Methods for Detecting Impurities in Glacial Acetic Acid
AUG 5, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Impurity Detection Background and Objectives
Glacial acetic acid, a widely used industrial chemical, requires stringent purity standards for various applications. The detection of impurities in this compound has been a critical focus in the chemical industry for decades. The evolution of impurity detection methods has closely followed advancements in analytical chemistry and instrumentation technology.
The primary objective of impurity detection in glacial acetic acid is to ensure product quality and safety across diverse industries, including pharmaceuticals, food processing, and polymer manufacturing. Accurate identification and quantification of impurities are essential for maintaining regulatory compliance and optimizing production processes.
Historically, impurity detection in glacial acetic acid relied on basic chemical tests and rudimentary spectroscopic techniques. As analytical capabilities advanced, more sophisticated methods emerged, including gas chromatography, high-performance liquid chromatography, and mass spectrometry. These technologies have significantly improved the sensitivity and specificity of impurity detection.
The development of impurity detection methods has been driven by increasingly stringent quality control requirements and the need to identify trace contaminants that can affect product performance or safety. Regulatory bodies, such as the FDA and EMA, have played a crucial role in setting standards for impurity levels and detection methodologies.
Recent technological trends in impurity detection focus on enhancing detection limits, improving analytical speed, and developing more robust and automated systems. There is a growing emphasis on real-time monitoring and process analytical technology (PAT) to enable continuous quality assurance during production.
The challenges in impurity detection for glacial acetic acid include the need for methods that can detect a wide range of potential contaminants, from inorganic species to organic compounds. Additionally, there is a constant push to lower detection limits while maintaining accuracy and precision.
Looking forward, the field of impurity detection in glacial acetic acid is expected to benefit from advancements in areas such as artificial intelligence for data analysis, miniaturized and portable analytical devices, and novel spectroscopic techniques. These developments aim to provide faster, more accurate, and more comprehensive impurity profiles, ultimately contributing to higher quality standards in acetic acid production and utilization.
The primary objective of impurity detection in glacial acetic acid is to ensure product quality and safety across diverse industries, including pharmaceuticals, food processing, and polymer manufacturing. Accurate identification and quantification of impurities are essential for maintaining regulatory compliance and optimizing production processes.
Historically, impurity detection in glacial acetic acid relied on basic chemical tests and rudimentary spectroscopic techniques. As analytical capabilities advanced, more sophisticated methods emerged, including gas chromatography, high-performance liquid chromatography, and mass spectrometry. These technologies have significantly improved the sensitivity and specificity of impurity detection.
The development of impurity detection methods has been driven by increasingly stringent quality control requirements and the need to identify trace contaminants that can affect product performance or safety. Regulatory bodies, such as the FDA and EMA, have played a crucial role in setting standards for impurity levels and detection methodologies.
Recent technological trends in impurity detection focus on enhancing detection limits, improving analytical speed, and developing more robust and automated systems. There is a growing emphasis on real-time monitoring and process analytical technology (PAT) to enable continuous quality assurance during production.
The challenges in impurity detection for glacial acetic acid include the need for methods that can detect a wide range of potential contaminants, from inorganic species to organic compounds. Additionally, there is a constant push to lower detection limits while maintaining accuracy and precision.
Looking forward, the field of impurity detection in glacial acetic acid is expected to benefit from advancements in areas such as artificial intelligence for data analysis, miniaturized and portable analytical devices, and novel spectroscopic techniques. These developments aim to provide faster, more accurate, and more comprehensive impurity profiles, ultimately contributing to higher quality standards in acetic acid production and utilization.
Market Demand Analysis for High-Purity Acetic Acid
The global market for high-purity acetic acid has been experiencing steady growth, driven by increasing demand from various industries such as pharmaceuticals, food and beverage, and electronics. The pharmaceutical sector, in particular, has emerged as a significant consumer of high-purity acetic acid, utilizing it in the production of various medications and active pharmaceutical ingredients (APIs).
In the food and beverage industry, high-purity acetic acid is widely used as a preservative and flavoring agent. The growing consumer preference for natural and clean-label products has further boosted the demand for high-quality acetic acid in this sector. Additionally, the electronics industry relies on high-purity acetic acid for manufacturing processes, particularly in the production of printed circuit boards and semiconductors.
The market for high-purity acetic acid is also influenced by the increasing focus on sustainability and environmental regulations. This has led to a rise in demand for bio-based acetic acid, which is produced through fermentation processes using renewable resources. The shift towards eco-friendly alternatives is expected to create new opportunities for market growth in the coming years.
Geographically, Asia-Pacific has emerged as the largest market for high-purity acetic acid, followed by North America and Europe. The rapid industrialization and growth of end-use industries in countries like China and India are driving the demand in the Asia-Pacific region. North America and Europe, on the other hand, are witnessing steady growth due to the presence of established pharmaceutical and chemical industries.
The market for high-purity acetic acid is characterized by stringent quality requirements, as impurities can significantly impact the performance and safety of end products. This has led to an increased focus on advanced purification techniques and quality control measures. Manufacturers are investing in research and development to improve production processes and develop more efficient methods for detecting and removing impurities in glacial acetic acid.
Looking ahead, the market for high-purity acetic acid is projected to continue its growth trajectory. Factors such as the expanding pharmaceutical industry, increasing demand for specialty chemicals, and the growing adoption of acetic acid in various applications are expected to drive market growth. However, challenges such as price volatility of raw materials and environmental concerns related to conventional production methods may impact market dynamics in the future.
In the food and beverage industry, high-purity acetic acid is widely used as a preservative and flavoring agent. The growing consumer preference for natural and clean-label products has further boosted the demand for high-quality acetic acid in this sector. Additionally, the electronics industry relies on high-purity acetic acid for manufacturing processes, particularly in the production of printed circuit boards and semiconductors.
The market for high-purity acetic acid is also influenced by the increasing focus on sustainability and environmental regulations. This has led to a rise in demand for bio-based acetic acid, which is produced through fermentation processes using renewable resources. The shift towards eco-friendly alternatives is expected to create new opportunities for market growth in the coming years.
Geographically, Asia-Pacific has emerged as the largest market for high-purity acetic acid, followed by North America and Europe. The rapid industrialization and growth of end-use industries in countries like China and India are driving the demand in the Asia-Pacific region. North America and Europe, on the other hand, are witnessing steady growth due to the presence of established pharmaceutical and chemical industries.
The market for high-purity acetic acid is characterized by stringent quality requirements, as impurities can significantly impact the performance and safety of end products. This has led to an increased focus on advanced purification techniques and quality control measures. Manufacturers are investing in research and development to improve production processes and develop more efficient methods for detecting and removing impurities in glacial acetic acid.
Looking ahead, the market for high-purity acetic acid is projected to continue its growth trajectory. Factors such as the expanding pharmaceutical industry, increasing demand for specialty chemicals, and the growing adoption of acetic acid in various applications are expected to drive market growth. However, challenges such as price volatility of raw materials and environmental concerns related to conventional production methods may impact market dynamics in the future.
Current Challenges in Glacial Acetic Acid Purity Testing
The detection of impurities in glacial acetic acid presents several significant challenges that hinder the development of reliable and efficient testing methods. One of the primary obstacles is the high corrosivity of glacial acetic acid, which limits the selection of materials for analytical instruments and sample handling equipment. This corrosive nature can lead to contamination of samples and damage to analytical devices, potentially compromising the accuracy of results.
Another major challenge is the low boiling point of glacial acetic acid, which is close to that of water. This similarity makes it difficult to separate acetic acid from water-based impurities using conventional distillation techniques. As a result, more sophisticated separation methods are required, adding complexity and cost to the testing process.
The detection of trace impurities poses a significant challenge due to the high purity levels required for industrial-grade glacial acetic acid. Identifying and quantifying contaminants at parts per million (ppm) or even parts per billion (ppb) levels demands highly sensitive analytical techniques. This requirement often necessitates the use of advanced instrumentation, such as gas chromatography-mass spectrometry (GC-MS) or high-performance liquid chromatography (HPLC), which can be expensive and require specialized expertise to operate and maintain.
Furthermore, the diverse nature of potential impurities in glacial acetic acid complicates the development of comprehensive testing methods. Contaminants can range from organic compounds like aldehydes and ketones to inorganic species such as metal ions. Each class of impurity may require different analytical approaches, making it challenging to develop a single, universal testing protocol.
The stability of glacial acetic acid samples during storage and analysis is another concern. Some impurities may react with acetic acid over time, altering the composition of the sample and potentially leading to inaccurate results. This instability necessitates careful sample handling and storage procedures, as well as rapid analysis to minimize the risk of compositional changes.
Lastly, the lack of standardized methods for impurity detection in glacial acetic acid across different industries and regulatory bodies creates inconsistencies in testing protocols and result interpretation. This absence of harmonized standards makes it difficult to compare results between different laboratories or manufacturers, hindering quality control efforts and potentially impacting the global trade of glacial acetic acid.
Another major challenge is the low boiling point of glacial acetic acid, which is close to that of water. This similarity makes it difficult to separate acetic acid from water-based impurities using conventional distillation techniques. As a result, more sophisticated separation methods are required, adding complexity and cost to the testing process.
The detection of trace impurities poses a significant challenge due to the high purity levels required for industrial-grade glacial acetic acid. Identifying and quantifying contaminants at parts per million (ppm) or even parts per billion (ppb) levels demands highly sensitive analytical techniques. This requirement often necessitates the use of advanced instrumentation, such as gas chromatography-mass spectrometry (GC-MS) or high-performance liquid chromatography (HPLC), which can be expensive and require specialized expertise to operate and maintain.
Furthermore, the diverse nature of potential impurities in glacial acetic acid complicates the development of comprehensive testing methods. Contaminants can range from organic compounds like aldehydes and ketones to inorganic species such as metal ions. Each class of impurity may require different analytical approaches, making it challenging to develop a single, universal testing protocol.
The stability of glacial acetic acid samples during storage and analysis is another concern. Some impurities may react with acetic acid over time, altering the composition of the sample and potentially leading to inaccurate results. This instability necessitates careful sample handling and storage procedures, as well as rapid analysis to minimize the risk of compositional changes.
Lastly, the lack of standardized methods for impurity detection in glacial acetic acid across different industries and regulatory bodies creates inconsistencies in testing protocols and result interpretation. This absence of harmonized standards makes it difficult to compare results between different laboratories or manufacturers, hindering quality control efforts and potentially impacting the global trade of glacial acetic acid.
Existing Impurity Detection Methods for Glacial Acetic Acid
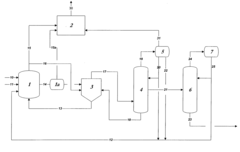
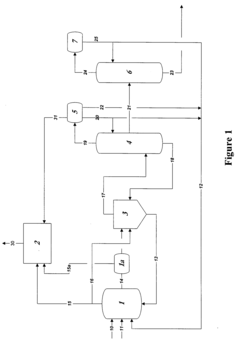
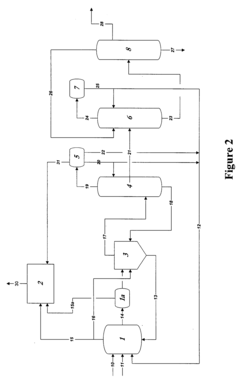
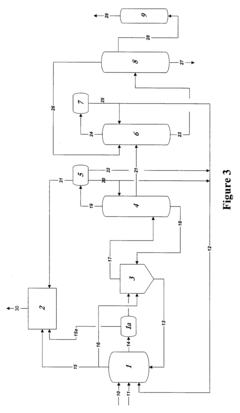
01 Purification methods for glacial acetic acid
Various purification techniques are employed to remove impurities from glacial acetic acid. These methods include distillation, crystallization, and adsorption processes. Advanced separation technologies such as membrane filtration and chromatography may also be used to achieve high-purity glacial acetic acid.- Purification methods for glacial acetic acid: Various purification methods are employed to remove impurities from glacial acetic acid. These methods include distillation, crystallization, and adsorption techniques. The purification process aims to reduce the presence of contaminants such as water, aldehydes, and other organic compounds, resulting in high-purity glacial acetic acid suitable for industrial and laboratory use.
- Impurity detection and analysis in glacial acetic acid: Analytical techniques are used to detect and quantify impurities in glacial acetic acid. These methods may include gas chromatography, mass spectrometry, and spectrophotometric analysis. Accurate impurity detection is crucial for quality control and ensuring the purity of glacial acetic acid for various applications.
- Equipment and systems for handling glacial acetic acid: Specialized equipment and systems are designed for the production, storage, and handling of glacial acetic acid. These systems often incorporate materials resistant to acetic acid corrosion and features to minimize contamination. Proper equipment design helps maintain the purity of glacial acetic acid throughout its lifecycle.
- Impurity removal in acetic acid production processes: Various production processes for acetic acid incorporate steps to minimize or remove impurities. These may include catalytic processes, oxidation methods, and fermentation techniques. The focus is on developing efficient production methods that yield high-purity glacial acetic acid with minimal impurities.
- Stabilization and preservation of glacial acetic acid purity: Methods and additives are used to stabilize glacial acetic acid and preserve its purity during storage and transportation. This may involve the use of antioxidants, moisture scavengers, or specialized packaging materials. These techniques help maintain the quality and purity of glacial acetic acid over extended periods.
02 Common impurities in glacial acetic acid
Glacial acetic acid may contain various impurities, including water, aldehydes, formic acid, and metal ions. These impurities can affect the quality and performance of the acid in industrial applications. Identifying and quantifying these impurities is crucial for quality control and process optimization.Expand Specific Solutions03 Analytical techniques for impurity detection
Several analytical methods are used to detect and measure impurities in glacial acetic acid. These include gas chromatography, high-performance liquid chromatography, mass spectrometry, and spectrophotometric techniques. These methods allow for precise quantification of trace impurities and help ensure product quality.Expand Specific Solutions04 Impact of impurities on industrial processes
Impurities in glacial acetic acid can significantly affect industrial processes that use this chemical. They may interfere with chemical reactions, reduce product yield, or cause equipment corrosion. Understanding the impact of specific impurities is essential for optimizing production processes and maintaining product quality.Expand Specific Solutions05 Specialized equipment for handling and purifying glacial acetic acid
Specialized equipment is designed for handling and purifying glacial acetic acid while minimizing impurity introduction. This includes corrosion-resistant storage tanks, specialized distillation columns, and advanced filtration systems. Such equipment helps maintain the purity of glacial acetic acid throughout its production and storage lifecycle.Expand Specific Solutions
Key Players in Analytical Chemistry and Acetic Acid Industry
The detection of impurities in glacial acetic acid is a mature field with established techniques, yet ongoing research continues to refine methods for increased sensitivity and efficiency. The market for this technology is moderate in size, primarily driven by the chemical, pharmaceutical, and food industries. Companies like LyondellBasell Acetyls LLC and Jiangsu Lianhuan Pharmaceutical Co., Ltd. are key players in acetic acid production, while research institutions such as Friedrich Schiller University of Jena and the University of Alicante contribute to advancing detection methods. The competitive landscape is characterized by a mix of established industrial players and academic institutions collaborating to improve impurity detection techniques, balancing cost-effectiveness with regulatory compliance.
LyondellBasell Acetyls LLC
Technical Solution: LyondellBasell Acetyls LLC has developed advanced methods for detecting impurities in glacial acetic acid, focusing on high-precision gas chromatography-mass spectrometry (GC-MS) techniques. Their approach involves using specialized column configurations and optimized temperature programs to separate and identify trace impurities with high sensitivity[1]. The company has also implemented online monitoring systems that utilize near-infrared (NIR) spectroscopy for real-time impurity detection during the production process[2]. This combination of offline and online analysis allows for comprehensive quality control and early detection of potential contaminants.
Strengths: High sensitivity and accuracy in impurity detection, real-time monitoring capabilities. Weaknesses: Potentially high equipment costs, requires specialized expertise for data interpretation.
Air Liquide SA
Technical Solution: Air Liquide has pioneered the use of Raman spectroscopy for impurity detection in glacial acetic acid. Their method employs surface-enhanced Raman scattering (SERS) substrates to amplify the signal of trace impurities, allowing for detection limits in the sub-ppm range[5]. The company has also developed a portable Raman spectrometer system for on-site analysis, enabling rapid quality control checks during production and transportation. In addition to spectroscopic methods, Air Liquide utilizes electrochemical sensors with specialized coatings to detect specific ionic impurities commonly found in acetic acid production processes[6]. This multi-modal approach provides a comprehensive impurity profile with minimal sample preparation.
Strengths: Rapid analysis times, potential for on-site and in-line monitoring. Weaknesses: May have limitations in detecting certain types of impurities, requires careful calibration and maintenance of SERS substrates.
Core Innovations in Trace Impurity Analysis
Control of impurities in product glacial acetic acid of rhodium-catalyzed methanol carbonylation
PatentInactiveUS20090187043A1
Innovation
- A method involving a rhodium-catalyzed carbonylation process with controlled water and iodide concentrations, combined with the use of a macroreticular strong-acid cation exchange resin and silver or mercury exchanged cation exchange substrates to manage impurities, ensuring high purity glacial acetic acid production.
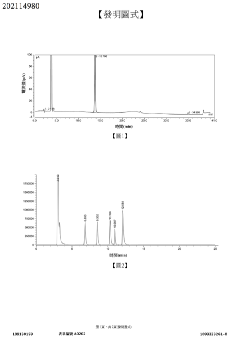
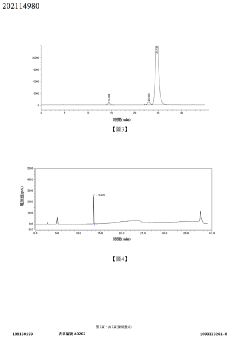
Impurity detection method for 2-((1s,2s,3r,6s,8s)-2-(aminomethyl)tricyclo[4210 3,8]nonane-2-yl)acetate benzenesulfonate or composition thereof
PatentActiveTW202114980A
Innovation
- Combination of HPLC-CAD and LC-MS methods for comprehensive impurity detection, including content, related substances, and chiral isomers.
- Achieved effective separation and quantitative detection with a degree of separation greater than 1.5, ensuring good specificity, repeatability, and stability.
- Significantly reduced detection costs, making the method suitable for industrial production.
Regulatory Standards for Acetic Acid Purity
Regulatory standards for acetic acid purity are crucial in ensuring the quality and safety of glacial acetic acid used in various industries. These standards are typically set by national and international regulatory bodies, such as the United States Pharmacopeia (USP), European Pharmacopoeia (EP), and International Conference on Harmonisation (ICH).
The USP, for instance, specifies that glacial acetic acid should contain not less than 99.5% (w/w) of CH3COOH. It also sets limits for various impurities, including formic acid, sulfur dioxide, and heavy metals. The EP has similar requirements, with a minimum purity of 99.5% and specific limits on impurities like aldehydes, formic acid, and non-volatile residues.
In addition to pharmacopoeial standards, industry-specific regulations may apply. For example, the food industry follows guidelines set by the Food and Drug Administration (FDA) in the United States or the European Food Safety Authority (EFSA) in Europe. These regulations often include specifications for acetic acid used as a food additive or processing aid.
Environmental regulations also play a role in acetic acid purity standards. The Environmental Protection Agency (EPA) in the United States and the European Chemicals Agency (ECHA) set limits on emissions and waste products associated with acetic acid production and use. These regulations indirectly influence purity standards by requiring manufacturers to control and monitor impurities throughout the production process.
Regulatory bodies often require specific analytical methods for detecting and quantifying impurities in glacial acetic acid. These methods may include gas chromatography, high-performance liquid chromatography, and spectrophotometric techniques. The choice of method depends on the nature of the impurity and the required detection limits.
Compliance with these regulatory standards necessitates rigorous quality control measures in acetic acid production facilities. Manufacturers must implement robust testing protocols and maintain detailed documentation of their quality assurance processes. Regular audits and inspections by regulatory agencies ensure ongoing compliance with established purity standards.
As scientific understanding and analytical capabilities advance, regulatory standards for acetic acid purity continue to evolve. Regulatory bodies periodically review and update their standards to reflect new knowledge about potential impurities and their health impacts. This ongoing process ensures that purity standards remain relevant and effective in protecting public health and maintaining product quality across various industries that rely on glacial acetic acid.
The USP, for instance, specifies that glacial acetic acid should contain not less than 99.5% (w/w) of CH3COOH. It also sets limits for various impurities, including formic acid, sulfur dioxide, and heavy metals. The EP has similar requirements, with a minimum purity of 99.5% and specific limits on impurities like aldehydes, formic acid, and non-volatile residues.
In addition to pharmacopoeial standards, industry-specific regulations may apply. For example, the food industry follows guidelines set by the Food and Drug Administration (FDA) in the United States or the European Food Safety Authority (EFSA) in Europe. These regulations often include specifications for acetic acid used as a food additive or processing aid.
Environmental regulations also play a role in acetic acid purity standards. The Environmental Protection Agency (EPA) in the United States and the European Chemicals Agency (ECHA) set limits on emissions and waste products associated with acetic acid production and use. These regulations indirectly influence purity standards by requiring manufacturers to control and monitor impurities throughout the production process.
Regulatory bodies often require specific analytical methods for detecting and quantifying impurities in glacial acetic acid. These methods may include gas chromatography, high-performance liquid chromatography, and spectrophotometric techniques. The choice of method depends on the nature of the impurity and the required detection limits.
Compliance with these regulatory standards necessitates rigorous quality control measures in acetic acid production facilities. Manufacturers must implement robust testing protocols and maintain detailed documentation of their quality assurance processes. Regular audits and inspections by regulatory agencies ensure ongoing compliance with established purity standards.
As scientific understanding and analytical capabilities advance, regulatory standards for acetic acid purity continue to evolve. Regulatory bodies periodically review and update their standards to reflect new knowledge about potential impurities and their health impacts. This ongoing process ensures that purity standards remain relevant and effective in protecting public health and maintaining product quality across various industries that rely on glacial acetic acid.
Environmental Impact of Impurity Detection Processes
The environmental impact of impurity detection processes in glacial acetic acid production is a critical consideration for sustainable manufacturing practices. Traditional detection methods often involve chemical reagents and solvents that can contribute to environmental pollution if not properly managed. Gas chromatography, a common technique for impurity analysis, requires the use of carrier gases and may result in the release of volatile organic compounds (VOCs) into the atmosphere.
Spectroscopic methods, such as UV-Vis and FTIR, generally have a lower environmental footprint as they do not require extensive sample preparation or chemical reagents. However, the disposal of cuvettes and other consumables used in these processes still needs to be addressed to minimize waste generation. High-performance liquid chromatography (HPLC), while effective for impurity detection, utilizes significant amounts of organic solvents that can pose environmental risks if not recycled or disposed of properly.
Recent advancements in green analytical chemistry have led to the development of more environmentally friendly detection methods. These include the use of supercritical fluid chromatography, which employs carbon dioxide as a mobile phase, reducing the reliance on harmful organic solvents. Additionally, the application of solid-phase microextraction techniques has shown promise in minimizing solvent consumption and waste generation during sample preparation.
The energy consumption associated with impurity detection processes is another environmental concern. Many analytical instruments require continuous operation and temperature control, leading to increased electricity usage and associated carbon emissions. Efforts to improve energy efficiency in laboratory equipment and the adoption of renewable energy sources can help mitigate these impacts.
Water usage in impurity detection processes, particularly for sample preparation and instrument cleaning, also contributes to the environmental footprint. Implementing water recycling systems and optimizing cleaning procedures can significantly reduce water consumption and wastewater generation. Furthermore, the proper treatment of wastewater containing trace amounts of acetic acid and other chemicals is essential to prevent contamination of water bodies.
As the chemical industry moves towards more sustainable practices, there is a growing emphasis on developing and implementing impurity detection methods with reduced environmental impact. This includes the exploration of non-destructive testing techniques, such as near-infrared spectroscopy and Raman spectroscopy, which require minimal sample preparation and generate little to no waste. The integration of these environmentally friendly detection methods into quality control processes not only benefits the ecosystem but also aligns with regulatory requirements and corporate sustainability goals.
Spectroscopic methods, such as UV-Vis and FTIR, generally have a lower environmental footprint as they do not require extensive sample preparation or chemical reagents. However, the disposal of cuvettes and other consumables used in these processes still needs to be addressed to minimize waste generation. High-performance liquid chromatography (HPLC), while effective for impurity detection, utilizes significant amounts of organic solvents that can pose environmental risks if not recycled or disposed of properly.
Recent advancements in green analytical chemistry have led to the development of more environmentally friendly detection methods. These include the use of supercritical fluid chromatography, which employs carbon dioxide as a mobile phase, reducing the reliance on harmful organic solvents. Additionally, the application of solid-phase microextraction techniques has shown promise in minimizing solvent consumption and waste generation during sample preparation.
The energy consumption associated with impurity detection processes is another environmental concern. Many analytical instruments require continuous operation and temperature control, leading to increased electricity usage and associated carbon emissions. Efforts to improve energy efficiency in laboratory equipment and the adoption of renewable energy sources can help mitigate these impacts.
Water usage in impurity detection processes, particularly for sample preparation and instrument cleaning, also contributes to the environmental footprint. Implementing water recycling systems and optimizing cleaning procedures can significantly reduce water consumption and wastewater generation. Furthermore, the proper treatment of wastewater containing trace amounts of acetic acid and other chemicals is essential to prevent contamination of water bodies.
As the chemical industry moves towards more sustainable practices, there is a growing emphasis on developing and implementing impurity detection methods with reduced environmental impact. This includes the exploration of non-destructive testing techniques, such as near-infrared spectroscopy and Raman spectroscopy, which require minimal sample preparation and generate little to no waste. The integration of these environmentally friendly detection methods into quality control processes not only benefits the ecosystem but also aligns with regulatory requirements and corporate sustainability goals.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!