Key Insights into Carbon Tetrachloride's Historical Utilization
JUL 2, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Historical Context
Carbon tetrachloride (CCl4) has a rich and complex history that spans over a century. First synthesized in the 1840s by French chemist Henri Victor Regnault, this compound quickly gained prominence due to its unique properties and versatile applications. Initially, CCl4 was primarily used as a solvent in various industrial processes, owing to its excellent ability to dissolve fats, oils, and other organic substances.
In the early 20th century, carbon tetrachloride found widespread use as a dry cleaning agent and a degreasing solvent in the manufacturing industry. Its non-flammability made it an attractive alternative to more volatile solvents, leading to its adoption in numerous industrial applications. During this period, CCl4 also became a popular fire extinguishing agent, particularly for electrical fires, due to its effectiveness in smothering flames without conducting electricity.
The 1920s and 1930s saw an expansion of CCl4's applications into the agricultural sector. It was widely used as a fumigant for grain storage and as a pesticide, helping to protect crops from insect infestations. This period also marked the beginning of its use in the production of chlorofluorocarbons (CFCs), which would later become a significant environmental concern.
During World War II, carbon tetrachloride played a crucial role in military applications. It was used in the production of smoke screens and as a cleaning agent for military equipment. The post-war era witnessed a surge in its industrial applications, particularly in the burgeoning plastics and electronics industries.
However, the 1970s marked a turning point in the history of CCl4 utilization. Growing awareness of its toxicity and potential environmental impacts led to increased scrutiny and regulation. Studies revealed its harmful effects on human health, including liver and kidney damage, as well as its contribution to ozone depletion. This led to a gradual phase-out of its use in many applications, particularly in consumer products.
The Montreal Protocol of 1987 further accelerated the decline of CCl4 usage by classifying it as an ozone-depleting substance. This international agreement set the stage for the global phase-out of CCl4 production and consumption, marking a significant shift in its historical utilization. Since then, alternative compounds and technologies have been developed to replace CCl4 in various applications, reflecting the evolving understanding of environmental and health concerns associated with chemical usage.
In the early 20th century, carbon tetrachloride found widespread use as a dry cleaning agent and a degreasing solvent in the manufacturing industry. Its non-flammability made it an attractive alternative to more volatile solvents, leading to its adoption in numerous industrial applications. During this period, CCl4 also became a popular fire extinguishing agent, particularly for electrical fires, due to its effectiveness in smothering flames without conducting electricity.
The 1920s and 1930s saw an expansion of CCl4's applications into the agricultural sector. It was widely used as a fumigant for grain storage and as a pesticide, helping to protect crops from insect infestations. This period also marked the beginning of its use in the production of chlorofluorocarbons (CFCs), which would later become a significant environmental concern.
During World War II, carbon tetrachloride played a crucial role in military applications. It was used in the production of smoke screens and as a cleaning agent for military equipment. The post-war era witnessed a surge in its industrial applications, particularly in the burgeoning plastics and electronics industries.
However, the 1970s marked a turning point in the history of CCl4 utilization. Growing awareness of its toxicity and potential environmental impacts led to increased scrutiny and regulation. Studies revealed its harmful effects on human health, including liver and kidney damage, as well as its contribution to ozone depletion. This led to a gradual phase-out of its use in many applications, particularly in consumer products.
The Montreal Protocol of 1987 further accelerated the decline of CCl4 usage by classifying it as an ozone-depleting substance. This international agreement set the stage for the global phase-out of CCl4 production and consumption, marking a significant shift in its historical utilization. Since then, alternative compounds and technologies have been developed to replace CCl4 in various applications, reflecting the evolving understanding of environmental and health concerns associated with chemical usage.
Market Analysis CCl4
Carbon tetrachloride (CCl4) has experienced significant market shifts throughout its history, driven by changing applications and regulatory pressures. Initially, CCl4 found widespread use as a solvent in various industries, including dry cleaning, metal degreasing, and fire extinguishing. Its non-flammable properties and effectiveness as a cleaning agent made it a popular choice in manufacturing processes.
The market for CCl4 peaked in the mid-20th century, with global production reaching substantial levels. However, the discovery of its ozone-depleting properties and potential health hazards led to a dramatic decline in demand. The Montreal Protocol, implemented in 1987, phased out the production and consumption of ozone-depleting substances, including CCl4, in developed countries.
This regulatory action resulted in a sharp contraction of the CCl4 market, particularly in North America and Europe. Many industries were forced to seek alternatives, leading to the development of new solvents and cleaning technologies. The dry cleaning sector, once a major consumer of CCl4, transitioned to perchloroethylene and other less harmful solvents.
Despite the overall decline, CCl4 continued to find limited use in certain applications. The chemical industry maintained demand for CCl4 as a feedstock in the production of other chlorinated compounds, such as hydrofluorocarbons (HFCs) and hydrochlorofluorocarbons (HCFCs). These substances were initially used as replacements for chlorofluorocarbons (CFCs) in refrigeration and air conditioning systems.
The pharmaceutical industry also retained some use of CCl4, primarily in the synthesis of certain drugs. However, stringent regulations and the push for greener chemistry have led to ongoing efforts to phase out its use in this sector as well.
In developing countries, the phase-out of CCl4 occurred more gradually, with some nations continuing to use it in various applications well into the 21st century. This created a geographical shift in the CCl4 market, with production and consumption moving from developed to developing economies.
The global market for CCl4 has continued to shrink, with current production levels significantly lower than historical peaks. Environmental concerns and health regulations remain the primary factors influencing market dynamics. The focus has shifted towards the management and disposal of existing CCl4 stocks, as well as the remediation of contaminated sites.
Looking forward, the CCl4 market is expected to further contract as alternative technologies and substances continue to replace its remaining applications. Research into more environmentally friendly and safer alternatives is ongoing, driven by both regulatory pressures and corporate sustainability initiatives.
The market for CCl4 peaked in the mid-20th century, with global production reaching substantial levels. However, the discovery of its ozone-depleting properties and potential health hazards led to a dramatic decline in demand. The Montreal Protocol, implemented in 1987, phased out the production and consumption of ozone-depleting substances, including CCl4, in developed countries.
This regulatory action resulted in a sharp contraction of the CCl4 market, particularly in North America and Europe. Many industries were forced to seek alternatives, leading to the development of new solvents and cleaning technologies. The dry cleaning sector, once a major consumer of CCl4, transitioned to perchloroethylene and other less harmful solvents.
Despite the overall decline, CCl4 continued to find limited use in certain applications. The chemical industry maintained demand for CCl4 as a feedstock in the production of other chlorinated compounds, such as hydrofluorocarbons (HFCs) and hydrochlorofluorocarbons (HCFCs). These substances were initially used as replacements for chlorofluorocarbons (CFCs) in refrigeration and air conditioning systems.
The pharmaceutical industry also retained some use of CCl4, primarily in the synthesis of certain drugs. However, stringent regulations and the push for greener chemistry have led to ongoing efforts to phase out its use in this sector as well.
In developing countries, the phase-out of CCl4 occurred more gradually, with some nations continuing to use it in various applications well into the 21st century. This created a geographical shift in the CCl4 market, with production and consumption moving from developed to developing economies.
The global market for CCl4 has continued to shrink, with current production levels significantly lower than historical peaks. Environmental concerns and health regulations remain the primary factors influencing market dynamics. The focus has shifted towards the management and disposal of existing CCl4 stocks, as well as the remediation of contaminated sites.
Looking forward, the CCl4 market is expected to further contract as alternative technologies and substances continue to replace its remaining applications. Research into more environmentally friendly and safer alternatives is ongoing, driven by both regulatory pressures and corporate sustainability initiatives.
CCl4 Usage Challenges
Carbon tetrachloride (CCl4) has faced numerous challenges throughout its historical utilization, primarily due to its environmental and health impacts. One of the most significant challenges has been its ozone-depleting properties. CCl4 was widely used as a refrigerant and in fire extinguishers, but its ability to deplete the ozone layer led to its phase-out under the Montreal Protocol in 1987. This international treaty aimed to protect the ozone layer by phasing out the production of numerous substances responsible for ozone depletion.
Another major challenge in CCl4 usage has been its toxicity to human health. Exposure to CCl4 can cause severe liver and kidney damage, as well as central nervous system depression. This toxicity led to restrictions on its use in consumer products and industrial applications, particularly in developed countries. The occupational hazards associated with CCl4 exposure in industrial settings also necessitated stringent safety measures and protective equipment for workers.
The environmental persistence of CCl4 has posed additional challenges. With a long atmospheric lifetime of approximately 26 years, CCl4 can accumulate in the environment and contribute to long-term ecological damage. This persistence has complicated efforts to mitigate its environmental impact, even after its production and use have been significantly reduced.
Regulatory compliance has been a ongoing challenge for industries that historically relied on CCl4. As regulations became more stringent, companies had to invest in alternative technologies and processes, often at significant cost. This transition period was particularly challenging for smaller businesses that lacked the resources to quickly adapt to new regulatory requirements.
The global nature of CCl4 production and use has presented challenges in terms of international cooperation and enforcement. While many developed countries have successfully phased out CCl4, some developing nations have continued its production and use, citing economic concerns. This disparity has led to challenges in achieving global reductions in CCl4 emissions and has required ongoing diplomatic efforts to ensure universal compliance with international agreements.
Lastly, the legacy of past CCl4 use continues to pose challenges. Contaminated sites, where CCl4 was produced or heavily used, require extensive and costly remediation efforts. The cleanup of these sites is often complicated by the chemical's ability to persist in soil and groundwater, necessitating long-term monitoring and treatment strategies.
Another major challenge in CCl4 usage has been its toxicity to human health. Exposure to CCl4 can cause severe liver and kidney damage, as well as central nervous system depression. This toxicity led to restrictions on its use in consumer products and industrial applications, particularly in developed countries. The occupational hazards associated with CCl4 exposure in industrial settings also necessitated stringent safety measures and protective equipment for workers.
The environmental persistence of CCl4 has posed additional challenges. With a long atmospheric lifetime of approximately 26 years, CCl4 can accumulate in the environment and contribute to long-term ecological damage. This persistence has complicated efforts to mitigate its environmental impact, even after its production and use have been significantly reduced.
Regulatory compliance has been a ongoing challenge for industries that historically relied on CCl4. As regulations became more stringent, companies had to invest in alternative technologies and processes, often at significant cost. This transition period was particularly challenging for smaller businesses that lacked the resources to quickly adapt to new regulatory requirements.
The global nature of CCl4 production and use has presented challenges in terms of international cooperation and enforcement. While many developed countries have successfully phased out CCl4, some developing nations have continued its production and use, citing economic concerns. This disparity has led to challenges in achieving global reductions in CCl4 emissions and has required ongoing diplomatic efforts to ensure universal compliance with international agreements.
Lastly, the legacy of past CCl4 use continues to pose challenges. Contaminated sites, where CCl4 was produced or heavily used, require extensive and costly remediation efforts. The cleanup of these sites is often complicated by the chemical's ability to persist in soil and groundwater, necessitating long-term monitoring and treatment strategies.
CCl4 Utilization Methods
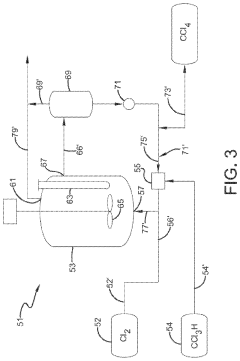
01 Production and purification of carbon tetrachloride
Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.- Production and purification of carbon tetrachloride: Various methods for producing and purifying carbon tetrachloride are described. These include chemical synthesis processes, distillation techniques, and purification methods to obtain high-quality carbon tetrachloride for industrial and laboratory use.
- Applications of carbon tetrachloride in chemical processes: Carbon tetrachloride is utilized in various chemical processes and reactions. It serves as a solvent, reagent, or intermediate in the production of other chemicals and materials. Its applications span across different industries, including pharmaceuticals and polymer manufacturing.
- Environmental and safety considerations: Due to its environmental impact and health hazards, there are concerns and regulations surrounding the use of carbon tetrachloride. Research focuses on developing alternatives, improving handling procedures, and implementing safety measures to mitigate risks associated with its use and disposal.
- Detection and analysis methods: Various analytical techniques and methods are developed for detecting and quantifying carbon tetrachloride in different matrices. These include spectroscopic methods, chromatography, and sensor-based approaches for environmental monitoring and quality control purposes.
- Historical uses and patents: Early patents and historical documents reveal the diverse applications of carbon tetrachloride in the past. These include its use as a cleaning agent, fire extinguishing medium, and in various industrial processes. Many of these applications have been phased out due to safety and environmental concerns.
02 Applications of carbon tetrachloride in chemical processes
Carbon tetrachloride is utilized in various chemical processes as a solvent, reagent, or intermediate. It finds applications in organic synthesis, extraction processes, and as a raw material for the production of other chlorinated compounds.Expand Specific Solutions03 Environmental and safety considerations
Due to its environmental impact and health hazards, research focuses on developing alternatives to carbon tetrachloride and methods for its safe handling, storage, and disposal. This includes techniques for detecting and monitoring carbon tetrachloride in various environments.Expand Specific Solutions04 Carbon tetrachloride in analytical chemistry
Carbon tetrachloride is used in various analytical techniques and procedures. It serves as a solvent in spectroscopy, chromatography, and other analytical methods for the identification and quantification of chemical compounds.Expand Specific Solutions05 Historical uses and patents related to carbon tetrachloride
Early patents and historical documents describe various applications of carbon tetrachloride, including its use as a fire extinguishing agent, cleaning solvent, and in the production of refrigerants. These patents provide insight into the evolution of carbon tetrachloride's industrial applications over time.Expand Specific Solutions
CCl4 Industry Leaders
The carbon tetrachloride market has evolved through distinct phases, from widespread industrial use to strict regulation due to environmental concerns. Currently, the market is in a mature stage with limited applications, primarily in niche industrial processes. The global market size is relatively small, estimated in the low hundreds of millions of dollars annually. Technologically, carbon tetrachloride production is well-established, with companies like Occidental Chemical Corp. and DuPont de Nemours, Inc. having long-standing expertise. However, research institutions such as the University of Florida and Central South University continue to explore alternative applications and safer substitutes, indicating ongoing technological development despite the compound's restricted use.
Occidental Chemical Corp.
Technical Solution: Occidental Chemical Corp. has been a significant player in the production and utilization of carbon tetrachloride. The company developed advanced purification techniques for CCl4, ensuring high-quality product for various industrial applications. Occidental's research focused on optimizing the chlorination process of methane and other hydrocarbons to produce CCl4 more efficiently [3]. They also pioneered the use of CCl4 in the production of perchloroethylene, a widely used dry cleaning solvent [4]. Occidental's expertise extended to the development of specialized storage and transportation systems for CCl4, addressing its corrosive nature and environmental concerns.
Strengths: Advanced purification techniques and efficient production processes for CCl4. Weaknesses: Dependence on a chemical with declining usage due to environmental regulations, necessitating diversification of product portfolio.
DuPont de Nemours, Inc.
Technical Solution: DuPont has been a key player in the historical utilization of carbon tetrachloride (CCl4). The company developed innovative production methods, including the chlorination of methane and carbon disulfide. DuPont's research led to the use of CCl4 as a precursor in the manufacture of chlorofluorocarbons (CFCs), which were widely used as refrigerants and aerosol propellants [1]. The company also pioneered the use of CCl4 as a solvent in various industrial processes, such as dry cleaning and metal degreasing [2]. DuPont's expertise in handling and processing CCl4 led to the development of safety protocols and equipment for its use in industrial settings.
Strengths: Extensive experience in CCl4 production and applications, leading to efficient processes and safety protocols. Weaknesses: Historical association with environmentally harmful substances, necessitating a shift in focus towards more sustainable alternatives.
CCl4 Key Patents
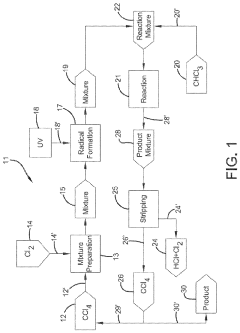
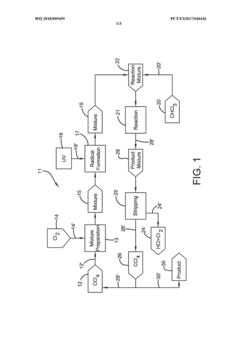
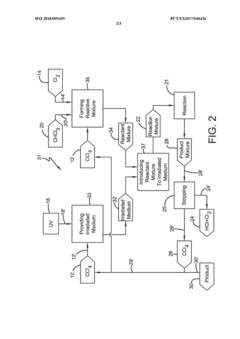
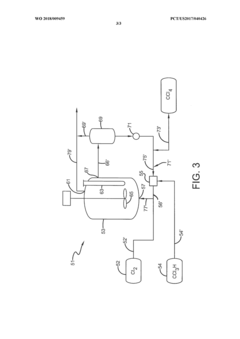
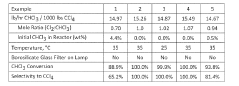
Photochlorination of partially-chlorinated chloromethanes to carbon tetrachloride
PatentActiveUS20240025823A1
Innovation
- A method involving the photochlorination of a chloromethanes stream containing chloroform, methyl chloride, and methylene chloride, combined with chlorine and additional carbon tetrachloride, and subjected to electromagnetic radiation to form carbon tetrachloride, achieving high conversion rates with reduced levels of unwanted chlorinated hydrocarbons.
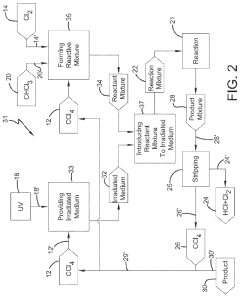
Photochlorination of chloroform to carbon tetrachloride
PatentWO2018009459A1
Innovation
- A photochlorination process involving the reaction of chlorine with chloroform in the presence of electromagnetic radiation within a carbon tetrachloride medium, maintaining low chloroform concentrations and stoichiometric levels of chlorine, to selectively convert chloroform to carbon tetrachloride, with the reaction mixture being well-mixed and subjected to UV light of specific wavelengths.
Environmental Impact CCl4
Carbon tetrachloride (CCl4) has had a significant environmental impact since its widespread industrial use began in the early 20th century. This compound's persistence in the atmosphere and its role in ozone depletion have been major concerns for environmental scientists and policymakers worldwide.
CCl4 is a potent ozone-depleting substance, with an ozone depletion potential (ODP) of 0.72, making it one of the most destructive chlorine-containing compounds. When released into the atmosphere, it can persist for decades, slowly breaking down and releasing chlorine atoms that catalyze the destruction of stratospheric ozone. This process contributes to the expansion of the ozone hole, particularly over polar regions, leading to increased ultraviolet radiation reaching the Earth's surface.
The compound's impact on global warming is also noteworthy. CCl4 is a greenhouse gas with a global warming potential (GWP) approximately 1,730 times that of carbon dioxide over a 100-year period. Although its atmospheric concentrations are much lower than CO2, its potency as a greenhouse gas means it contributes significantly to climate change on a per-molecule basis.
In aquatic environments, CCl4 can be particularly harmful. It is highly toxic to many aquatic organisms, including fish and invertebrates, even at low concentrations. Its persistence in water bodies and tendency to bioaccumulate in aquatic food chains can lead to long-term ecological damage. Groundwater contamination by CCl4 from industrial sites and improper disposal has been a significant issue, posing risks to both human health and ecosystems.
Soil contamination is another area of concern. CCl4 can persist in soil for extended periods, particularly in anaerobic conditions. This persistence can lead to long-term contamination of soil ecosystems and potential leaching into groundwater systems. The compound's mobility in soil further exacerbates its spread and environmental impact.
The recognition of these environmental impacts led to the phasing out of CCl4 under the Montreal Protocol. Since the late 1980s, global efforts have been made to reduce its production and use. While these efforts have been largely successful, with atmospheric concentrations of CCl4 declining since the mid-1990s, the compound's long atmospheric lifetime means its environmental effects will continue for many years.
Recent studies have highlighted unexpected sources of CCl4 emissions, including inadvertent production during industrial processes and emissions from contaminated sites. These findings underscore the need for continued monitoring and research to fully understand and mitigate the ongoing environmental impact of this compound.
CCl4 is a potent ozone-depleting substance, with an ozone depletion potential (ODP) of 0.72, making it one of the most destructive chlorine-containing compounds. When released into the atmosphere, it can persist for decades, slowly breaking down and releasing chlorine atoms that catalyze the destruction of stratospheric ozone. This process contributes to the expansion of the ozone hole, particularly over polar regions, leading to increased ultraviolet radiation reaching the Earth's surface.
The compound's impact on global warming is also noteworthy. CCl4 is a greenhouse gas with a global warming potential (GWP) approximately 1,730 times that of carbon dioxide over a 100-year period. Although its atmospheric concentrations are much lower than CO2, its potency as a greenhouse gas means it contributes significantly to climate change on a per-molecule basis.
In aquatic environments, CCl4 can be particularly harmful. It is highly toxic to many aquatic organisms, including fish and invertebrates, even at low concentrations. Its persistence in water bodies and tendency to bioaccumulate in aquatic food chains can lead to long-term ecological damage. Groundwater contamination by CCl4 from industrial sites and improper disposal has been a significant issue, posing risks to both human health and ecosystems.
Soil contamination is another area of concern. CCl4 can persist in soil for extended periods, particularly in anaerobic conditions. This persistence can lead to long-term contamination of soil ecosystems and potential leaching into groundwater systems. The compound's mobility in soil further exacerbates its spread and environmental impact.
The recognition of these environmental impacts led to the phasing out of CCl4 under the Montreal Protocol. Since the late 1980s, global efforts have been made to reduce its production and use. While these efforts have been largely successful, with atmospheric concentrations of CCl4 declining since the mid-1990s, the compound's long atmospheric lifetime means its environmental effects will continue for many years.
Recent studies have highlighted unexpected sources of CCl4 emissions, including inadvertent production during industrial processes and emissions from contaminated sites. These findings underscore the need for continued monitoring and research to fully understand and mitigate the ongoing environmental impact of this compound.
CCl4 Regulations
The regulation of carbon tetrachloride (CCl4) has evolved significantly over the past decades, reflecting growing awareness of its environmental and health impacts. In the early 20th century, CCl4 was widely used in various applications with minimal restrictions. However, as scientific evidence emerged about its ozone-depleting properties and potential health hazards, regulatory frameworks began to tighten.
The Montreal Protocol, signed in 1987, marked a turning point in CCl4 regulation. This international treaty aimed to phase out the production of ozone-depleting substances, including CCl4. Signatories agreed to gradually reduce and eventually eliminate the production and consumption of CCl4, with developed countries taking the lead in this effort.
In the United States, the Environmental Protection Agency (EPA) has played a crucial role in regulating CCl4. The Clean Air Act Amendments of 1990 incorporated the provisions of the Montreal Protocol into U.S. law, leading to strict controls on CCl4 production and use. The EPA classified CCl4 as a hazardous air pollutant and implemented regulations to minimize its emissions from industrial sources.
The European Union has also implemented stringent regulations on CCl4. The Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, enacted in 2007, includes CCl4 in its list of substances of very high concern. This classification imposes strict requirements on the manufacture, import, and use of CCl4 within the EU.
Globally, the United Nations Environment Programme (UNEP) has been instrumental in coordinating international efforts to monitor and control CCl4 emissions. UNEP's Ozone Secretariat oversees the implementation of the Montreal Protocol and tracks global progress in phasing out ozone-depleting substances, including CCl4.
Despite these regulatory efforts, challenges remain in completely eliminating CCl4 emissions. Recent studies have identified unexpected sources of CCl4 in the atmosphere, suggesting that some production or emissions may be occurring outside of regulatory frameworks. This has led to renewed efforts to strengthen monitoring and enforcement mechanisms.
The future of CCl4 regulation is likely to focus on addressing these remaining sources of emissions and ensuring compliance with existing regulations. There is also growing interest in developing alternative substances and technologies to replace the remaining uses of CCl4 in industrial processes. As scientific understanding of CCl4's environmental impacts continues to evolve, regulatory frameworks may need to adapt to address new concerns and emerging challenges.
The Montreal Protocol, signed in 1987, marked a turning point in CCl4 regulation. This international treaty aimed to phase out the production of ozone-depleting substances, including CCl4. Signatories agreed to gradually reduce and eventually eliminate the production and consumption of CCl4, with developed countries taking the lead in this effort.
In the United States, the Environmental Protection Agency (EPA) has played a crucial role in regulating CCl4. The Clean Air Act Amendments of 1990 incorporated the provisions of the Montreal Protocol into U.S. law, leading to strict controls on CCl4 production and use. The EPA classified CCl4 as a hazardous air pollutant and implemented regulations to minimize its emissions from industrial sources.
The European Union has also implemented stringent regulations on CCl4. The Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, enacted in 2007, includes CCl4 in its list of substances of very high concern. This classification imposes strict requirements on the manufacture, import, and use of CCl4 within the EU.
Globally, the United Nations Environment Programme (UNEP) has been instrumental in coordinating international efforts to monitor and control CCl4 emissions. UNEP's Ozone Secretariat oversees the implementation of the Montreal Protocol and tracks global progress in phasing out ozone-depleting substances, including CCl4.
Despite these regulatory efforts, challenges remain in completely eliminating CCl4 emissions. Recent studies have identified unexpected sources of CCl4 in the atmosphere, suggesting that some production or emissions may be occurring outside of regulatory frameworks. This has led to renewed efforts to strengthen monitoring and enforcement mechanisms.
The future of CCl4 regulation is likely to focus on addressing these remaining sources of emissions and ensuring compliance with existing regulations. There is also growing interest in developing alternative substances and technologies to replace the remaining uses of CCl4 in industrial processes. As scientific understanding of CCl4's environmental impacts continues to evolve, regulatory frameworks may need to adapt to address new concerns and emerging challenges.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!