Microcapsule Shell Materials And Release Kinetics Tuning
AUG 29, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microcapsule Technology Background and Objectives
Microcapsule technology has evolved significantly over the past several decades, transitioning from simple encapsulation methods to sophisticated engineered systems with precisely controlled properties. The concept of microencapsulation was first introduced in the 1950s for carbonless copy paper applications, but has since expanded into numerous industries including pharmaceuticals, agriculture, food, textiles, and cosmetics. This technological evolution has been driven by the increasing demand for controlled release systems that can protect active ingredients and deliver them at specific rates under predetermined conditions.
The fundamental principle of microcapsules involves a core material surrounded by a protective shell or matrix. The shell material selection has progressed from simple natural polymers to complex synthetic materials with tailored properties. Early encapsulation relied primarily on gelatin, gum arabic, and other natural polymers, while modern systems incorporate sophisticated materials including polyesters, polyamides, polyurethanes, and various copolymers with specific functionalities.
Recent advancements in material science have enabled the development of stimuli-responsive microcapsule shells that can respond to environmental triggers such as pH, temperature, light, magnetic fields, or specific chemical compounds. This represents a significant leap forward from traditional passive release mechanisms that relied solely on diffusion or shell degradation. The ability to tune release kinetics has become increasingly important as applications demand more precise control over when and where active ingredients are released.
The primary objective of current research in microcapsule technology is to develop shell materials and architectures that allow for precise control over release kinetics while maintaining stability during storage and processing. This includes the development of multi-layered shells, composite materials, and hybrid organic-inorganic structures that can provide multiple functionalities simultaneously. Another critical goal is to enhance the scalability and cost-effectiveness of production methods to facilitate broader commercial adoption.
Looking forward, the field is moving toward more sustainable and environmentally friendly shell materials derived from renewable resources, as well as biodegradable options that leave no harmful residues. Additionally, there is growing interest in developing "smart" microcapsules capable of autonomous decision-making based on environmental conditions, potentially incorporating elements of nanotechnology and molecular recognition to achieve unprecedented levels of control over release profiles.
The convergence of microcapsule technology with other emerging fields such as 3D printing, microfluidics, and artificial intelligence is expected to open new frontiers in customized delivery systems with applications ranging from personalized medicine to advanced materials with self-healing properties. These developments aim to address the increasing complexity of release requirements in modern applications while maintaining practical manufacturability.
The fundamental principle of microcapsules involves a core material surrounded by a protective shell or matrix. The shell material selection has progressed from simple natural polymers to complex synthetic materials with tailored properties. Early encapsulation relied primarily on gelatin, gum arabic, and other natural polymers, while modern systems incorporate sophisticated materials including polyesters, polyamides, polyurethanes, and various copolymers with specific functionalities.
Recent advancements in material science have enabled the development of stimuli-responsive microcapsule shells that can respond to environmental triggers such as pH, temperature, light, magnetic fields, or specific chemical compounds. This represents a significant leap forward from traditional passive release mechanisms that relied solely on diffusion or shell degradation. The ability to tune release kinetics has become increasingly important as applications demand more precise control over when and where active ingredients are released.
The primary objective of current research in microcapsule technology is to develop shell materials and architectures that allow for precise control over release kinetics while maintaining stability during storage and processing. This includes the development of multi-layered shells, composite materials, and hybrid organic-inorganic structures that can provide multiple functionalities simultaneously. Another critical goal is to enhance the scalability and cost-effectiveness of production methods to facilitate broader commercial adoption.
Looking forward, the field is moving toward more sustainable and environmentally friendly shell materials derived from renewable resources, as well as biodegradable options that leave no harmful residues. Additionally, there is growing interest in developing "smart" microcapsules capable of autonomous decision-making based on environmental conditions, potentially incorporating elements of nanotechnology and molecular recognition to achieve unprecedented levels of control over release profiles.
The convergence of microcapsule technology with other emerging fields such as 3D printing, microfluidics, and artificial intelligence is expected to open new frontiers in customized delivery systems with applications ranging from personalized medicine to advanced materials with self-healing properties. These developments aim to address the increasing complexity of release requirements in modern applications while maintaining practical manufacturability.
Market Applications and Demand Analysis
The global market for microcapsule technology has witnessed substantial growth across diverse industries, driven by the increasing demand for controlled release systems and enhanced product performance. The market size for microencapsulation was valued at approximately $8.5 billion in 2021 and is projected to reach $15.2 billion by 2027, growing at a CAGR of 10.2% during the forecast period.
In the pharmaceutical sector, microcapsules with tunable release kinetics have revolutionized drug delivery systems, enabling precise control over medication release rates and improving therapeutic efficacy. This application segment holds the largest market share at 32%, with particular demand for pH-responsive and enzymatically degradable shell materials that can target specific areas of the gastrointestinal tract or respond to specific biological triggers.
The food and beverage industry represents the second-largest application area, accounting for 28% of the market. Here, microcapsules are extensively used for flavor encapsulation, nutrient protection, and extending shelf life. Consumer preference for clean-label products has driven innovation in natural shell materials such as modified starches, proteins, and cellulose derivatives that offer controlled release properties while meeting regulatory requirements.
Agricultural applications have emerged as the fastest-growing segment with a 13.5% annual growth rate. Smart delivery systems for fertilizers, pesticides, and growth promoters utilizing biodegradable microcapsule shells have gained significant traction due to their ability to reduce environmental impact while improving efficacy through controlled release mechanisms.
The cosmetics and personal care industry has embraced microcapsule technology for delivering active ingredients, fragrances, and moisturizers with precise timing and targeting. This sector represents 17% of the market, with particular interest in stimuli-responsive shell materials that can release contents in response to temperature, pH, or mechanical stress.
Regional analysis indicates North America and Europe currently dominate the market with 35% and 30% shares respectively, primarily due to advanced R&D infrastructure and early adoption. However, the Asia-Pacific region is experiencing the highest growth rate at 12.3% annually, driven by expanding pharmaceutical and cosmetic industries in China, Japan, and India.
Key market drivers include increasing consumer demand for products with enhanced performance characteristics, growing preference for sustainable and biodegradable materials, and technological advancements enabling more precise control over release kinetics. The ability to tune release profiles through shell material selection and modification represents a critical competitive advantage for manufacturers across all application sectors.
In the pharmaceutical sector, microcapsules with tunable release kinetics have revolutionized drug delivery systems, enabling precise control over medication release rates and improving therapeutic efficacy. This application segment holds the largest market share at 32%, with particular demand for pH-responsive and enzymatically degradable shell materials that can target specific areas of the gastrointestinal tract or respond to specific biological triggers.
The food and beverage industry represents the second-largest application area, accounting for 28% of the market. Here, microcapsules are extensively used for flavor encapsulation, nutrient protection, and extending shelf life. Consumer preference for clean-label products has driven innovation in natural shell materials such as modified starches, proteins, and cellulose derivatives that offer controlled release properties while meeting regulatory requirements.
Agricultural applications have emerged as the fastest-growing segment with a 13.5% annual growth rate. Smart delivery systems for fertilizers, pesticides, and growth promoters utilizing biodegradable microcapsule shells have gained significant traction due to their ability to reduce environmental impact while improving efficacy through controlled release mechanisms.
The cosmetics and personal care industry has embraced microcapsule technology for delivering active ingredients, fragrances, and moisturizers with precise timing and targeting. This sector represents 17% of the market, with particular interest in stimuli-responsive shell materials that can release contents in response to temperature, pH, or mechanical stress.
Regional analysis indicates North America and Europe currently dominate the market with 35% and 30% shares respectively, primarily due to advanced R&D infrastructure and early adoption. However, the Asia-Pacific region is experiencing the highest growth rate at 12.3% annually, driven by expanding pharmaceutical and cosmetic industries in China, Japan, and India.
Key market drivers include increasing consumer demand for products with enhanced performance characteristics, growing preference for sustainable and biodegradable materials, and technological advancements enabling more precise control over release kinetics. The ability to tune release profiles through shell material selection and modification represents a critical competitive advantage for manufacturers across all application sectors.
Current Shell Materials and Technical Challenges
Microcapsule shell materials have evolved significantly over the past decades, with current technologies employing a diverse range of polymeric, inorganic, and hybrid materials. Synthetic polymers such as polyurea, polyurethane, melamine-formaldehyde, and poly(methyl methacrylate) remain dominant in commercial applications due to their established manufacturing processes and predictable performance characteristics. These materials offer good mechanical stability and moderate barrier properties, making them suitable for encapsulating various active ingredients in consumer products, pharmaceuticals, and agricultural formulations.
Natural polymers including gelatin, alginate, chitosan, and cellulose derivatives have gained increasing attention due to their biocompatibility, biodegradability, and sustainability advantages. These materials are particularly valuable in food, pharmaceutical, and cosmetic applications where safety profiles and environmental considerations are paramount. However, their widespread adoption faces challenges related to batch-to-batch variability, limited mechanical strength, and higher production costs compared to synthetic alternatives.
Inorganic shell materials such as silica, calcium carbonate, and various metal oxides represent another important category, offering exceptional barrier properties, thermal stability, and controlled porosity. These materials excel in applications requiring protection against harsh environmental conditions or precise release kinetics, though they often require more complex synthesis procedures and may present challenges in biodegradability.
Despite these advances, significant technical challenges persist in the field of microcapsule shell materials. Achieving precise control over shell thickness and uniformity remains difficult, particularly in large-scale production environments. This variability directly impacts release kinetics, stability, and overall performance consistency. Current manufacturing processes often struggle to maintain tight quality control parameters across production batches.
Permeability control represents another major challenge, as existing shell materials frequently exhibit either excessive or insufficient barrier properties for specific applications. The ability to fine-tune permeability characteristics without compromising mechanical integrity continues to elude many current formulations. This limitation restricts the development of sophisticated release profiles needed for advanced applications in drug delivery, self-healing materials, and smart textiles.
Compatibility issues between shell materials and core substances present ongoing difficulties, particularly with highly reactive, volatile, or sensitive active ingredients. Current encapsulation technologies often require compromise between optimal shell properties and core stability, limiting the range of substances that can be effectively encapsulated and protected.
Scale-up challenges further complicate commercial implementation, as laboratory-scale successes frequently encounter significant hurdles during industrial production. Process parameters that work effectively at small scales often require substantial modification for large-scale manufacturing, impacting product consistency and economic viability.
Natural polymers including gelatin, alginate, chitosan, and cellulose derivatives have gained increasing attention due to their biocompatibility, biodegradability, and sustainability advantages. These materials are particularly valuable in food, pharmaceutical, and cosmetic applications where safety profiles and environmental considerations are paramount. However, their widespread adoption faces challenges related to batch-to-batch variability, limited mechanical strength, and higher production costs compared to synthetic alternatives.
Inorganic shell materials such as silica, calcium carbonate, and various metal oxides represent another important category, offering exceptional barrier properties, thermal stability, and controlled porosity. These materials excel in applications requiring protection against harsh environmental conditions or precise release kinetics, though they often require more complex synthesis procedures and may present challenges in biodegradability.
Despite these advances, significant technical challenges persist in the field of microcapsule shell materials. Achieving precise control over shell thickness and uniformity remains difficult, particularly in large-scale production environments. This variability directly impacts release kinetics, stability, and overall performance consistency. Current manufacturing processes often struggle to maintain tight quality control parameters across production batches.
Permeability control represents another major challenge, as existing shell materials frequently exhibit either excessive or insufficient barrier properties for specific applications. The ability to fine-tune permeability characteristics without compromising mechanical integrity continues to elude many current formulations. This limitation restricts the development of sophisticated release profiles needed for advanced applications in drug delivery, self-healing materials, and smart textiles.
Compatibility issues between shell materials and core substances present ongoing difficulties, particularly with highly reactive, volatile, or sensitive active ingredients. Current encapsulation technologies often require compromise between optimal shell properties and core stability, limiting the range of substances that can be effectively encapsulated and protected.
Scale-up challenges further complicate commercial implementation, as laboratory-scale successes frequently encounter significant hurdles during industrial production. Process parameters that work effectively at small scales often require substantial modification for large-scale manufacturing, impacting product consistency and economic viability.
Current Shell Material Selection Strategies
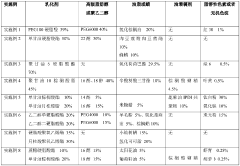
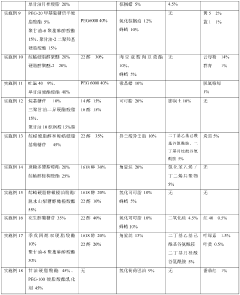
01 Controlled release mechanisms for microcapsules
Various mechanisms can be employed to control the release of active ingredients from microcapsules. These include pH-responsive release, temperature-triggered release, and enzymatic degradation of the capsule wall. By carefully selecting the shell materials and designing the microcapsule structure, the release kinetics can be tailored for specific applications, allowing for sustained or targeted delivery of the encapsulated compounds.- Factors affecting microcapsule release kinetics: Various factors can influence the release kinetics of microcapsules, including the shell material composition, core-to-shell ratio, particle size, and environmental conditions. The selection of appropriate shell materials and manufacturing techniques can help control the release rate of encapsulated active ingredients. Understanding these factors is crucial for designing microcapsules with desired release profiles for specific applications.
- Stimuli-responsive release mechanisms: Microcapsules can be designed to release their contents in response to specific stimuli, such as pH changes, temperature variations, mechanical stress, or enzymatic activity. These smart release systems allow for targeted and controlled delivery of active ingredients. By incorporating responsive polymers or functional groups into the microcapsule shell, the release kinetics can be precisely tailored to respond to environmental triggers at the desired location or time.
- Mathematical modeling of release kinetics: Mathematical models are used to predict and analyze the release kinetics of microcapsules. These models incorporate various parameters such as diffusion coefficients, partition coefficients, and degradation rates to simulate the release behavior under different conditions. Common models include zero-order kinetics, first-order kinetics, Higuchi model, and Korsmeyer-Peppas model. These mathematical approaches help in optimizing microcapsule formulations and predicting their performance in real-world applications.
- Novel shell materials for controlled release: Innovative shell materials are being developed to achieve better control over microcapsule release kinetics. These materials include biodegradable polymers, composite materials, and hybrid organic-inorganic structures. The selection of shell materials significantly impacts the permeability, stability, and degradation rate of microcapsules, thereby influencing the release profile of encapsulated substances. Advanced shell materials can provide sustained release, pulsatile release, or targeted release depending on the application requirements.
- Analytical techniques for studying release kinetics: Various analytical methods are employed to study and characterize the release kinetics of microcapsules. These techniques include spectroscopy, chromatography, microscopy, and rheological measurements. In vitro release testing under simulated conditions helps predict in vivo performance. Advanced imaging techniques allow for real-time monitoring of release processes, providing insights into the mechanisms governing release kinetics and enabling the development of more effective microcapsule systems.
02 Shell material composition effects on release profiles
The composition of the microcapsule shell material significantly influences the release kinetics of the encapsulated substances. Different polymers, such as polysaccharides, proteins, and synthetic polymers, exhibit varying permeability, degradation rates, and responsiveness to environmental stimuli. By adjusting the shell material composition or creating multi-layered shells, the release rate can be precisely controlled to achieve desired pharmacokinetic or application profiles.Expand Specific Solutions03 Mathematical modeling of microcapsule release kinetics
Mathematical models are essential tools for predicting and analyzing the release kinetics of active ingredients from microcapsules. Various models, including zero-order, first-order, Higuchi, and Korsmeyer-Peppas models, can be applied to describe different release mechanisms. These models help in understanding the diffusion processes, erosion mechanisms, and other factors affecting release rates, enabling the optimization of microcapsule formulations for specific applications.Expand Specific Solutions04 Environmental factors influencing release kinetics
Environmental conditions significantly impact the release kinetics of microcapsules. Factors such as temperature, pH, ionic strength, and the presence of specific enzymes or chemicals can trigger or modify the release rate. Understanding these environmental influences allows for the design of smart microcapsules that respond to specific conditions, enabling targeted release in particular environments or controlled release over time under varying conditions.Expand Specific Solutions05 Novel techniques for measuring and characterizing release kinetics
Advanced analytical techniques are being developed to accurately measure and characterize the release kinetics of microcapsules. These include spectroscopic methods, chromatographic techniques, microscopy, and real-time monitoring systems. Such techniques provide valuable insights into the release mechanisms, allowing for better design and optimization of microcapsule formulations. Additionally, high-throughput screening methods enable rapid evaluation of multiple formulations under various conditions.Expand Specific Solutions
Leading Companies and Research Institutions
The microcapsule shell materials and release kinetics tuning market is in a growth phase, with an estimated global value exceeding $7 billion and projected annual growth of 10-12%. The competitive landscape features established chemical giants like BASF, Bayer, and Firmenich alongside specialized players such as Encapsys LLC, which pioneered microencapsulation technology. Technical maturity varies across applications, with agricultural and personal care sectors showing higher sophistication. Leading innovators BASF and Encapsys have developed advanced controlled-release technologies, while academic institutions like Tsinghua University and Max Planck Society contribute fundamental research. The industry is witnessing increased focus on sustainable, biodegradable shell materials and precise release mechanisms, driving partnerships between established companies and research institutions to accelerate commercialization.
Encapsys LLC
Technical Solution: Encapsys LLC has developed a specialized microcapsule technology platform focused on in-situ polymerization techniques for creating highly controlled shell materials. Their core technology utilizes melamine-formaldehyde and urea-formaldehyde chemistry with proprietary modifications to enhance shell performance characteristics. Encapsys has pioneered precise control over shell wall thickness (typically 0.1-2μm) and porosity through manipulation of reaction kinetics and surfactant systems during the encapsulation process. Their technology enables the creation of pressure-sensitive microcapsules with calibrated burst strength profiles, allowing for targeted release upon specific mechanical triggers. Additionally, Encapsys has developed hybrid organic-inorganic shell materials incorporating silica nanoparticles to enhance thermal stability and mechanical strength while maintaining controlled permeability characteristics. Their manufacturing process allows for narrow particle size distributions (coefficient of variation <15%) which ensures consistent release performance across batches.
Strengths: Highly specialized in pressure-sensitive release mechanisms; excellent manufacturing consistency and scalability. Weaknesses: Limited flexibility in creating biodegradable formulations; some shell chemistries have regulatory restrictions in certain applications.
Firmenich SA
Technical Solution: Firmenich SA has developed a proprietary microcapsule technology called "EncapSure" specifically designed for fragrance and flavor applications. Their approach focuses on multi-layered shell architectures using combinations of natural and synthetic polymers including modified starches, proteins, and specialized copolymers. Firmenich's technology enables precise control over fragrance release through engineered shell porosity and thickness gradients (typically 0.5-5μm). Their innovation includes temperature-phased release systems that can deliver different fragrance notes at specific temperature thresholds, creating dynamic sensory experiences. The company has also pioneered moisture-activated release mechanisms using hydrophilic polymers that swell upon contact with water, creating controlled rupture or increased permeability. Additionally, Firmenich has developed friction-responsive capsules that release fragrances gradually during mechanical interaction, ideal for textile and personal care applications.
Strengths: Specialized expertise in sensory applications allows for highly refined consumer experiences; excellent compatibility with complex fragrance molecules. Weaknesses: Limited application beyond fragrance/flavor industries; some natural polymer shells show batch-to-batch variability in performance characteristics.
Key Patents in Release Kinetics Control
Microcapsule shell and method for preparing microcapsules
PatentWO2024045003A1
Innovation
- The microcapsule shell material is made of high-grade fatty alcohol or polyethylene glycol, grease or wax, emulsifier, etc., with a melting point higher than 50°C. It absorbs water and swells and becomes soft but does not dissolve. It can be easily applied under pressure to release the core material. And uses non-ionic emulsifiers and harmless raw materials.
Controlled release, biodegradable core-shell microcapsule compositions
PatentWO2020209907A1
Innovation
- Development of biodegradable core-shell microcapsules using natural biopolymers cross-linked with specific cross-linking agents, which provide stable fragrance retention for at least four weeks at elevated temperatures and controlled release upon triggering conditions such as friction, pH change, or temperature changes.
Regulatory Framework for Microcapsule Applications
The regulatory landscape governing microcapsule applications spans multiple jurisdictions and sectors, creating a complex framework that manufacturers and researchers must navigate. In the United States, the Food and Drug Administration (FDA) oversees microcapsules used in pharmaceutical, food, and cosmetic applications through different regulatory pathways. For pharmaceutical applications, microcapsules are regulated under the Federal Food, Drug, and Cosmetic Act, requiring extensive safety and efficacy data through clinical trials before market approval.
The European Medicines Agency (EMA) employs a similar but distinct approach, with additional emphasis on environmental impact assessments for novel shell materials. The European Food Safety Authority (EFSA) maintains specific guidelines for food-grade microcapsules, particularly focusing on migration limits of shell components into food matrices.
Regulatory considerations specifically addressing shell materials and release kinetics have evolved significantly over the past decade. Materials must meet biocompatibility standards when used in medical applications, with ISO 10993 serving as the international benchmark for biological evaluation. Natural polymers generally face fewer regulatory hurdles compared to synthetic materials, though consistency and sourcing documentation requirements remain stringent.
Release kinetics tuning presents unique regulatory challenges, as controlled-release mechanisms must demonstrate predictable behavior under various environmental conditions. The FDA's Modified Release Solid Oral Dosage Forms guidance specifically addresses the validation requirements for release mechanisms, including stability testing across temperature and pH ranges relevant to the intended application.
International harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) have established guidelines for stability testing (Q1A) and specifications (Q6A) that impact microcapsule development. These standards help streamline global regulatory submissions but require comprehensive characterization of release profiles.
Emerging regulations increasingly focus on nanoscale aspects of microcapsules, with both the FDA and EMA developing specific frameworks for nanomaterials used in shell construction. Additionally, sustainability considerations are becoming regulatory factors, with several jurisdictions implementing restrictions on persistent, bioaccumulative materials commonly used in conventional microcapsule formulations.
Compliance strategies for novel shell materials typically involve early regulatory consultation, comprehensive characterization of material properties, and thorough documentation of release kinetics under various stress conditions. Regulatory pathways may differ significantly depending on the intended application, with medical devices, pharmaceuticals, and consumer products each following distinct approval processes.
The European Medicines Agency (EMA) employs a similar but distinct approach, with additional emphasis on environmental impact assessments for novel shell materials. The European Food Safety Authority (EFSA) maintains specific guidelines for food-grade microcapsules, particularly focusing on migration limits of shell components into food matrices.
Regulatory considerations specifically addressing shell materials and release kinetics have evolved significantly over the past decade. Materials must meet biocompatibility standards when used in medical applications, with ISO 10993 serving as the international benchmark for biological evaluation. Natural polymers generally face fewer regulatory hurdles compared to synthetic materials, though consistency and sourcing documentation requirements remain stringent.
Release kinetics tuning presents unique regulatory challenges, as controlled-release mechanisms must demonstrate predictable behavior under various environmental conditions. The FDA's Modified Release Solid Oral Dosage Forms guidance specifically addresses the validation requirements for release mechanisms, including stability testing across temperature and pH ranges relevant to the intended application.
International harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) have established guidelines for stability testing (Q1A) and specifications (Q6A) that impact microcapsule development. These standards help streamline global regulatory submissions but require comprehensive characterization of release profiles.
Emerging regulations increasingly focus on nanoscale aspects of microcapsules, with both the FDA and EMA developing specific frameworks for nanomaterials used in shell construction. Additionally, sustainability considerations are becoming regulatory factors, with several jurisdictions implementing restrictions on persistent, bioaccumulative materials commonly used in conventional microcapsule formulations.
Compliance strategies for novel shell materials typically involve early regulatory consultation, comprehensive characterization of material properties, and thorough documentation of release kinetics under various stress conditions. Regulatory pathways may differ significantly depending on the intended application, with medical devices, pharmaceuticals, and consumer products each following distinct approval processes.
Sustainability and Biodegradability Considerations
The sustainability and biodegradability of microcapsule shell materials have become increasingly critical considerations in modern formulation development across various industries. As environmental regulations tighten globally and consumer awareness grows, the environmental impact of microencapsulation technologies can no longer be treated as secondary to performance metrics.
Traditional microcapsule shell materials such as formaldehyde-based resins, polyurethanes, and certain synthetic polymers present significant end-of-life challenges, with persistence in the environment ranging from decades to centuries. This environmental persistence has prompted a paradigm shift toward bio-based and biodegradable alternatives that maintain functional performance while reducing ecological footprint.
Recent advances in biodegradable shell materials include polylactic acid (PLA), polyhydroxyalkanoates (PHAs), modified celluloses, and chitosan-based formulations. These materials offer controlled degradation pathways that can be tuned to specific application requirements. For instance, PLA-based microcapsules can be engineered to maintain stability during product shelf-life but degrade within weeks to months after disposal, compared to years for conventional materials.
The release kinetics of active ingredients can be strategically coupled with the biodegradation profile of the shell material. This synergistic approach allows for complete utilization of the encapsulated active before environmental breakdown of the shell begins. Research indicates that incorporating enzymes or pH-responsive elements into biodegradable shells can trigger sequential release and degradation processes, optimizing both functional performance and environmental outcomes.
Life cycle assessment (LCA) studies comparing traditional and biodegradable microcapsule systems reveal significant reductions in environmental impact metrics, including carbon footprint (30-60% reduction), aquatic toxicity (up to 80% reduction), and resource depletion. However, these benefits must be balanced against potential challenges in manufacturing scalability and cost implications, which currently represent a 15-40% premium over conventional systems.
Regulatory frameworks are evolving to address these materials specifically. The European Union's Single-Use Plastics Directive and similar regulations in North America and Asia are creating market drivers for biodegradable microcapsule technologies. Industry leaders are responding by establishing biodegradability targets for their encapsulation technologies, with several major players committing to 100% biodegradable or compostable formulations by 2030.
The intersection of release kinetics tuning and biodegradability presents both technical challenges and innovation opportunities. Emerging research focuses on biomimetic approaches that replicate natural encapsulation and release mechanisms found in biological systems, potentially offering breakthrough solutions that optimize both functional performance and environmental sustainability.
Traditional microcapsule shell materials such as formaldehyde-based resins, polyurethanes, and certain synthetic polymers present significant end-of-life challenges, with persistence in the environment ranging from decades to centuries. This environmental persistence has prompted a paradigm shift toward bio-based and biodegradable alternatives that maintain functional performance while reducing ecological footprint.
Recent advances in biodegradable shell materials include polylactic acid (PLA), polyhydroxyalkanoates (PHAs), modified celluloses, and chitosan-based formulations. These materials offer controlled degradation pathways that can be tuned to specific application requirements. For instance, PLA-based microcapsules can be engineered to maintain stability during product shelf-life but degrade within weeks to months after disposal, compared to years for conventional materials.
The release kinetics of active ingredients can be strategically coupled with the biodegradation profile of the shell material. This synergistic approach allows for complete utilization of the encapsulated active before environmental breakdown of the shell begins. Research indicates that incorporating enzymes or pH-responsive elements into biodegradable shells can trigger sequential release and degradation processes, optimizing both functional performance and environmental outcomes.
Life cycle assessment (LCA) studies comparing traditional and biodegradable microcapsule systems reveal significant reductions in environmental impact metrics, including carbon footprint (30-60% reduction), aquatic toxicity (up to 80% reduction), and resource depletion. However, these benefits must be balanced against potential challenges in manufacturing scalability and cost implications, which currently represent a 15-40% premium over conventional systems.
Regulatory frameworks are evolving to address these materials specifically. The European Union's Single-Use Plastics Directive and similar regulations in North America and Asia are creating market drivers for biodegradable microcapsule technologies. Industry leaders are responding by establishing biodegradability targets for their encapsulation technologies, with several major players committing to 100% biodegradable or compostable formulations by 2030.
The intersection of release kinetics tuning and biodegradability presents both technical challenges and innovation opportunities. Emerging research focuses on biomimetic approaches that replicate natural encapsulation and release mechanisms found in biological systems, potentially offering breakthrough solutions that optimize both functional performance and environmental sustainability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!