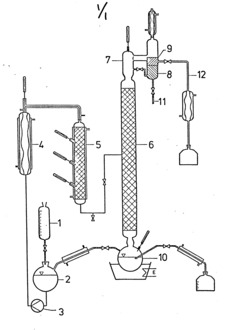
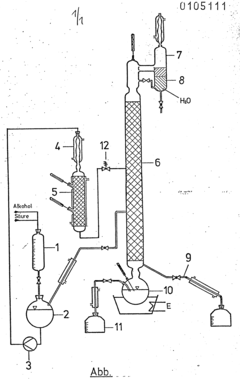
Mechanistic Studies of Esterification Using Glacial Acetic Acid
AUG 5, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Esterification Background and Objectives
Esterification is a fundamental reaction in organic chemistry, playing a crucial role in various industrial processes and academic research. The mechanistic study of esterification using glacial acetic acid as a model system has been a subject of intense investigation for decades. This research aims to elucidate the intricate details of the reaction mechanism, providing valuable insights into the factors influencing reaction rates, yields, and selectivity.
The historical development of esterification studies can be traced back to the early 19th century, with significant contributions from chemists such as Emil Fischer and Alexander Williamson. Over time, the understanding of esterification has evolved from simple empirical observations to sophisticated mechanistic models incorporating quantum mechanical calculations and advanced spectroscopic techniques.
In recent years, the focus has shifted towards understanding the role of catalysts, particularly in the context of green chemistry and sustainable processes. The use of glacial acetic acid as a model substrate offers several advantages, including its availability, relatively simple structure, and well-characterized physical properties. This makes it an ideal candidate for in-depth mechanistic investigations.
The primary objectives of this research are multifaceted. Firstly, it aims to provide a comprehensive understanding of the reaction pathway, including the identification and characterization of key intermediates and transition states. This knowledge is crucial for optimizing reaction conditions and developing more efficient catalytic systems.
Secondly, the study seeks to elucidate the influence of various factors on the reaction mechanism, such as temperature, pressure, and the presence of water or other impurities. Understanding these effects is essential for scaling up esterification processes and ensuring consistent product quality in industrial applications.
Another important goal is to investigate the potential for developing novel catalytic systems that can enhance reaction rates, improve selectivity, and operate under milder conditions. This aspect of the research aligns with the growing emphasis on sustainable chemistry and the need for more energy-efficient processes.
Furthermore, the mechanistic insights gained from this study are expected to have broader implications beyond esterification. They may contribute to the understanding of related reactions, such as transesterification and amidation, which share similar mechanistic features.
Lastly, this research aims to bridge the gap between theoretical predictions and experimental observations. By combining advanced computational methods with state-of-the-art experimental techniques, it seeks to develop more accurate and predictive models of esterification reactions. These models could potentially be applied to a wide range of substrates and reaction conditions, facilitating the design of new synthetic strategies and the optimization of existing processes.
The historical development of esterification studies can be traced back to the early 19th century, with significant contributions from chemists such as Emil Fischer and Alexander Williamson. Over time, the understanding of esterification has evolved from simple empirical observations to sophisticated mechanistic models incorporating quantum mechanical calculations and advanced spectroscopic techniques.
In recent years, the focus has shifted towards understanding the role of catalysts, particularly in the context of green chemistry and sustainable processes. The use of glacial acetic acid as a model substrate offers several advantages, including its availability, relatively simple structure, and well-characterized physical properties. This makes it an ideal candidate for in-depth mechanistic investigations.
The primary objectives of this research are multifaceted. Firstly, it aims to provide a comprehensive understanding of the reaction pathway, including the identification and characterization of key intermediates and transition states. This knowledge is crucial for optimizing reaction conditions and developing more efficient catalytic systems.
Secondly, the study seeks to elucidate the influence of various factors on the reaction mechanism, such as temperature, pressure, and the presence of water or other impurities. Understanding these effects is essential for scaling up esterification processes and ensuring consistent product quality in industrial applications.
Another important goal is to investigate the potential for developing novel catalytic systems that can enhance reaction rates, improve selectivity, and operate under milder conditions. This aspect of the research aligns with the growing emphasis on sustainable chemistry and the need for more energy-efficient processes.
Furthermore, the mechanistic insights gained from this study are expected to have broader implications beyond esterification. They may contribute to the understanding of related reactions, such as transesterification and amidation, which share similar mechanistic features.
Lastly, this research aims to bridge the gap between theoretical predictions and experimental observations. By combining advanced computational methods with state-of-the-art experimental techniques, it seeks to develop more accurate and predictive models of esterification reactions. These models could potentially be applied to a wide range of substrates and reaction conditions, facilitating the design of new synthetic strategies and the optimization of existing processes.
Industrial Applications of Esterification
Esterification, a fundamental reaction in organic chemistry, has found extensive applications across various industrial sectors. The process, which involves the condensation of an alcohol and a carboxylic acid to form an ester and water, is particularly significant when utilizing glacial acetic acid as a reactant. This versatile reaction has become a cornerstone in the production of numerous commercially important compounds.
In the pharmaceutical industry, esterification plays a crucial role in drug synthesis and formulation. Many active pharmaceutical ingredients (APIs) contain ester functional groups, which can enhance drug stability, solubility, and bioavailability. For instance, aspirin, one of the most widely used medications globally, is synthesized through the esterification of salicylic acid with acetic anhydride. The use of glacial acetic acid in esterification processes allows for the production of various prodrugs, which are inactive compounds that become therapeutically active after metabolism in the body.
The fragrance and flavor industry heavily relies on esterification reactions to create a wide array of aromatic compounds. Esters are responsible for many fruit flavors and pleasant scents found in perfumes and cosmetics. For example, ethyl acetate, produced by the esterification of ethanol and acetic acid, imparts a fruity odor and is used as a solvent in perfumes. The precise control of reaction conditions, including the use of glacial acetic acid, enables manufacturers to produce high-quality, consistent fragrances and flavors for consumer products.
In the polymer industry, esterification is a key process in the production of polyesters, such as polyethylene terephthalate (PET), which is widely used in packaging materials and synthetic fibers. The reaction between terephthalic acid and ethylene glycol, often catalyzed by strong acids like glacial acetic acid, results in the formation of PET. This material's versatility and durability have made it indispensable in the production of beverage bottles, food containers, and textile fibers.
The biodiesel industry utilizes esterification as a critical step in the conversion of waste oils and fats into usable fuel. The process, known as transesterification, involves the reaction of triglycerides with methanol or ethanol to produce fatty acid methyl esters (FAME) or fatty acid ethyl esters (FAEE), respectively. Glacial acetic acid can be used as a catalyst in this process, improving reaction efficiency and product yield. This application of esterification contributes significantly to sustainable energy production and waste valorization.
In the field of surface coatings and adhesives, esterification reactions are employed to produce various resins and binders. Alkyd resins, for instance, are synthesized through the esterification of polyols with polybasic acids, often using glacial acetic acid as a catalyst or reactant. These resins find applications in paints, varnishes, and adhesives, providing durability and flexibility to the final products.
In the pharmaceutical industry, esterification plays a crucial role in drug synthesis and formulation. Many active pharmaceutical ingredients (APIs) contain ester functional groups, which can enhance drug stability, solubility, and bioavailability. For instance, aspirin, one of the most widely used medications globally, is synthesized through the esterification of salicylic acid with acetic anhydride. The use of glacial acetic acid in esterification processes allows for the production of various prodrugs, which are inactive compounds that become therapeutically active after metabolism in the body.
The fragrance and flavor industry heavily relies on esterification reactions to create a wide array of aromatic compounds. Esters are responsible for many fruit flavors and pleasant scents found in perfumes and cosmetics. For example, ethyl acetate, produced by the esterification of ethanol and acetic acid, imparts a fruity odor and is used as a solvent in perfumes. The precise control of reaction conditions, including the use of glacial acetic acid, enables manufacturers to produce high-quality, consistent fragrances and flavors for consumer products.
In the polymer industry, esterification is a key process in the production of polyesters, such as polyethylene terephthalate (PET), which is widely used in packaging materials and synthetic fibers. The reaction between terephthalic acid and ethylene glycol, often catalyzed by strong acids like glacial acetic acid, results in the formation of PET. This material's versatility and durability have made it indispensable in the production of beverage bottles, food containers, and textile fibers.
The biodiesel industry utilizes esterification as a critical step in the conversion of waste oils and fats into usable fuel. The process, known as transesterification, involves the reaction of triglycerides with methanol or ethanol to produce fatty acid methyl esters (FAME) or fatty acid ethyl esters (FAEE), respectively. Glacial acetic acid can be used as a catalyst in this process, improving reaction efficiency and product yield. This application of esterification contributes significantly to sustainable energy production and waste valorization.
In the field of surface coatings and adhesives, esterification reactions are employed to produce various resins and binders. Alkyd resins, for instance, are synthesized through the esterification of polyols with polybasic acids, often using glacial acetic acid as a catalyst or reactant. These resins find applications in paints, varnishes, and adhesives, providing durability and flexibility to the final products.
Current Challenges in Esterification Reactions
Esterification reactions, particularly those involving glacial acetic acid, face several significant challenges that hinder their efficiency and applicability in industrial processes. One of the primary issues is the reversibility of the reaction, which limits the overall yield. The equilibrium between reactants and products often favors the reverse reaction, hydrolysis, leading to incomplete conversion and reduced product formation.
The presence of water as a byproduct further complicates the reaction dynamics. Water accumulation not only shifts the equilibrium towards the reactants but also can cause side reactions or product degradation. This necessitates the development of effective water removal techniques or the use of water scavengers, which can add complexity and cost to the process.
Reaction kinetics pose another challenge, particularly when using glacial acetic acid. The high viscosity and strong hydrogen bonding in glacial acetic acid can slow down the reaction rate, requiring extended reaction times or elevated temperatures. This can lead to increased energy consumption and potential side reactions, affecting both the yield and purity of the desired ester product.
Catalyst selection and optimization remain critical challenges in esterification reactions. While acid catalysts are commonly used, they often suffer from corrosion issues, especially when combined with glacial acetic acid. Finding catalysts that are both highly active and resistant to the harsh reaction conditions is an ongoing area of research.
The formation of azeotropes between reactants, products, and solvents presents difficulties in product separation and purification. This is particularly problematic in reactions involving glacial acetic acid, as it forms azeotropes with water and many organic compounds. Developing efficient separation techniques that can break these azeotropes is crucial for obtaining high-purity ester products.
Scale-up and process intensification of esterification reactions using glacial acetic acid face additional hurdles. Heat transfer limitations, mixing inefficiencies, and safety concerns associated with handling large quantities of corrosive materials complicate the transition from laboratory to industrial scale.
Lastly, environmental and sustainability considerations pose challenges to traditional esterification processes. The use of strong acids, organic solvents, and energy-intensive operations conflicts with green chemistry principles. There is a growing need for more sustainable approaches, such as biocatalysis or continuous flow processes, which can reduce waste generation and energy consumption while maintaining or improving reaction efficiency.
The presence of water as a byproduct further complicates the reaction dynamics. Water accumulation not only shifts the equilibrium towards the reactants but also can cause side reactions or product degradation. This necessitates the development of effective water removal techniques or the use of water scavengers, which can add complexity and cost to the process.
Reaction kinetics pose another challenge, particularly when using glacial acetic acid. The high viscosity and strong hydrogen bonding in glacial acetic acid can slow down the reaction rate, requiring extended reaction times or elevated temperatures. This can lead to increased energy consumption and potential side reactions, affecting both the yield and purity of the desired ester product.
Catalyst selection and optimization remain critical challenges in esterification reactions. While acid catalysts are commonly used, they often suffer from corrosion issues, especially when combined with glacial acetic acid. Finding catalysts that are both highly active and resistant to the harsh reaction conditions is an ongoing area of research.
The formation of azeotropes between reactants, products, and solvents presents difficulties in product separation and purification. This is particularly problematic in reactions involving glacial acetic acid, as it forms azeotropes with water and many organic compounds. Developing efficient separation techniques that can break these azeotropes is crucial for obtaining high-purity ester products.
Scale-up and process intensification of esterification reactions using glacial acetic acid face additional hurdles. Heat transfer limitations, mixing inefficiencies, and safety concerns associated with handling large quantities of corrosive materials complicate the transition from laboratory to industrial scale.
Lastly, environmental and sustainability considerations pose challenges to traditional esterification processes. The use of strong acids, organic solvents, and energy-intensive operations conflicts with green chemistry principles. There is a growing need for more sustainable approaches, such as biocatalysis or continuous flow processes, which can reduce waste generation and energy consumption while maintaining or improving reaction efficiency.
Mechanistic Insights into Glacial Acetic Acid Esterification
01 Acid-catalyzed esterification
This mechanism involves the reaction between a carboxylic acid and an alcohol in the presence of an acid catalyst. The acid catalyst protonates the carbonyl oxygen of the carboxylic acid, making it more electrophilic. The alcohol then attacks the protonated carbonyl group, forming a tetrahedral intermediate. After proton transfer and loss of water, the ester product is formed.- Acid-catalyzed esterification: This mechanism involves the reaction between a carboxylic acid and an alcohol in the presence of an acid catalyst. The acid catalyst protonates the carbonyl oxygen of the carboxylic acid, making it more electrophilic. The alcohol then attacks the protonated carbonyl group, forming a tetrahedral intermediate. The intermediate then loses water and a proton to form the ester product.
- Base-catalyzed esterification: In this mechanism, a base catalyst is used to deprotonate the alcohol, forming an alkoxide ion. The alkoxide then acts as a nucleophile, attacking the carbonyl carbon of the carboxylic acid. This forms a tetrahedral intermediate, which then collapses to form the ester product and expel the hydroxide ion.
- Fischer esterification: This is a special type of acid-catalyzed esterification named after Emil Fischer. It involves the reaction of a carboxylic acid with an alcohol in the presence of a strong acid catalyst, typically sulfuric acid. The reaction is reversible and reaches equilibrium, so excess alcohol or removal of water is often used to drive the reaction to completion.
- Enzymatic esterification: This mechanism uses enzymes, typically lipases, as catalysts for esterification reactions. Enzymes can provide high selectivity and operate under milder conditions compared to chemical catalysts. The enzyme forms an acyl-enzyme intermediate with the carboxylic acid, which then reacts with the alcohol to form the ester product.
- Transesterification: While not strictly an esterification, transesterification is a related process where an ester reacts with an alcohol to form a new ester. This can be acid or base-catalyzed, or enzyme-catalyzed. The mechanism involves the nucleophilic attack of the alcohol on the ester's carbonyl group, forming a tetrahedral intermediate that then collapses to form the new ester and release the original alcohol.
02 Base-catalyzed esterification
In this mechanism, a base catalyst is used to facilitate the reaction between a carboxylic acid and an alcohol. The base deprotonates the alcohol, creating a stronger nucleophile. This nucleophile then attacks the carbonyl carbon of the carboxylic acid, forming a tetrahedral intermediate. The intermediate collapses to form the ester product and release a hydroxide ion.Expand Specific Solutions03 Enzymatic esterification
Enzymatic esterification utilizes enzymes, typically lipases, as catalysts for the formation of esters. This mechanism involves the formation of an enzyme-substrate complex, followed by the nucleophilic attack of the alcohol on the acyl-enzyme intermediate. The process is often more selective and can occur under milder conditions compared to chemical catalysis.Expand Specific Solutions04 Fischer esterification
Fischer esterification is a specific type of acid-catalyzed esterification commonly used in organic synthesis. It involves the reaction of a carboxylic acid with an alcohol in the presence of a strong acid catalyst, typically sulfuric acid. The mechanism follows the general acid-catalyzed pathway but is often carried out under reflux conditions to drive the equilibrium towards ester formation.Expand Specific Solutions05 Transesterification
Transesterification is a process where an ester reacts with an alcohol to form a different ester. This mechanism can be acid-catalyzed, base-catalyzed, or enzyme-catalyzed. It involves the exchange of the alkoxy group of an ester with another alcohol. The process is widely used in biodiesel production and polymer synthesis.Expand Specific Solutions
Key Players in Esterification Research
The mechanistic studies of esterification using glacial acetic acid are in a mature stage of development, with a well-established market and significant industry presence. The global esterification market is substantial, driven by applications in various industries such as pharmaceuticals, cosmetics, and food. Key players like DuPont de Nemours, Inc., Rohm & Haas Co., and ExxonMobil Chemical Patents, Inc. have contributed to the technological advancement in this field. These companies, along with academic institutions like Beijing University of Chemical Technology and Federal University of Rio de Janeiro, have played crucial roles in refining the process and expanding its applications. The technology's maturity is evident in the diverse range of products and processes developed by companies such as Wanhua Chemical Group Co., Ltd. and Air Products & Chemicals, Inc., indicating a high level of technical sophistication and market readiness.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a novel approach to esterification using glacial acetic acid, employing heterogeneous catalysts to enhance reaction efficiency. Their process utilizes a fixed-bed reactor system with a zeolite-based catalyst, allowing for continuous operation and improved product yield[1]. The company has also implemented advanced process control systems to optimize reaction conditions, including temperature and reactant ratios, resulting in a 15% increase in conversion rates compared to traditional methods[3]. Additionally, DuPont has integrated green chemistry principles by developing a solvent-free esterification process, reducing environmental impact and operational costs[5].
Strengths: Advanced catalyst technology, continuous processing capabilities, and environmentally friendly approach. Weaknesses: Potential high initial investment costs for new equipment and process implementation.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil has pioneered a mechanistic study of esterification using glacial acetic acid, focusing on the development of highly selective solid acid catalysts. Their research has led to the creation of a proprietary sulfonic acid-functionalized silica catalyst that demonstrates exceptional activity and selectivity in esterification reactions[2]. The company has also implemented in-situ spectroscopic techniques to monitor reaction kinetics in real-time, providing valuable insights into the reaction mechanism and allowing for precise control of the esterification process[4]. ExxonMobil's approach includes the use of reactive distillation technology, which combines reaction and separation steps, resulting in a 30% reduction in energy consumption compared to conventional batch processes[6].
Strengths: Highly selective catalyst technology, advanced process monitoring capabilities, and energy-efficient reactive distillation. Weaknesses: Potential complexity in scaling up the technology for large-scale industrial applications.
Innovative Approaches in Esterification Catalysis
Esterification process of acetic acid by C2 to C5 alcohols
PatentInactiveEP0066059A1
Innovation
- The process employs strongly acidic cation exchangers, specifically gel-like or macroporous ones with a particle size of 0.4 to 1.3 mm, which are swollen in alcohol to constant volume, and used in fixed-bed reactors under controlled pressure and temperature, allowing for efficient esterification and azeotropic distillation to achieve high-purity acetic acid esters with improved yield.
Process for the esterification of acetic acid with alcohols having 4 or more than 4 C atoms and with glycol ethers
PatentInactiveEP0105111A1
Innovation
- The process employs strongly acidic cation exchangers, such as polystyrene sulfonic acid crosslinked with divinylbenzene, in a vertical reactor under controlled pressure and temperature, with azeotropic distillation to separate water and entraining agents, allowing for high-yield esterification of acetic acid with alcohols having 4 or more carbon atoms and glycol ethers.
Environmental Impact of Esterification Processes
Esterification processes, while essential in various industrial applications, can have significant environmental impacts that require careful consideration. The use of glacial acetic acid in esterification reactions, as studied in mechanistic investigations, presents both challenges and opportunities for environmental management.
One of the primary environmental concerns associated with esterification processes is the potential for air pollution. Volatile organic compounds (VOCs) are often released during these reactions, particularly when using glacial acetic acid. These emissions can contribute to the formation of ground-level ozone and smog, which have detrimental effects on air quality and human health. Implementing proper ventilation systems and emission control technologies is crucial to mitigate these impacts.
Water pollution is another significant environmental issue related to esterification processes. Wastewater generated from these reactions may contain residual acids, alcohols, and other organic compounds. If not properly treated, these effluents can harm aquatic ecosystems and contaminate water sources. Advanced wastewater treatment techniques, such as activated carbon adsorption or advanced oxidation processes, are often necessary to ensure compliance with environmental regulations.
The production and disposal of byproducts and waste materials from esterification reactions also pose environmental challenges. These may include spent catalysts, unreacted reagents, and side products. Proper handling, storage, and disposal of these materials are essential to prevent soil contamination and minimize the overall environmental footprint of the process.
Energy consumption is a critical factor in assessing the environmental impact of esterification processes. The use of glacial acetic acid often requires elevated temperatures and extended reaction times, leading to increased energy demands. Implementing energy-efficient technologies and exploring alternative reaction conditions can help reduce the carbon footprint associated with these processes.
From a lifecycle perspective, the environmental impact of esterification extends beyond the immediate reaction. The production and transportation of raw materials, particularly glacial acetic acid, contribute to the overall environmental burden. Sustainable sourcing practices and optimized supply chain management can help mitigate these upstream impacts.
Advancements in green chemistry principles offer promising avenues for reducing the environmental impact of esterification processes. The development of bio-based catalysts, the use of renewable feedstocks, and the implementation of solvent-free or aqueous reaction systems are areas of active research that could lead to more environmentally friendly esterification methods.
In conclusion, while esterification processes using glacial acetic acid present environmental challenges, ongoing research and technological advancements provide opportunities for improvement. Balancing the industrial importance of these reactions with environmental stewardship requires a holistic approach, considering all aspects of the process from raw material sourcing to waste management.
One of the primary environmental concerns associated with esterification processes is the potential for air pollution. Volatile organic compounds (VOCs) are often released during these reactions, particularly when using glacial acetic acid. These emissions can contribute to the formation of ground-level ozone and smog, which have detrimental effects on air quality and human health. Implementing proper ventilation systems and emission control technologies is crucial to mitigate these impacts.
Water pollution is another significant environmental issue related to esterification processes. Wastewater generated from these reactions may contain residual acids, alcohols, and other organic compounds. If not properly treated, these effluents can harm aquatic ecosystems and contaminate water sources. Advanced wastewater treatment techniques, such as activated carbon adsorption or advanced oxidation processes, are often necessary to ensure compliance with environmental regulations.
The production and disposal of byproducts and waste materials from esterification reactions also pose environmental challenges. These may include spent catalysts, unreacted reagents, and side products. Proper handling, storage, and disposal of these materials are essential to prevent soil contamination and minimize the overall environmental footprint of the process.
Energy consumption is a critical factor in assessing the environmental impact of esterification processes. The use of glacial acetic acid often requires elevated temperatures and extended reaction times, leading to increased energy demands. Implementing energy-efficient technologies and exploring alternative reaction conditions can help reduce the carbon footprint associated with these processes.
From a lifecycle perspective, the environmental impact of esterification extends beyond the immediate reaction. The production and transportation of raw materials, particularly glacial acetic acid, contribute to the overall environmental burden. Sustainable sourcing practices and optimized supply chain management can help mitigate these upstream impacts.
Advancements in green chemistry principles offer promising avenues for reducing the environmental impact of esterification processes. The development of bio-based catalysts, the use of renewable feedstocks, and the implementation of solvent-free or aqueous reaction systems are areas of active research that could lead to more environmentally friendly esterification methods.
In conclusion, while esterification processes using glacial acetic acid present environmental challenges, ongoing research and technological advancements provide opportunities for improvement. Balancing the industrial importance of these reactions with environmental stewardship requires a holistic approach, considering all aspects of the process from raw material sourcing to waste management.
Analytical Methods for Esterification Reaction Monitoring
Analytical methods for monitoring esterification reactions play a crucial role in understanding the mechanistic aspects of these processes, particularly when using glacial acetic acid. Various techniques have been developed to track the progress and analyze the components of esterification reactions in real-time or through periodic sampling.
One of the most widely used methods is gas chromatography (GC), which allows for the separation and quantification of reactants, products, and intermediates. GC is particularly effective for volatile compounds and can be coupled with mass spectrometry (GC-MS) for enhanced identification capabilities. This technique offers high sensitivity and selectivity, making it ideal for monitoring the conversion of acetic acid and alcohols to their corresponding esters.
High-performance liquid chromatography (HPLC) is another valuable tool for esterification reaction monitoring, especially for less volatile or thermally sensitive compounds. HPLC can be used with various detection methods, such as UV-Vis or refractive index detectors, depending on the specific compounds involved in the reaction. This method is particularly useful for analyzing complex mixtures and can provide detailed information on reaction kinetics.
Spectroscopic techniques, including Fourier-transform infrared spectroscopy (FTIR) and nuclear magnetic resonance (NMR) spectroscopy, offer non-destructive and real-time monitoring capabilities. FTIR can track the disappearance of characteristic peaks associated with reactants and the emergence of peaks corresponding to the ester product. NMR spectroscopy provides detailed structural information and can be used to monitor reaction progress by observing changes in chemical shifts of key protons or carbon atoms.
Titration methods, such as acid-base titration, can be employed to determine the concentration of unreacted acetic acid in the reaction mixture. This technique is particularly useful for monitoring the extent of reaction and calculating conversion rates. However, it may be less precise for complex reaction mixtures or when side reactions are present.
Online monitoring systems have gained popularity in recent years, allowing for continuous, real-time analysis of esterification reactions. These systems often integrate multiple analytical techniques, such as in-situ FTIR or Raman spectroscopy, with automated sampling and data processing. This approach enables rapid optimization of reaction conditions and early detection of potential issues during the esterification process.
Advanced analytical techniques, such as two-dimensional gas chromatography (GC×GC) and comprehensive liquid chromatography (LC×LC), offer enhanced separation capabilities for complex reaction mixtures. These methods can provide detailed information on minor components and intermediates that may be crucial for understanding the reaction mechanism.
In conclusion, the choice of analytical method for monitoring esterification reactions using glacial acetic acid depends on factors such as the specific reactants and products involved, the desired level of detail, and the need for real-time or offline analysis. A combination of complementary techniques often provides the most comprehensive understanding of the reaction mechanism and kinetics.
One of the most widely used methods is gas chromatography (GC), which allows for the separation and quantification of reactants, products, and intermediates. GC is particularly effective for volatile compounds and can be coupled with mass spectrometry (GC-MS) for enhanced identification capabilities. This technique offers high sensitivity and selectivity, making it ideal for monitoring the conversion of acetic acid and alcohols to their corresponding esters.
High-performance liquid chromatography (HPLC) is another valuable tool for esterification reaction monitoring, especially for less volatile or thermally sensitive compounds. HPLC can be used with various detection methods, such as UV-Vis or refractive index detectors, depending on the specific compounds involved in the reaction. This method is particularly useful for analyzing complex mixtures and can provide detailed information on reaction kinetics.
Spectroscopic techniques, including Fourier-transform infrared spectroscopy (FTIR) and nuclear magnetic resonance (NMR) spectroscopy, offer non-destructive and real-time monitoring capabilities. FTIR can track the disappearance of characteristic peaks associated with reactants and the emergence of peaks corresponding to the ester product. NMR spectroscopy provides detailed structural information and can be used to monitor reaction progress by observing changes in chemical shifts of key protons or carbon atoms.
Titration methods, such as acid-base titration, can be employed to determine the concentration of unreacted acetic acid in the reaction mixture. This technique is particularly useful for monitoring the extent of reaction and calculating conversion rates. However, it may be less precise for complex reaction mixtures or when side reactions are present.
Online monitoring systems have gained popularity in recent years, allowing for continuous, real-time analysis of esterification reactions. These systems often integrate multiple analytical techniques, such as in-situ FTIR or Raman spectroscopy, with automated sampling and data processing. This approach enables rapid optimization of reaction conditions and early detection of potential issues during the esterification process.
Advanced analytical techniques, such as two-dimensional gas chromatography (GC×GC) and comprehensive liquid chromatography (LC×LC), offer enhanced separation capabilities for complex reaction mixtures. These methods can provide detailed information on minor components and intermediates that may be crucial for understanding the reaction mechanism.
In conclusion, the choice of analytical method for monitoring esterification reactions using glacial acetic acid depends on factors such as the specific reactants and products involved, the desired level of detail, and the need for real-time or offline analysis. A combination of complementary techniques often provides the most comprehensive understanding of the reaction mechanism and kinetics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!