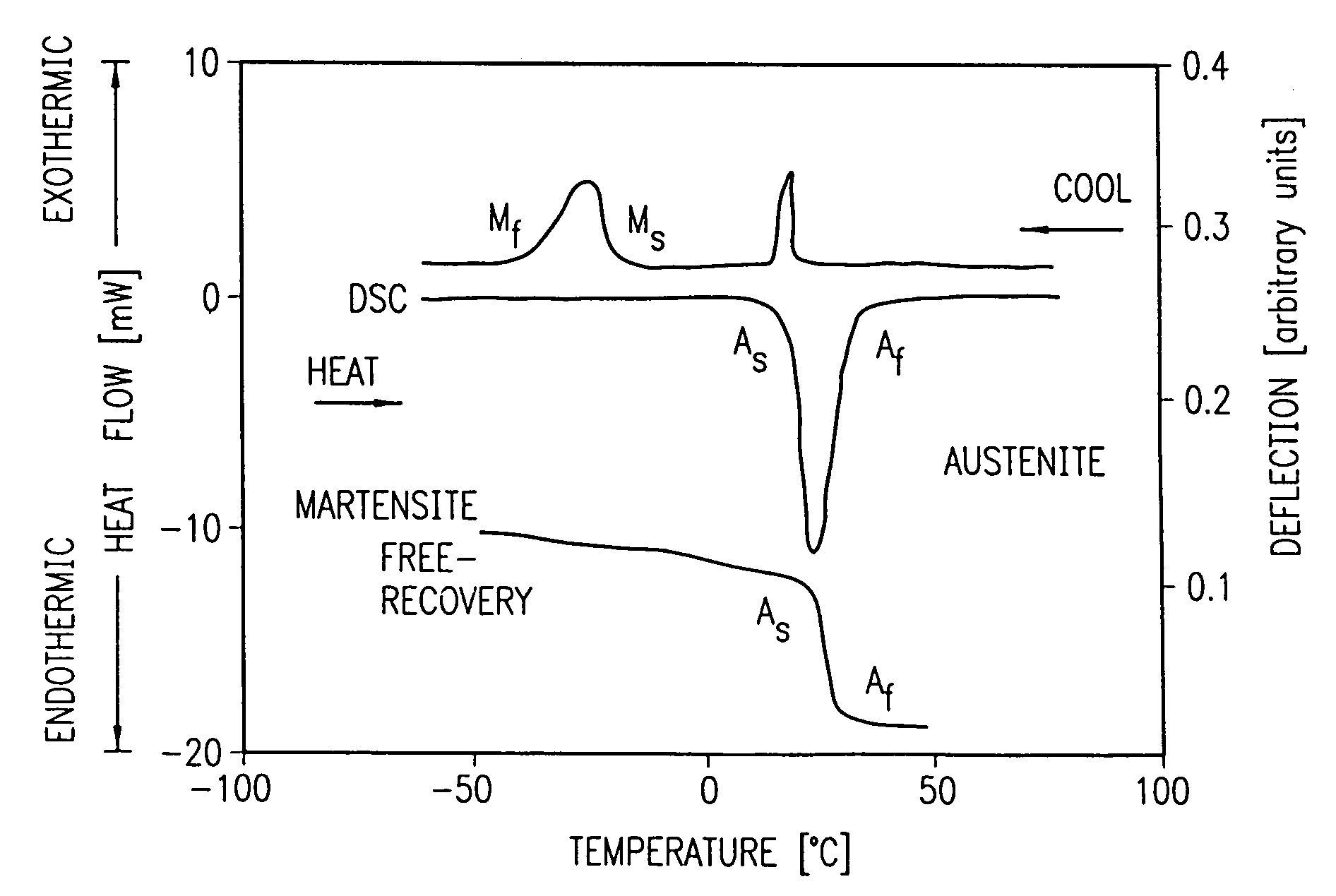
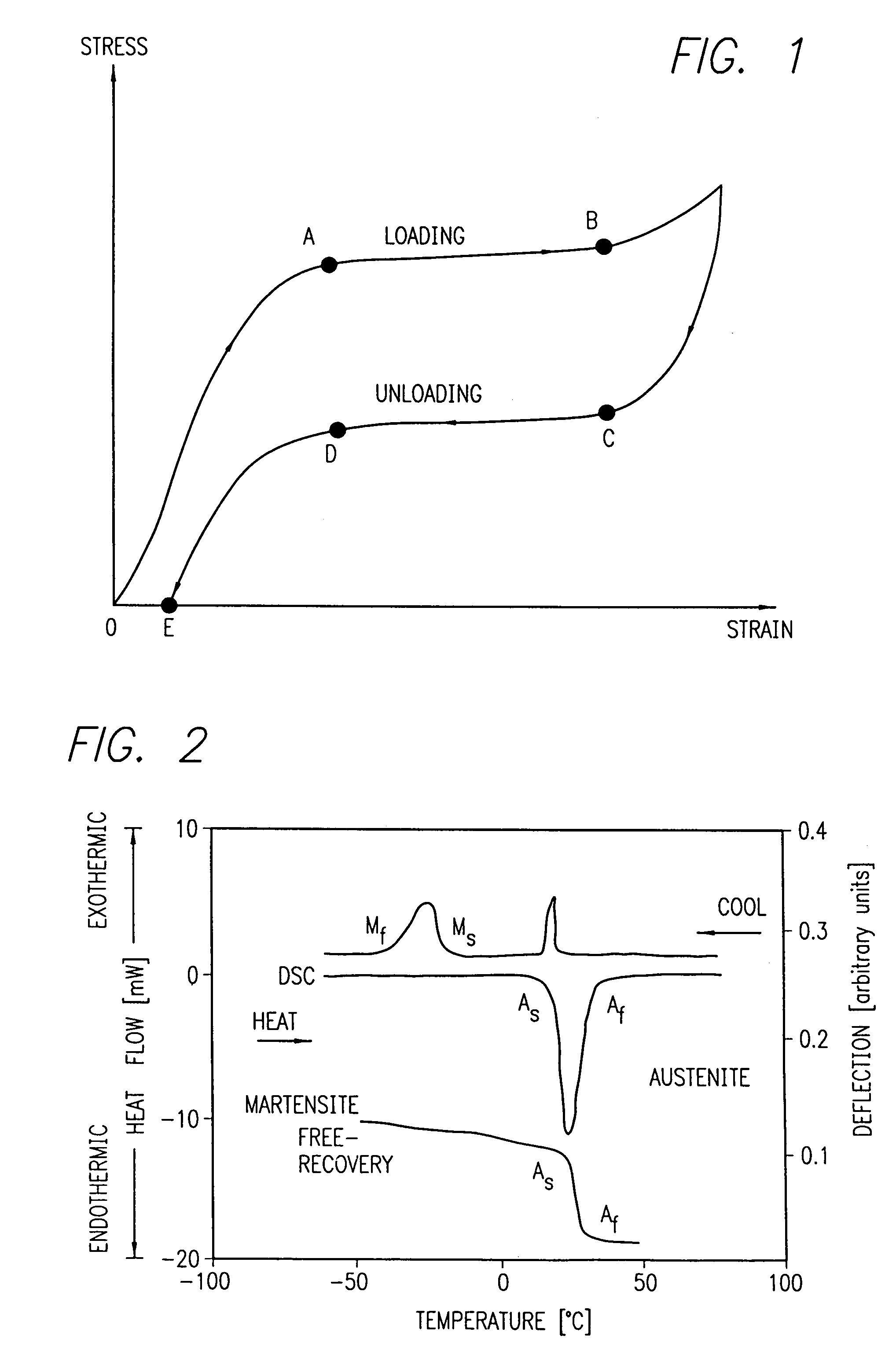
Nitinol, a nickel-titanium alloy, has revolutionized the field of assistive devices with its unique shape memory and superelastic properties. However, the current state of Nitinol technology in this domain faces several challenges that hinder its widespread adoption and optimal utilization.
One of the primary challenges is the complex manufacturing process of Nitinol-based components. The material's sensitivity to temperature and stress during fabrication requires precise control and specialized equipment, leading to higher production costs and limited scalability. This complexity often results in inconsistencies in the final product's performance, particularly in maintaining desired shape memory characteristics across different batches.
Another significant challenge lies in the biocompatibility of Nitinol for long-term use in assistive devices. While generally considered safe, concerns persist regarding the potential release of nickel ions, which may cause allergic reactions or other health issues in some users. Researchers are actively working on surface treatments and coatings to mitigate this risk, but a universally accepted solution remains elusive.
The integration of Nitinol with other materials in assistive devices presents another hurdle. Achieving reliable and durable connections between Nitinol components and traditional materials like plastics or other metals often requires innovative joining techniques. This integration challenge can affect the overall performance and longevity of assistive devices, particularly those subjected to frequent stress and movement.
Furthermore, the behavior of Nitinol under various environmental conditions and long-term use scenarios is not fully understood. Factors such as cyclic loading, corrosion, and fatigue can impact the material's performance over time, potentially compromising the reliability and safety of assistive devices. More comprehensive studies and real-world data are needed to address these concerns and develop predictive models for Nitinol's long-term behavior.
The customization of Nitinol properties for specific assistive device applications remains a challenge. While the material offers a range of shape memory and superelastic behaviors, fine-tuning these properties to meet the diverse needs of different users and device types requires extensive research and development. This customization challenge often leads to compromises in device design or limits the potential benefits of Nitinol in improving ergonomics.
Lastly, the regulatory landscape for Nitinol-based assistive devices presents obstacles to innovation and market entry. The unique properties of Nitinol often necessitate specialized testing and approval processes, which can be time-consuming and costly for manufacturers. This regulatory burden can slow down the introduction of new, potentially beneficial assistive devices to the market.
One of the primary challenges is the complex manufacturing process of Nitinol-based components. The material's sensitivity to temperature and stress during fabrication requires precise control and specialized equipment, leading to higher production costs and limited scalability. This complexity often results in inconsistencies in the final product's performance, particularly in maintaining desired shape memory characteristics across different batches.
Another significant challenge lies in the biocompatibility of Nitinol for long-term use in assistive devices. While generally considered safe, concerns persist regarding the potential release of nickel ions, which may cause allergic reactions or other health issues in some users. Researchers are actively working on surface treatments and coatings to mitigate this risk, but a universally accepted solution remains elusive.
The integration of Nitinol with other materials in assistive devices presents another hurdle. Achieving reliable and durable connections between Nitinol components and traditional materials like plastics or other metals often requires innovative joining techniques. This integration challenge can affect the overall performance and longevity of assistive devices, particularly those subjected to frequent stress and movement.
Furthermore, the behavior of Nitinol under various environmental conditions and long-term use scenarios is not fully understood. Factors such as cyclic loading, corrosion, and fatigue can impact the material's performance over time, potentially compromising the reliability and safety of assistive devices. More comprehensive studies and real-world data are needed to address these concerns and develop predictive models for Nitinol's long-term behavior.
The customization of Nitinol properties for specific assistive device applications remains a challenge. While the material offers a range of shape memory and superelastic behaviors, fine-tuning these properties to meet the diverse needs of different users and device types requires extensive research and development. This customization challenge often leads to compromises in device design or limits the potential benefits of Nitinol in improving ergonomics.
Lastly, the regulatory landscape for Nitinol-based assistive devices presents obstacles to innovation and market entry. The unique properties of Nitinol often necessitate specialized testing and approval processes, which can be time-consuming and costly for manufacturers. This regulatory burden can slow down the introduction of new, potentially beneficial assistive devices to the market.
Current Nitinol Applications in Assistive Devices
01 Shape memory properties in ergonomic applications
Nitinol's shape memory properties are utilized in ergonomic designs to create adaptive and responsive products. These applications include self-adjusting seating, temperature-responsive grips, and customizable support structures that can conform to individual user needs, enhancing comfort and reducing strain in various environments.- Shape memory properties in ergonomic applications: Nitinol's shape memory properties are utilized in ergonomic designs to create adaptive and responsive products. These applications leverage Nitinol's ability to return to a predetermined shape when heated, allowing for customizable and adjustable ergonomic solutions in various fields such as medical devices, furniture, and consumer products.
- Superelasticity in ergonomic design: The superelastic properties of Nitinol are exploited in ergonomic designs to provide flexibility and durability. This characteristic allows for the creation of products that can withstand repeated stress and strain while maintaining their shape, making them ideal for applications in sports equipment, eyewear frames, and orthopedic devices.
- Nitinol in medical ergonomics: Nitinol is extensively used in medical devices and instruments to enhance ergonomics for both patients and healthcare professionals. Its unique properties enable the development of minimally invasive surgical tools, implants, and prosthetics that adapt to the body's anatomy, improving comfort and functionality.
- Ergonomic actuators and sensors: Nitinol-based actuators and sensors are incorporated into ergonomic designs to create smart, responsive systems. These components can detect changes in temperature or stress and respond accordingly, enabling the development of adaptive ergonomic solutions in various applications, including automotive interiors and wearable technology.
- Manufacturing processes for ergonomic Nitinol products: Specialized manufacturing techniques are employed to produce Nitinol-based ergonomic products. These processes include precision shaping, heat treatment, and surface modification to optimize the material's properties for specific ergonomic applications. Advanced manufacturing methods ensure consistent performance and reliability in the final products.
02 Medical devices and instruments
Nitinol is extensively used in medical devices and instruments due to its biocompatibility and unique properties. Ergonomic applications include minimally invasive surgical tools, dental instruments, and orthopedic implants that can adapt to body temperature and anatomy, improving patient comfort and surgical outcomes.Expand Specific Solutions03 Actuators and sensors in ergonomic systems
Nitinol-based actuators and sensors are integrated into ergonomic systems to create smart, responsive environments. These can include automatic adjustments in workstations, vehicle seating, and prosthetics, enhancing user comfort and reducing the risk of repetitive strain injuries.Expand Specific Solutions04 Wearable technology and smart textiles
Nitinol is incorporated into wearable technology and smart textiles to create ergonomic clothing and accessories. Applications include self-adjusting garments, supportive footwear, and adaptive sports equipment that can respond to body movement and environmental conditions for improved comfort and performance.Expand Specific Solutions05 Ergonomic design optimization using Nitinol
Researchers and designers use computational methods and simulations to optimize the use of Nitinol in ergonomic products. This includes analyzing stress distribution, fatigue resistance, and shape recovery to create more efficient and comfortable designs for various applications, from consumer products to industrial equipment.Expand Specific Solutions
Key Players in Nitinol-based Assistive Technology
The market for Nitinol-based assistive devices is in a growth phase, driven by increasing demand for ergonomic and adaptive medical technologies. The global market size for shape memory alloys, including Nitinol, is projected to reach $18 billion by 2025. While the technology is mature in certain applications, there is ongoing innovation in ergonomic assistive devices. Key players like Abbott Laboratories, Boston Scientific, and W. L. Gore & Associates are leading research and development efforts, leveraging Nitinol's unique properties to enhance device flexibility and adaptability. Emerging companies such as Neurvana Medical and Anaconda Biomed are also contributing to technological advancements, particularly in neurovascular applications.
W. L. Gore & Associates, Inc.
Technical Solution: W. L. Gore & Associates has made significant strides in incorporating Nitinol into their medical device portfolio, particularly in improving the ergonomics of assistive devices. Their approach leverages Nitinol's unique properties in combination with their expertise in advanced materials. Gore has developed hybrid structures that combine Nitinol frameworks with their proprietary ePTFE (expanded polytetrafluoroethylene) materials, creating devices with enhanced flexibility and biocompatibility[8]. This synergy allows for the creation of assistive devices that are both durable and comfortable for long-term use. The company has also focused on optimizing the thermomechanical processing of Nitinol to achieve precise shape-setting capabilities, enabling the production of complex geometries that better conform to anatomical structures[9]. Gore's research extends to the development of Nitinol-based actuators for soft robotics applications in assistive technologies, potentially revolutionizing the field of prosthetics and orthotics[10].
Strengths: Innovative material combinations, expertise in thermomechanical processing, and applications in soft robotics. Weaknesses: Complexity in manufacturing processes and potential limitations in scalability for certain applications.
Abbott Laboratories
Technical Solution: Abbott Laboratories has developed innovative Nitinol-based devices for improving ergonomics in assistive medical technologies. Their approach involves using superelastic Nitinol alloys in stents and other implantable devices. These Nitinol-based devices can change shape in response to body temperature, allowing for minimally invasive procedures and improved patient comfort[1]. Abbott has also incorporated Nitinol into catheter-based delivery systems, enhancing flexibility and control during interventional procedures[2]. The company's research focuses on optimizing Nitinol's unique properties to create self-expanding structures that adapt to individual patient anatomies, significantly improving the ergonomics of various assistive devices[3].
Strengths: Advanced shape memory capabilities, minimally invasive applications, and adaptability to patient-specific needs. Weaknesses: Higher production costs and potential for nickel sensitivity in some patients.
Innovative Nitinol Properties for Ergonomic Enhancement
Devices for controlling the unloading of superelastic and shape memory orthopedic implants
PatentActiveUS20170100163A1
Innovation
- The development of devices with adjustable retaining mechanisms, such as internal pins and delivery devices, allows surgeons to control the deformation and recoverable strain of Nitinol staples, screws, plates, and intramedullary implants, enabling precise application and maintenance of compressive loads on bone fragments during healing.
Avoiding stress-induced martensitic transformation in nickel titanium alloys used in medical devices
PatentInactiveUS7175655B1
Innovation
- A process involving heating a shape memory alloy to a temperature above its martensite deformation temperature (Md) to deform it into a restrained shape in an austenitic state, allowing it to be cooled and stored within a delivery system, such as a catheter, without inducing stress-induced martensite, ensuring the alloy remains in an austenitic state during deployment in the body.
Regulatory Framework for Nitinol Medical Devices
The regulatory framework for Nitinol medical devices is a complex and evolving landscape that plays a crucial role in ensuring the safety and efficacy of assistive devices incorporating this unique shape memory alloy. In the United States, the Food and Drug Administration (FDA) is the primary regulatory body overseeing the approval and monitoring of Nitinol-based medical devices. The FDA classifies these devices based on their intended use and risk level, with most Nitinol devices falling under Class II or Class III categories.
For Class II devices, manufacturers typically need to submit a 510(k) premarket notification, demonstrating that their device is substantially equivalent to a predicate device already on the market. This process involves providing detailed information on the device's design, manufacturing processes, and performance characteristics. Special attention is given to the unique properties of Nitinol, such as its superelasticity and shape memory effects, which must be thoroughly documented and validated.
Class III devices, which are considered high-risk, require a more rigorous premarket approval (PMA) process. This involves extensive clinical trials and comprehensive safety and efficacy data. For Nitinol devices in this category, manufacturers must provide in-depth analysis of the material's behavior under various physiological conditions and demonstrate long-term biocompatibility.
In the European Union, Nitinol medical devices fall under the purview of the Medical Device Regulation (MDR), which came into full effect in May 2021. The MDR has introduced more stringent requirements for clinical evidence, post-market surveillance, and traceability. Manufacturers must obtain CE marking to market their devices in the EU, which involves a conformity assessment procedure that varies depending on the device classification.
Both the FDA and EU regulatory frameworks emphasize the importance of quality management systems in the production of Nitinol devices. Manufacturers are required to implement and maintain robust quality control measures throughout the entire product lifecycle, from design and development to production and post-market surveillance.
Specific to Nitinol, regulatory bodies pay close attention to the material's unique characteristics and potential risks. This includes scrutiny of the alloy composition, heat treatment processes, surface finish, and potential for nickel leaching. Manufacturers must provide detailed information on these aspects and demonstrate that their devices meet stringent safety standards.
As the field of assistive devices continues to evolve, regulatory frameworks are adapting to address new challenges and technologies. There is an increasing focus on the long-term performance and durability of Nitinol devices, as well as their interaction with other materials and technologies in complex medical systems. Regulatory bodies are also placing greater emphasis on real-world evidence and post-market data to ensure the ongoing safety and effectiveness of these devices.
For Class II devices, manufacturers typically need to submit a 510(k) premarket notification, demonstrating that their device is substantially equivalent to a predicate device already on the market. This process involves providing detailed information on the device's design, manufacturing processes, and performance characteristics. Special attention is given to the unique properties of Nitinol, such as its superelasticity and shape memory effects, which must be thoroughly documented and validated.
Class III devices, which are considered high-risk, require a more rigorous premarket approval (PMA) process. This involves extensive clinical trials and comprehensive safety and efficacy data. For Nitinol devices in this category, manufacturers must provide in-depth analysis of the material's behavior under various physiological conditions and demonstrate long-term biocompatibility.
In the European Union, Nitinol medical devices fall under the purview of the Medical Device Regulation (MDR), which came into full effect in May 2021. The MDR has introduced more stringent requirements for clinical evidence, post-market surveillance, and traceability. Manufacturers must obtain CE marking to market their devices in the EU, which involves a conformity assessment procedure that varies depending on the device classification.
Both the FDA and EU regulatory frameworks emphasize the importance of quality management systems in the production of Nitinol devices. Manufacturers are required to implement and maintain robust quality control measures throughout the entire product lifecycle, from design and development to production and post-market surveillance.
Specific to Nitinol, regulatory bodies pay close attention to the material's unique characteristics and potential risks. This includes scrutiny of the alloy composition, heat treatment processes, surface finish, and potential for nickel leaching. Manufacturers must provide detailed information on these aspects and demonstrate that their devices meet stringent safety standards.
As the field of assistive devices continues to evolve, regulatory frameworks are adapting to address new challenges and technologies. There is an increasing focus on the long-term performance and durability of Nitinol devices, as well as their interaction with other materials and technologies in complex medical systems. Regulatory bodies are also placing greater emphasis on real-world evidence and post-market data to ensure the ongoing safety and effectiveness of these devices.
Biocompatibility and Safety Considerations
Nitinol, a nickel-titanium alloy, has gained significant attention in the development of assistive devices due to its unique properties. However, its use in medical applications necessitates a thorough examination of biocompatibility and safety considerations. The biocompatibility of Nitinol is primarily influenced by its surface properties and the potential release of nickel ions.
Surface treatments play a crucial role in enhancing the biocompatibility of Nitinol. Techniques such as electropolishing, passivation, and the application of protective coatings can significantly reduce the risk of nickel ion release and improve the material's overall biocompatibility. These treatments create a stable titanium oxide layer on the surface, which acts as a barrier against corrosion and nickel leaching.
The potential release of nickel ions from Nitinol devices is a primary safety concern, as nickel is known to cause allergic reactions in some individuals. Studies have shown that properly treated Nitinol exhibits minimal nickel release, often below the threshold for inducing allergic responses. However, long-term studies are still needed to fully understand the implications of prolonged exposure in various physiological environments.
Mechanical properties of Nitinol, such as its superelasticity and shape memory effect, contribute to its safety profile in assistive devices. These properties allow for the design of devices that can withstand repeated stress without fatigue failure, reducing the risk of mechanical breakdown and potential harm to users. Additionally, the ability of Nitinol to conform to body contours can minimize tissue irritation and improve overall comfort.
Regulatory bodies, including the FDA, have established guidelines for the use of Nitinol in medical devices. These guidelines address issues such as material purity, manufacturing processes, and testing protocols to ensure the safety and efficacy of Nitinol-based assistive devices. Manufacturers must adhere to these standards and conduct rigorous biocompatibility testing before bringing products to market.
The integration of Nitinol in assistive devices also requires consideration of its interaction with other materials and components. Galvanic corrosion can occur when Nitinol is in contact with dissimilar metals, potentially compromising the device's integrity and safety. Proper design and material selection are essential to mitigate these risks and ensure long-term performance and safety.
In conclusion, while Nitinol offers significant advantages in improving the ergonomics of assistive devices, its use must be carefully evaluated from a biocompatibility and safety perspective. Ongoing research and adherence to regulatory standards are crucial to fully harness the potential of Nitinol while ensuring the well-being of users.
Surface treatments play a crucial role in enhancing the biocompatibility of Nitinol. Techniques such as electropolishing, passivation, and the application of protective coatings can significantly reduce the risk of nickel ion release and improve the material's overall biocompatibility. These treatments create a stable titanium oxide layer on the surface, which acts as a barrier against corrosion and nickel leaching.
The potential release of nickel ions from Nitinol devices is a primary safety concern, as nickel is known to cause allergic reactions in some individuals. Studies have shown that properly treated Nitinol exhibits minimal nickel release, often below the threshold for inducing allergic responses. However, long-term studies are still needed to fully understand the implications of prolonged exposure in various physiological environments.
Mechanical properties of Nitinol, such as its superelasticity and shape memory effect, contribute to its safety profile in assistive devices. These properties allow for the design of devices that can withstand repeated stress without fatigue failure, reducing the risk of mechanical breakdown and potential harm to users. Additionally, the ability of Nitinol to conform to body contours can minimize tissue irritation and improve overall comfort.
Regulatory bodies, including the FDA, have established guidelines for the use of Nitinol in medical devices. These guidelines address issues such as material purity, manufacturing processes, and testing protocols to ensure the safety and efficacy of Nitinol-based assistive devices. Manufacturers must adhere to these standards and conduct rigorous biocompatibility testing before bringing products to market.
The integration of Nitinol in assistive devices also requires consideration of its interaction with other materials and components. Galvanic corrosion can occur when Nitinol is in contact with dissimilar metals, potentially compromising the device's integrity and safety. Proper design and material selection are essential to mitigate these risks and ensure long-term performance and safety.
In conclusion, while Nitinol offers significant advantages in improving the ergonomics of assistive devices, its use must be carefully evaluated from a biocompatibility and safety perspective. Ongoing research and adherence to regulatory standards are crucial to fully harness the potential of Nitinol while ensuring the well-being of users.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!