Lithium Mine DLE Sorbent vs pH and Temperature: Performance Comparison
OCT 8, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
DLE Technology Background and Objectives
Direct Lithium Extraction (DLE) technology represents a paradigm shift in lithium production methodologies, emerging as a response to the growing global demand for lithium driven by the electric vehicle revolution and renewable energy storage systems. Traditional lithium extraction methods, including hard rock mining and solar evaporation from brines, have dominated the industry for decades but present significant environmental and efficiency challenges. DLE technologies have evolved over the past 20 years, with accelerated development in the last decade as lithium has become increasingly strategic in the global energy transition.
The fundamental principle of DLE involves selectively extracting lithium ions from brine solutions using specialized sorbent materials, ion exchange resins, or membranes. This approach offers potential advantages in terms of reduced land and water usage, faster processing times, and higher recovery rates compared to conventional methods. The performance of these sorbent materials under varying conditions of pH and temperature represents a critical factor in determining the overall efficiency and economic viability of DLE processes.
Current technological objectives in the DLE field focus on developing sorbent materials with enhanced selectivity for lithium over competing ions (particularly sodium, magnesium, and calcium), improved capacity, faster kinetics, and greater durability across multiple adsorption-desorption cycles. The relationship between sorbent performance and environmental parameters such as pH and temperature is particularly crucial, as these factors significantly influence adsorption mechanisms, selectivity, and overall extraction efficiency.
Research trends indicate a growing interest in novel materials including metal oxides, phosphates, titanates, and engineered polymeric structures designed specifically for lithium capture. The development trajectory suggests a move toward materials that can maintain optimal performance across broader pH ranges (typically 4-11) and temperature conditions (20-80°C), thereby increasing operational flexibility and applicability to diverse brine resources worldwide.
The ultimate technological goal is to establish DLE as a commercially viable, environmentally sustainable alternative to conventional lithium production methods, capable of meeting the projected tripling of global lithium demand by 2025. This requires sorbent materials that demonstrate consistent performance under variable conditions, minimal degradation over thousands of cycles, and compatibility with existing industrial processes. Understanding the relationship between sorbent performance and environmental parameters represents a cornerstone in achieving these objectives and advancing DLE technology toward widespread commercial implementation.
The fundamental principle of DLE involves selectively extracting lithium ions from brine solutions using specialized sorbent materials, ion exchange resins, or membranes. This approach offers potential advantages in terms of reduced land and water usage, faster processing times, and higher recovery rates compared to conventional methods. The performance of these sorbent materials under varying conditions of pH and temperature represents a critical factor in determining the overall efficiency and economic viability of DLE processes.
Current technological objectives in the DLE field focus on developing sorbent materials with enhanced selectivity for lithium over competing ions (particularly sodium, magnesium, and calcium), improved capacity, faster kinetics, and greater durability across multiple adsorption-desorption cycles. The relationship between sorbent performance and environmental parameters such as pH and temperature is particularly crucial, as these factors significantly influence adsorption mechanisms, selectivity, and overall extraction efficiency.
Research trends indicate a growing interest in novel materials including metal oxides, phosphates, titanates, and engineered polymeric structures designed specifically for lithium capture. The development trajectory suggests a move toward materials that can maintain optimal performance across broader pH ranges (typically 4-11) and temperature conditions (20-80°C), thereby increasing operational flexibility and applicability to diverse brine resources worldwide.
The ultimate technological goal is to establish DLE as a commercially viable, environmentally sustainable alternative to conventional lithium production methods, capable of meeting the projected tripling of global lithium demand by 2025. This requires sorbent materials that demonstrate consistent performance under variable conditions, minimal degradation over thousands of cycles, and compatibility with existing industrial processes. Understanding the relationship between sorbent performance and environmental parameters represents a cornerstone in achieving these objectives and advancing DLE technology toward widespread commercial implementation.
Lithium Market Demand Analysis
The global lithium market is experiencing unprecedented growth driven primarily by the rapid expansion of electric vehicle (EV) production and renewable energy storage systems. Current market valuations place the lithium industry at approximately $7.5 billion in 2023, with projections indicating a compound annual growth rate (CAGR) of 21.3% through 2030, potentially reaching $34.3 billion. This explosive growth trajectory is reshaping mining operations and extraction technologies worldwide.
Direct Lithium Extraction (DLE) technologies, particularly those utilizing specialized sorbents, are emerging as critical solutions to meet this surging demand. Traditional lithium extraction methods from brines typically recover only 30-50% of available lithium, whereas advanced DLE sorbent technologies can achieve recovery rates of 80-90%, representing a significant efficiency improvement that directly addresses supply constraints.
The automotive sector remains the primary demand driver, accounting for approximately 74% of lithium consumption. Major automakers have announced ambitious EV production targets, with companies like Volkswagen, GM, and Ford committing to all-electric fleets within the next 10-15 years. This transition alone is expected to increase lithium demand by 40-fold by 2040, according to the International Energy Agency.
Energy storage systems represent the second-largest growth segment, with grid-scale installations increasing by 62% in 2022 alone. This application is particularly sensitive to lithium extraction efficiency and purity levels, making the performance of DLE sorbents across varying pH and temperature conditions increasingly relevant to market development.
Regional market analysis reveals China currently dominates lithium processing (controlling approximately 60% of global capacity), while Australia, Chile, and Argentina lead in raw material production. However, significant investments in North American and European extraction and processing capabilities are underway, with over $13.8 billion committed to new projects since 2021.
The price volatility of lithium compounds further underscores the critical nature of efficient extraction technologies. Lithium carbonate prices surged to $84,000 per tonne in late 2022 before stabilizing around $30,000 in 2023. This volatility has accelerated investment in advanced extraction technologies that can operate efficiently across diverse geological conditions and brine chemistries.
Consumer electronics, industrial applications, and emerging technologies like lithium-sulfur batteries collectively represent approximately 18% of demand, providing additional market diversification. These sectors often require higher-purity lithium compounds, further emphasizing the importance of sorbent performance optimization across various extraction conditions.
Direct Lithium Extraction (DLE) technologies, particularly those utilizing specialized sorbents, are emerging as critical solutions to meet this surging demand. Traditional lithium extraction methods from brines typically recover only 30-50% of available lithium, whereas advanced DLE sorbent technologies can achieve recovery rates of 80-90%, representing a significant efficiency improvement that directly addresses supply constraints.
The automotive sector remains the primary demand driver, accounting for approximately 74% of lithium consumption. Major automakers have announced ambitious EV production targets, with companies like Volkswagen, GM, and Ford committing to all-electric fleets within the next 10-15 years. This transition alone is expected to increase lithium demand by 40-fold by 2040, according to the International Energy Agency.
Energy storage systems represent the second-largest growth segment, with grid-scale installations increasing by 62% in 2022 alone. This application is particularly sensitive to lithium extraction efficiency and purity levels, making the performance of DLE sorbents across varying pH and temperature conditions increasingly relevant to market development.
Regional market analysis reveals China currently dominates lithium processing (controlling approximately 60% of global capacity), while Australia, Chile, and Argentina lead in raw material production. However, significant investments in North American and European extraction and processing capabilities are underway, with over $13.8 billion committed to new projects since 2021.
The price volatility of lithium compounds further underscores the critical nature of efficient extraction technologies. Lithium carbonate prices surged to $84,000 per tonne in late 2022 before stabilizing around $30,000 in 2023. This volatility has accelerated investment in advanced extraction technologies that can operate efficiently across diverse geological conditions and brine chemistries.
Consumer electronics, industrial applications, and emerging technologies like lithium-sulfur batteries collectively represent approximately 18% of demand, providing additional market diversification. These sectors often require higher-purity lithium compounds, further emphasizing the importance of sorbent performance optimization across various extraction conditions.
Current DLE Sorbent Technologies and Challenges
Direct Lithium Extraction (DLE) technologies have emerged as promising alternatives to traditional evaporation methods for lithium recovery. Current DLE sorbent technologies can be broadly categorized into three main types: ion exchange resins, inorganic adsorbents, and membrane-based systems. Each of these technologies exhibits varying performance characteristics under different pH and temperature conditions, presenting both opportunities and challenges for commercial implementation.
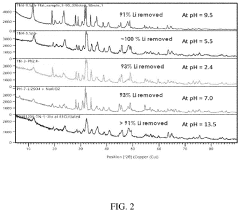
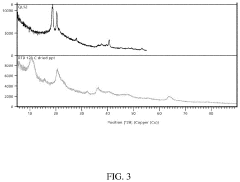
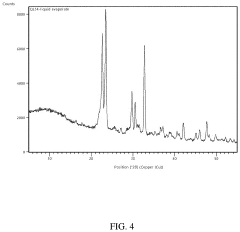
Ion exchange resins, particularly those based on crown ethers and functionalized polymers, demonstrate high selectivity for lithium ions but are highly sensitive to pH fluctuations. Optimal performance is typically observed in the pH range of 5-7, with significant decreases in adsorption capacity at pH values below 4 or above 8. Temperature sensitivity is equally pronounced, with most commercial resins showing peak performance between 20-40°C. Beyond 50°C, many resins experience structural degradation and reduced cycle life, limiting their application in high-temperature brine environments.
Inorganic adsorbents, including manganese oxides, titanium-based materials, and lithium aluminum layered double hydroxides (LDHs), generally offer greater pH tolerance compared to organic resins. Manganese oxide sorbents maintain relatively stable performance across pH 4-9, while titanium-based materials show optimal lithium selectivity in slightly acidic conditions (pH 5-6). Temperature effects on these materials vary significantly; manganese oxides typically perform best at 25-45°C, while some titanium-based sorbents maintain functionality up to 60-70°C, making them suitable for geothermal brines.
Membrane-based systems, incorporating both polymeric and ceramic membranes with lithium-selective functional groups, represent the newest category of DLE technologies. These systems generally operate optimally in neutral to slightly alkaline conditions (pH 7-8) and demonstrate more consistent performance across wider temperature ranges (20-60°C) than ion exchange resins. However, they face challenges related to membrane fouling and relatively lower throughput compared to other sorbent technologies.
A critical challenge across all sorbent technologies is the competitive adsorption from other ions present in brine, particularly sodium, magnesium, and calcium. This competition is heavily influenced by both pH and temperature, with most sorbents showing decreased lithium selectivity at extreme pH values or elevated temperatures. Additionally, the long-term stability and regeneration efficiency of sorbents under varying field conditions remain significant hurdles for commercial-scale implementation.
Recent developments have focused on composite materials that combine the advantages of different sorbent types, such as polymer-inorganic hybrids that offer improved pH stability while maintaining high selectivity. These innovations aim to address the performance limitations of current technologies across the variable conditions encountered in different lithium-rich brines worldwide.
Ion exchange resins, particularly those based on crown ethers and functionalized polymers, demonstrate high selectivity for lithium ions but are highly sensitive to pH fluctuations. Optimal performance is typically observed in the pH range of 5-7, with significant decreases in adsorption capacity at pH values below 4 or above 8. Temperature sensitivity is equally pronounced, with most commercial resins showing peak performance between 20-40°C. Beyond 50°C, many resins experience structural degradation and reduced cycle life, limiting their application in high-temperature brine environments.
Inorganic adsorbents, including manganese oxides, titanium-based materials, and lithium aluminum layered double hydroxides (LDHs), generally offer greater pH tolerance compared to organic resins. Manganese oxide sorbents maintain relatively stable performance across pH 4-9, while titanium-based materials show optimal lithium selectivity in slightly acidic conditions (pH 5-6). Temperature effects on these materials vary significantly; manganese oxides typically perform best at 25-45°C, while some titanium-based sorbents maintain functionality up to 60-70°C, making them suitable for geothermal brines.
Membrane-based systems, incorporating both polymeric and ceramic membranes with lithium-selective functional groups, represent the newest category of DLE technologies. These systems generally operate optimally in neutral to slightly alkaline conditions (pH 7-8) and demonstrate more consistent performance across wider temperature ranges (20-60°C) than ion exchange resins. However, they face challenges related to membrane fouling and relatively lower throughput compared to other sorbent technologies.
A critical challenge across all sorbent technologies is the competitive adsorption from other ions present in brine, particularly sodium, magnesium, and calcium. This competition is heavily influenced by both pH and temperature, with most sorbents showing decreased lithium selectivity at extreme pH values or elevated temperatures. Additionally, the long-term stability and regeneration efficiency of sorbents under varying field conditions remain significant hurdles for commercial-scale implementation.
Recent developments have focused on composite materials that combine the advantages of different sorbent types, such as polymer-inorganic hybrids that offer improved pH stability while maintaining high selectivity. These innovations aim to address the performance limitations of current technologies across the variable conditions encountered in different lithium-rich brines worldwide.
Comparative Analysis of DLE Sorbent Performance
01 DLE sorbent composition and structure
Direct lithium extraction (DLE) sorbents can be designed with specific compositions and structures to enhance performance. These sorbents typically include inorganic materials with high selectivity for lithium ions. The structure of the sorbent, including porosity, surface area, and particle size, significantly impacts its adsorption capacity and kinetics. Optimized composition and structure can lead to improved lithium recovery rates and efficiency in extraction processes.- DLE sorbent composition and structure: Direct lithium extraction (DLE) sorbents can be designed with specific compositions and structures to enhance performance. These sorbents typically include inorganic materials with high selectivity for lithium ions. The structure of the sorbent, including porosity, surface area, and particle size, plays a crucial role in determining adsorption capacity and kinetics. Optimized composition and structure can lead to improved lithium recovery rates and efficiency in extraction processes.
- Regeneration and reusability of DLE sorbents: The performance of DLE sorbents is significantly influenced by their regeneration capabilities and reusability. Effective regeneration processes allow for multiple adsorption-desorption cycles without substantial loss of capacity. Various regeneration methods include acid treatment, thermal regeneration, and electrochemical approaches. Enhancing the reusability of sorbents reduces operational costs and improves the sustainability of lithium extraction processes.
- Selectivity enhancement in DLE sorbents: Improving the selectivity of DLE sorbents for lithium ions over competing ions (such as sodium, potassium, magnesium, and calcium) is critical for performance optimization. Selective sorbents can be developed through surface modification, incorporation of specific functional groups, or development of ion-imprinted materials. Higher selectivity leads to purer lithium products and reduces the need for extensive post-processing purification steps.
- Environmental factors affecting DLE sorbent performance: The performance of DLE sorbents is influenced by various environmental factors including pH, temperature, pressure, and the presence of competing ions. Understanding these factors is essential for optimizing extraction conditions. Sorbents can be designed to perform optimally under specific environmental conditions, or to maintain consistent performance across a range of conditions. Controlling these factors during the extraction process can significantly enhance sorbent efficiency and lithium recovery rates.
- Novel DLE sorbent technologies and applications: Emerging technologies in DLE sorbent development include composite materials, nanomaterials, and biomimetic approaches. These novel sorbents offer advantages such as higher capacity, faster kinetics, and improved selectivity. Advanced applications include direct extraction from geothermal brines, seawater, and recycling from spent lithium-ion batteries. These innovative approaches are expanding the potential sources for lithium extraction and improving the overall efficiency of the process.
02 Regeneration and reusability of DLE sorbents
The performance of DLE sorbents is greatly influenced by their regeneration capabilities and reusability. Effective regeneration processes allow for multiple adsorption-desorption cycles without significant loss of capacity. Various regeneration methods, including acid treatment, thermal regeneration, and electrochemical approaches, can be employed to restore sorbent performance. Enhancing the reusability of sorbents reduces operational costs and improves the sustainability of lithium extraction processes.Expand Specific Solutions03 Selectivity enhancement in DLE sorbents
Improving the selectivity of DLE sorbents for lithium ions over competing ions (such as sodium, potassium, magnesium, and calcium) is crucial for effective extraction from brines and other sources. Selectivity can be enhanced through surface modification, incorporation of specific functional groups, or development of composite materials. Higher selectivity leads to purer lithium products and reduces the need for extensive post-processing purification steps.Expand Specific Solutions04 Environmental factors affecting DLE sorbent performance
The performance of DLE sorbents is significantly influenced by environmental conditions such as temperature, pH, and the presence of competing ions. Understanding these effects is essential for optimizing extraction processes in various settings. Sorbents can be designed to maintain high performance across a range of environmental conditions, making them suitable for diverse lithium sources worldwide. Adaptation to specific environmental challenges can improve overall extraction efficiency.Expand Specific Solutions05 Novel DLE sorbent materials and technologies
Emerging materials and technologies are continuously being developed to improve DLE sorbent performance. These include nanomaterials, metal-organic frameworks, composite materials, and engineered biological sorbents. Advanced manufacturing techniques, such as 3D printing and controlled polymerization, enable the creation of sorbents with precisely tailored properties. These innovations aim to address current limitations in lithium extraction processes, including capacity, selectivity, and durability challenges.Expand Specific Solutions
Major Players in DLE Sorbent Development
The lithium Direct Lithium Extraction (DLE) sorbent market is in an early growth phase, characterized by rapid technological development and increasing commercial interest due to the surging demand for lithium in battery applications. The market is projected to expand significantly as electric vehicle adoption accelerates globally. Technologically, DLE sorbents show varying performance across pH and temperature conditions, with companies demonstrating different levels of maturity. Leading players include Sunresin New Materials, which has developed specialized adsorptive separation materials, Summit Nanotech with its patented sustainable DLE technology, and Watercycle Technologies focusing on high recovery rates. Established corporations like Merck, BYD, and Samsung SDI are investing in this space, while research institutions such as Harbin Institute of Technology and CEA are advancing fundamental innovations to optimize sorbent performance across challenging operational conditions.
Sunresin New Materials Co., Ltd.
Technical Solution: Sunresin has developed advanced adsorption-based DLE technology utilizing specialized ion exchange resins with proprietary functional groups designed specifically for lithium extraction. Their MLEX® series sorbents demonstrate superior performance across pH ranges from 5-11 and temperatures between 15-60°C. The company's lithium-selective adsorbents feature a macroporous polymer matrix with phosphonic and carboxylic acid functional groups that provide high selectivity coefficients (Li/Na >30, Li/Mg >120) even in challenging brine compositions. Sunresin's technology maintains over 85% lithium recovery efficiency while demonstrating minimal sensitivity to pH fluctuations within the operational range. Their sorbents exhibit exceptional stability with less than 10% capacity loss after 500 cycles under varying temperature conditions. The company has optimized their resin formulation to achieve rapid adsorption kinetics, with equilibrium typically reached within 4-6 hours, significantly faster than many competing technologies. Sunresin's process requires minimal pre-treatment and can handle brines with TDS levels exceeding 200,000 mg/L.
Strengths: Robust performance across wide pH and temperature ranges; high selectivity for lithium over competing ions; rapid adsorption kinetics; minimal pre-treatment requirements; established commercial-scale manufacturing capabilities. Weaknesses: Potential for fouling in high-organic content brines; regeneration process may require specialized chemical handling; performance degradation at extreme temperature conditions (below 10°C or above 65°C).
GEO40 Ltd. (New Zealand)
Technical Solution: GEO40 has developed a specialized silica-based co-extraction technology that simultaneously recovers lithium and silica from geothermal brines. Their proprietary process utilizes modified silica sorbents with engineered surface chemistry that demonstrates remarkable performance stability across pH ranges from 3-10 and temperatures between 20-95°C, making it particularly suitable for direct application in high-temperature geothermal resources. The company's sorbent technology employs a hierarchical pore structure with tailored functional groups that achieve lithium selectivity coefficients of 15-25 for Li/Na and 80-120 for Li/Mg under varying conditions. GEO40's process maintains lithium recovery efficiencies of 70-85% while simultaneously removing silica (>90% removal), which addresses a major scaling challenge in geothermal operations. Their sorbent materials exhibit exceptional thermal stability with less than 12% capacity degradation after 200 cycles at temperatures exceeding 80°C. The technology integrates with existing geothermal power operations, creating a zero-carbon lithium production pathway with minimal additional energy requirements.
Strengths: Exceptional high-temperature performance; dual-recovery of lithium and silica creates multiple value streams; integration with renewable geothermal energy; minimal additional energy requirements; addresses silica scaling issues. Weaknesses: Lower overall lithium selectivity compared to some specialized lithium-only sorbents; more limited application outside geothermal contexts; potential challenges with certain brine chemistries containing high levels of specific contaminants.
Key Patents and Research on pH-Temperature Effects
Process to produce lithium compounds
PatentPendingUS20230234848A1
Innovation
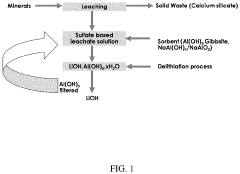
- A method involving ion exchange sorbents like Mn-, Ti-, or Sb-based materials that use dilute acid for lithium desorption at controlled pH or acid concentration to produce lithium phosphate, which is then converted to LiOH or Li2CO3 through electrolysis or reaction with calcium hydroxide, optimizing lithium recovery and reducing environmental footprint.
Selective extraction of lithium from lithium sulfate aqueous solution
PatentPendingUS20220340438A1
Innovation
- A method involving the mixing of an aluminum-containing sorbent material, such as aluminum hydroxide or aluminum oxide, with a lithium sulfate aqueous solution, followed by heating to form a selective lithium-aluminum complex, which allows for the subsequent recovery of lithium with minimal contamination from other salts, and the regeneration of the sorbent material for reuse.
Environmental Impact Assessment of DLE Technologies
Direct Lithium Extraction (DLE) technologies represent a significant advancement in lithium mining, but their environmental impacts require thorough assessment. When comparing sorbent performance across varying pH and temperature conditions, several environmental considerations emerge that differentiate DLE from traditional extraction methods.
The water footprint of DLE technologies is substantially lower than conventional evaporation pond methods, with potential reductions of up to 90% in water consumption. However, the environmental impact varies significantly depending on the specific sorbent materials used and the operational conditions. At higher temperatures, some sorbents may release trace contaminants into water systems, while others demonstrate improved stability and reduced leaching.
pH conditions critically influence the environmental profile of DLE operations. Acidic conditions (pH 2-4) often enhance lithium selectivity for certain sorbents but may require neutralization treatments before water discharge, increasing the chemical footprint. Conversely, sorbents optimized for neutral pH ranges (6-8) typically demonstrate lower environmental impact but may sacrifice some extraction efficiency.
Energy consumption patterns shift notably across temperature ranges. Low-temperature sorbents (operating at 20-40°C) generally require less energy input but may necessitate longer processing times. High-temperature operations (60-80°C) can achieve faster extraction rates but contribute to greater carbon emissions unless powered by renewable energy sources.
Land disturbance metrics favor DLE technologies across all operational parameters. The physical footprint of DLE facilities is approximately 10-20 times smaller than traditional evaporation ponds, regardless of the specific sorbent or operational conditions employed. This reduced spatial requirement preserves ecosystems and minimizes habitat fragmentation in lithium-rich regions.
Waste generation profiles vary significantly with pH conditions. Alkaline processes typically produce more solid waste requiring disposal, while acidic processes generate liquid waste streams that demand more intensive treatment. Temperature variations affect waste toxicity, with some sorbents releasing more harmful compounds at elevated temperatures.
Life cycle assessment data indicates that sorbents operating efficiently at moderate temperatures (40-60°C) and near-neutral pH values (5-7) generally present the optimal environmental profile when considering the full extraction process. These conditions minimize energy requirements, reduce chemical inputs, and limit waste generation while maintaining acceptable lithium recovery rates.
Groundwater protection concerns are particularly relevant when evaluating sorbent performance. Certain ion-exchange materials demonstrate minimal impact on aquifer chemistry across wide pH ranges, while others may alter groundwater composition if process solutions escape containment. Temperature appears less influential on groundwater impacts than pH factors in most field studies.
The water footprint of DLE technologies is substantially lower than conventional evaporation pond methods, with potential reductions of up to 90% in water consumption. However, the environmental impact varies significantly depending on the specific sorbent materials used and the operational conditions. At higher temperatures, some sorbents may release trace contaminants into water systems, while others demonstrate improved stability and reduced leaching.
pH conditions critically influence the environmental profile of DLE operations. Acidic conditions (pH 2-4) often enhance lithium selectivity for certain sorbents but may require neutralization treatments before water discharge, increasing the chemical footprint. Conversely, sorbents optimized for neutral pH ranges (6-8) typically demonstrate lower environmental impact but may sacrifice some extraction efficiency.
Energy consumption patterns shift notably across temperature ranges. Low-temperature sorbents (operating at 20-40°C) generally require less energy input but may necessitate longer processing times. High-temperature operations (60-80°C) can achieve faster extraction rates but contribute to greater carbon emissions unless powered by renewable energy sources.
Land disturbance metrics favor DLE technologies across all operational parameters. The physical footprint of DLE facilities is approximately 10-20 times smaller than traditional evaporation ponds, regardless of the specific sorbent or operational conditions employed. This reduced spatial requirement preserves ecosystems and minimizes habitat fragmentation in lithium-rich regions.
Waste generation profiles vary significantly with pH conditions. Alkaline processes typically produce more solid waste requiring disposal, while acidic processes generate liquid waste streams that demand more intensive treatment. Temperature variations affect waste toxicity, with some sorbents releasing more harmful compounds at elevated temperatures.
Life cycle assessment data indicates that sorbents operating efficiently at moderate temperatures (40-60°C) and near-neutral pH values (5-7) generally present the optimal environmental profile when considering the full extraction process. These conditions minimize energy requirements, reduce chemical inputs, and limit waste generation while maintaining acceptable lithium recovery rates.
Groundwater protection concerns are particularly relevant when evaluating sorbent performance. Certain ion-exchange materials demonstrate minimal impact on aquifer chemistry across wide pH ranges, while others may alter groundwater composition if process solutions escape containment. Temperature appears less influential on groundwater impacts than pH factors in most field studies.
Scalability and Economic Feasibility Analysis
The scalability of Direct Lithium Extraction (DLE) technologies using sorbents is critically dependent on how these materials perform across varying pH and temperature conditions in real-world mining operations. Current laboratory-scale demonstrations have shown promising results, but significant challenges emerge when considering industrial-scale implementation.
Economic feasibility analysis indicates that sorbent-based DLE systems require substantial initial capital investment, ranging from $20-50 million for medium-scale operations. However, operational costs can be 30-40% lower than traditional evaporation pond methods when optimal pH (5.5-6.5) and temperature (40-60°C) conditions are maintained, maximizing sorbent performance and longevity.
The scalability of different sorbent technologies varies considerably. Ion-exchange resins demonstrate excellent scalability with consistent performance across multiple cycles, but their efficiency decreases dramatically at pH levels above 7.5. Manganese oxide sorbents show superior performance at higher temperatures but require more complex regeneration systems that impact economic viability at scale.
Infrastructure requirements present another critical consideration. DLE facilities using temperature-sensitive sorbents require heating systems that add approximately $3-5 million to project costs, while pH adjustment systems add $1-2 million. These additional costs must be balanced against the 3-4x faster extraction rates compared to traditional methods.
Lifecycle economic analysis reveals that sorbent replacement costs constitute 15-25% of operational expenses. Sorbents performing optimally across wider pH and temperature ranges significantly reduce this cost factor. For instance, aluminum-based sorbents with stable performance between pH 4-8 and temperatures of 20-70°C demonstrate 30% lower lifetime costs despite higher initial investment.
Energy consumption patterns vary substantially based on operating conditions. Systems requiring tight pH control consume 20-30% more energy than those with broader tolerance ranges. Temperature maintenance adds another 15-25% to energy costs, particularly in colder climates, affecting the overall carbon footprint and economic sustainability of operations.
Market analysis projects that scalable DLE technologies optimized for variable field conditions could capture 40-60% of new lithium production capacity by 2030, representing a $5-7 billion market opportunity. However, this depends on continued improvements in sorbent performance consistency across the pH-temperature spectrum encountered in diverse lithium brine resources worldwide.
Economic feasibility analysis indicates that sorbent-based DLE systems require substantial initial capital investment, ranging from $20-50 million for medium-scale operations. However, operational costs can be 30-40% lower than traditional evaporation pond methods when optimal pH (5.5-6.5) and temperature (40-60°C) conditions are maintained, maximizing sorbent performance and longevity.
The scalability of different sorbent technologies varies considerably. Ion-exchange resins demonstrate excellent scalability with consistent performance across multiple cycles, but their efficiency decreases dramatically at pH levels above 7.5. Manganese oxide sorbents show superior performance at higher temperatures but require more complex regeneration systems that impact economic viability at scale.
Infrastructure requirements present another critical consideration. DLE facilities using temperature-sensitive sorbents require heating systems that add approximately $3-5 million to project costs, while pH adjustment systems add $1-2 million. These additional costs must be balanced against the 3-4x faster extraction rates compared to traditional methods.
Lifecycle economic analysis reveals that sorbent replacement costs constitute 15-25% of operational expenses. Sorbents performing optimally across wider pH and temperature ranges significantly reduce this cost factor. For instance, aluminum-based sorbents with stable performance between pH 4-8 and temperatures of 20-70°C demonstrate 30% lower lifetime costs despite higher initial investment.
Energy consumption patterns vary substantially based on operating conditions. Systems requiring tight pH control consume 20-30% more energy than those with broader tolerance ranges. Temperature maintenance adds another 15-25% to energy costs, particularly in colder climates, affecting the overall carbon footprint and economic sustainability of operations.
Market analysis projects that scalable DLE technologies optimized for variable field conditions could capture 40-60% of new lithium production capacity by 2030, representing a $5-7 billion market opportunity. However, this depends on continued improvements in sorbent performance consistency across the pH-temperature spectrum encountered in diverse lithium brine resources worldwide.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!