Microreactors for Drug Screening and Personalized Medicine Applications
SEP 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microreactor Technology Evolution and Objectives
Microreactors represent a transformative technology in the field of drug discovery and personalized medicine, evolving significantly over the past three decades. Initially developed in the 1990s as simple flow chemistry tools, microreactors have progressed from basic channel designs to sophisticated integrated systems capable of mimicking complex biological environments. This evolution has been driven by advancements in microfabrication techniques, materials science, and the integration of sensing technologies.
The technological trajectory of microreactors has been marked by several key milestones. Early systems focused primarily on improving reaction efficiency and reducing reagent consumption. By the early 2000s, the integration of multiple reaction chambers and detection systems enabled more complex screening applications. The past decade has witnessed the emergence of organ-on-a-chip and human-on-a-chip platforms, representing a paradigm shift toward biomimetic systems that can replicate human physiological responses.
Current microreactor technology aims to bridge the gap between traditional in vitro testing and clinical outcomes by providing more physiologically relevant drug screening platforms. This approach addresses the limitations of conventional drug development processes, which often fail to predict drug efficacy and toxicity accurately in humans, resulting in high attrition rates during clinical trials.
The primary objectives of microreactor technology in drug screening and personalized medicine applications are multifaceted. First, these systems aim to enhance the predictive power of preclinical testing by creating more accurate models of human physiology and disease states. Second, they seek to enable high-throughput screening with minimal sample volumes, making them particularly valuable for rare patient samples or expensive compounds.
Another critical objective is the development of patient-specific testing platforms that can inform personalized treatment decisions. By incorporating patient-derived cells or tissues into microreactor systems, clinicians can potentially predict individual responses to specific therapies before administration, optimizing treatment outcomes while minimizing adverse effects.
Looking forward, the field is moving toward fully automated, AI-integrated microreactor systems capable of autonomous experimentation and data analysis. The ultimate goal is to create accessible, standardized platforms that can be widely deployed in clinical settings, enabling real-time treatment optimization based on individual patient characteristics and disease progression patterns.
The convergence of microfluidics, tissue engineering, and artificial intelligence is expected to drive the next generation of microreactor technologies, potentially revolutionizing the drug development pipeline and enabling truly personalized medicine approaches that account for the biological complexity and variability of human patients.
The technological trajectory of microreactors has been marked by several key milestones. Early systems focused primarily on improving reaction efficiency and reducing reagent consumption. By the early 2000s, the integration of multiple reaction chambers and detection systems enabled more complex screening applications. The past decade has witnessed the emergence of organ-on-a-chip and human-on-a-chip platforms, representing a paradigm shift toward biomimetic systems that can replicate human physiological responses.
Current microreactor technology aims to bridge the gap between traditional in vitro testing and clinical outcomes by providing more physiologically relevant drug screening platforms. This approach addresses the limitations of conventional drug development processes, which often fail to predict drug efficacy and toxicity accurately in humans, resulting in high attrition rates during clinical trials.
The primary objectives of microreactor technology in drug screening and personalized medicine applications are multifaceted. First, these systems aim to enhance the predictive power of preclinical testing by creating more accurate models of human physiology and disease states. Second, they seek to enable high-throughput screening with minimal sample volumes, making them particularly valuable for rare patient samples or expensive compounds.
Another critical objective is the development of patient-specific testing platforms that can inform personalized treatment decisions. By incorporating patient-derived cells or tissues into microreactor systems, clinicians can potentially predict individual responses to specific therapies before administration, optimizing treatment outcomes while minimizing adverse effects.
Looking forward, the field is moving toward fully automated, AI-integrated microreactor systems capable of autonomous experimentation and data analysis. The ultimate goal is to create accessible, standardized platforms that can be widely deployed in clinical settings, enabling real-time treatment optimization based on individual patient characteristics and disease progression patterns.
The convergence of microfluidics, tissue engineering, and artificial intelligence is expected to drive the next generation of microreactor technologies, potentially revolutionizing the drug development pipeline and enabling truly personalized medicine approaches that account for the biological complexity and variability of human patients.
Market Analysis for Drug Screening Microreactors
The global market for drug screening microreactors is experiencing robust growth, driven by increasing demand for more efficient and cost-effective drug discovery processes. Currently valued at approximately $1.2 billion, this market segment is projected to grow at a compound annual growth rate (CAGR) of 8.5% over the next five years, potentially reaching $1.8 billion by 2028.
Pharmaceutical companies represent the largest market segment, accounting for nearly 45% of the total market share. These organizations are increasingly adopting microreactor technologies to accelerate their drug discovery pipelines and reduce the substantial costs associated with bringing new drugs to market, which can exceed $2.6 billion per successful compound.
Academic research institutions constitute the second-largest market segment at 30%, followed by contract research organizations (CROs) at 15%. The remaining 10% is distributed among biotechnology startups and government research facilities. Geographically, North America leads with 40% market share, followed by Europe (30%), Asia-Pacific (25%), and rest of the world (5%).
The personalized medicine application subset is showing particularly strong momentum, growing at 12% annually. This acceleration is fueled by advances in genomics and the increasing recognition that patient-specific drug responses can significantly impact treatment outcomes. Microreactors enable rapid testing of drug candidates against patient-derived cells, providing crucial data for personalized treatment protocols.
Key market drivers include the pressing need to reduce drug development timelines, which currently average 10-15 years from concept to market. Microreactors can potentially compress early-stage screening by 30-40%, representing significant time and cost savings. Additionally, regulatory pressures to decrease animal testing are propelling interest in microreactor platforms that can provide more human-relevant data earlier in the development process.
Market restraints include the high initial investment costs for microreactor systems, which can range from $50,000 for basic systems to over $500,000 for fully automated high-throughput platforms. Technical challenges related to standardization and validation of microreactor-based assays also present barriers to wider adoption, particularly in regulated environments.
Emerging trends include integration with artificial intelligence for data analysis, development of organ-on-chip systems that better mimic human physiology, and increasing focus on environmentally sustainable screening methods that reduce chemical waste. The convergence of microfluidics with 3D cell culture technologies is creating new market opportunities, particularly for complex disease models like cancer and neurodegenerative disorders.
Pharmaceutical companies represent the largest market segment, accounting for nearly 45% of the total market share. These organizations are increasingly adopting microreactor technologies to accelerate their drug discovery pipelines and reduce the substantial costs associated with bringing new drugs to market, which can exceed $2.6 billion per successful compound.
Academic research institutions constitute the second-largest market segment at 30%, followed by contract research organizations (CROs) at 15%. The remaining 10% is distributed among biotechnology startups and government research facilities. Geographically, North America leads with 40% market share, followed by Europe (30%), Asia-Pacific (25%), and rest of the world (5%).
The personalized medicine application subset is showing particularly strong momentum, growing at 12% annually. This acceleration is fueled by advances in genomics and the increasing recognition that patient-specific drug responses can significantly impact treatment outcomes. Microreactors enable rapid testing of drug candidates against patient-derived cells, providing crucial data for personalized treatment protocols.
Key market drivers include the pressing need to reduce drug development timelines, which currently average 10-15 years from concept to market. Microreactors can potentially compress early-stage screening by 30-40%, representing significant time and cost savings. Additionally, regulatory pressures to decrease animal testing are propelling interest in microreactor platforms that can provide more human-relevant data earlier in the development process.
Market restraints include the high initial investment costs for microreactor systems, which can range from $50,000 for basic systems to over $500,000 for fully automated high-throughput platforms. Technical challenges related to standardization and validation of microreactor-based assays also present barriers to wider adoption, particularly in regulated environments.
Emerging trends include integration with artificial intelligence for data analysis, development of organ-on-chip systems that better mimic human physiology, and increasing focus on environmentally sustainable screening methods that reduce chemical waste. The convergence of microfluidics with 3D cell culture technologies is creating new market opportunities, particularly for complex disease models like cancer and neurodegenerative disorders.
Technical Challenges in Microreactor Development
Despite significant advancements in microreactor technology for drug screening and personalized medicine, several critical technical challenges persist that impede widespread implementation. Material compatibility represents a fundamental obstacle, as microreactors must withstand various solvents, reagents, and biological samples without degradation or contamination. Traditional materials like polydimethylsiloxane (PDMS) suffer from absorption issues with hydrophobic compounds, while glass and silicon alternatives present manufacturing complexity and higher costs.
Scaling and parallelization present another significant hurdle. While microreactors excel at single reaction optimization, transitioning to high-throughput screening requires sophisticated multiplexing strategies. Current parallelization approaches often compromise the precision of individual reaction control or introduce cross-contamination risks between channels, limiting true high-throughput capabilities.
Integration of sensing and detection systems within the confined microreactor environment poses substantial challenges. Real-time monitoring of reactions requires miniaturized sensors that maintain sensitivity and specificity without disrupting fluid dynamics. The development of non-invasive optical, electrochemical, and spectroscopic detection methods compatible with microfluidic architectures remains technically demanding.
Surface fouling and clogging represent persistent operational challenges. Protein adsorption, cell adhesion, and precipitation of reaction products can alter surface properties and fluid dynamics, compromising reproducibility. Current surface modification strategies provide only temporary solutions and often lack long-term stability under continuous operation conditions.
Temperature and reaction control at microscale dimensions introduce unique challenges. Achieving uniform temperature distribution across microchannels while enabling rapid temperature transitions for kinetic studies requires sophisticated thermal management solutions. Heat dissipation issues can lead to undesired temperature gradients that affect reaction outcomes and reproducibility.
Interfacing microreactors with existing laboratory infrastructure presents compatibility issues. Standardization remains limited, with diverse connection formats, control systems, and data outputs complicating integration into established workflows. The lack of plug-and-play solutions increases implementation barriers for clinical and pharmaceutical laboratories.
Long-term stability and reproducibility challenges persist, particularly for personalized medicine applications where consistent performance across patient samples is critical. Batch-to-batch variability in microreactor fabrication and performance drift over extended operation periods undermine confidence in translating research findings to clinical applications.
Scaling and parallelization present another significant hurdle. While microreactors excel at single reaction optimization, transitioning to high-throughput screening requires sophisticated multiplexing strategies. Current parallelization approaches often compromise the precision of individual reaction control or introduce cross-contamination risks between channels, limiting true high-throughput capabilities.
Integration of sensing and detection systems within the confined microreactor environment poses substantial challenges. Real-time monitoring of reactions requires miniaturized sensors that maintain sensitivity and specificity without disrupting fluid dynamics. The development of non-invasive optical, electrochemical, and spectroscopic detection methods compatible with microfluidic architectures remains technically demanding.
Surface fouling and clogging represent persistent operational challenges. Protein adsorption, cell adhesion, and precipitation of reaction products can alter surface properties and fluid dynamics, compromising reproducibility. Current surface modification strategies provide only temporary solutions and often lack long-term stability under continuous operation conditions.
Temperature and reaction control at microscale dimensions introduce unique challenges. Achieving uniform temperature distribution across microchannels while enabling rapid temperature transitions for kinetic studies requires sophisticated thermal management solutions. Heat dissipation issues can lead to undesired temperature gradients that affect reaction outcomes and reproducibility.
Interfacing microreactors with existing laboratory infrastructure presents compatibility issues. Standardization remains limited, with diverse connection formats, control systems, and data outputs complicating integration into established workflows. The lack of plug-and-play solutions increases implementation barriers for clinical and pharmaceutical laboratories.
Long-term stability and reproducibility challenges persist, particularly for personalized medicine applications where consistent performance across patient samples is critical. Batch-to-batch variability in microreactor fabrication and performance drift over extended operation periods undermine confidence in translating research findings to clinical applications.
Current Microreactor Solutions for Drug Screening
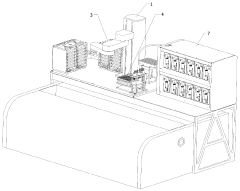
01 Design and fabrication of microreactors
Microreactors are miniaturized reaction systems with characteristic dimensions in the micrometer range. The design and fabrication of these devices involve various materials and techniques to create microchannels, mixing zones, and reaction chambers. Advanced manufacturing methods such as micromachining, lithography, and 3D printing are employed to create precise microstructures that enable efficient mixing, heat transfer, and controlled reaction environments at the microscale.- Design and fabrication of microreactors: Microreactors are miniaturized reaction systems with characteristic dimensions in the micrometer range. The design and fabrication of these devices involve various materials and techniques to create microchannels, mixing zones, and reaction chambers. Advanced manufacturing methods such as micromachining, lithography, and 3D printing are employed to produce precise microstructures with controlled flow patterns. These designs optimize surface-to-volume ratios and enable efficient heat and mass transfer for improved reaction control.
- Chemical synthesis applications in microreactors: Microreactors offer significant advantages for chemical synthesis processes, including enhanced reaction control, improved yields, and reduced waste. They enable precise temperature control, efficient mixing, and shorter reaction times compared to conventional batch reactors. These systems are particularly valuable for hazardous reactions, as the small volumes minimize risks. Continuous flow chemistry in microreactors allows for scalable production through numbering-up rather than scaling-up, maintaining the benefits of microscale processing at industrial production levels.
- Microfluidic systems for biological applications: Microreactors designed for biological applications incorporate specialized features for cell culture, enzymatic reactions, and bioanalytical processes. These systems can mimic physiological conditions and provide controlled microenvironments for studying cellular responses. Lab-on-a-chip devices integrate multiple laboratory functions on a single platform, enabling complex biological assays with minimal sample volumes. Applications include point-of-care diagnostics, drug screening, tissue engineering, and personalized medicine approaches that require precise handling of biological materials.
- Process intensification and control systems: Microreactors enable significant process intensification through enhanced heat and mass transfer capabilities. Advanced control systems integrate sensors, actuators, and feedback mechanisms to monitor and regulate reaction parameters in real-time. These systems can automatically adjust flow rates, temperature, and other variables to maintain optimal conditions. Process analytical technology (PAT) incorporated into microreactor setups allows for continuous monitoring of reaction progress and product quality, supporting quality-by-design approaches in manufacturing processes.
- Novel microreactor materials and structures: Innovative materials and structural designs are being developed to enhance microreactor performance. These include catalytic coatings on channel walls, membrane-integrated reactors for selective separation, and multifunctional reactor designs that combine reaction and separation processes. Smart materials that respond to external stimuli can create adaptive microreactor environments. Three-dimensional microstructures with complex geometries improve mixing efficiency and reaction control, while novel fabrication techniques enable the creation of hierarchical structures with features at multiple length scales for optimized performance.
02 Chemical synthesis applications in microreactors
Microreactors offer significant advantages for chemical synthesis processes, including enhanced reaction control, improved safety for hazardous reactions, and increased yield and selectivity. These devices enable precise control of reaction parameters such as temperature, pressure, and residence time, making them ideal for fine chemical production, pharmaceutical synthesis, and development of new chemical entities. The small volumes involved also reduce reagent consumption and waste generation, contributing to greener chemistry practices.Expand Specific Solutions03 Microfluidic systems for biological applications
Microreactors designed for biological applications incorporate specialized features for handling cells, enzymes, and other biological materials. These systems enable rapid screening of biological interactions, cell culture in controlled microenvironments, and enzymatic reactions with precise control. Applications include point-of-care diagnostics, drug discovery platforms, tissue engineering, and biomolecular analysis. The integration of detection systems allows for real-time monitoring of biological processes at the microscale.Expand Specific Solutions04 Process intensification and scale-up strategies
Microreactors facilitate process intensification by enhancing mass and heat transfer rates, leading to more efficient chemical processes. Scale-up strategies include numbering-up (parallel operation of multiple identical microreactors) rather than traditional size scaling, maintaining the advantageous characteristics of microscale operations. This approach enables consistent product quality and process parameters from laboratory to production scale. Advanced control systems and modular designs allow for flexible manufacturing capabilities and rapid process optimization.Expand Specific Solutions05 Integration of catalysts and functional materials
Microreactors can be enhanced by integrating catalysts and functional materials directly into the reactor structure. Techniques include wall coating, packed bed configurations, and immobilized enzyme systems. These integrated catalytic microreactors offer advantages such as reduced catalyst consumption, enhanced catalyst stability, and improved reaction efficiency. Novel materials including nanostructured catalysts, smart polymers, and stimuli-responsive surfaces can be incorporated to create advanced reactor functionalities for specialized applications.Expand Specific Solutions
Leading Companies in Microreactor Industry
The microreactor technology for drug screening and personalized medicine is in an early growth phase, with market size projected to expand significantly due to increasing demand for precision medicine solutions. The technology is advancing from research to commercial applications, with varying levels of maturity across players. Leading pharmaceutical companies like Bristol Myers Squibb are integrating microreactors into drug discovery workflows, while specialized biotechnology firms such as Xilis and Standard BioTools are developing proprietary platforms. Academic institutions (Duke University, Tsinghua University) are driving fundamental research, while established technology companies (Intel, Samsung, STMicroelectronics) contribute enabling components. Collaborative ecosystems between academia, healthcare providers, and industry are forming to accelerate development and clinical implementation of these microfluidic technologies for personalized treatment approaches.
Illumina, Inc.
Technical Solution: Illumina has integrated microreactor technology into their next-generation sequencing (NGS) workflows for personalized medicine applications. Their technology utilizes microfluidic chips containing thousands of nanoliter-scale reaction chambers for massively parallel DNA amplification and sequencing reactions. For drug screening applications, Illumina's platform enables comprehensive genomic profiling of patient samples to identify biomarkers that predict drug response. Their TruSight Oncology 500 assay, which leverages microreactor technology, can detect multiple classes of genomic alterations across 523 cancer-relevant genes from minimal sample input (as little as 40 ng DNA). The company has also developed microfluidic-based liquid biopsy technologies that can detect circulating tumor DNA at concentrations as low as 0.1% variant allele frequency, enabling non-invasive monitoring of treatment response. Illumina's recent acquisition of GRAIL further extends their microreactor capabilities into early cancer detection through multi-cancer screening, analyzing cell-free DNA methylation patterns across approximately 100,000 genomic regions to identify cancer signals with over 99.5% specificity.
Strengths: Industry-leading sequencing accuracy and throughput; comprehensive genomic profiling capabilities; extensive clinical validation data supporting utility in treatment selection. Weaknesses: High equipment and per-sample costs may limit accessibility; complex data analysis requirements; primarily focused on genomic aspects rather than functional drug screening.
Corning, Inc.
Technical Solution: Corning has developed a comprehensive suite of microreactor technologies for drug screening and personalized medicine applications. Their Corning® Microcarrier Spheroid Microplates feature U-shaped wells with an ultra-low attachment surface that promotes the formation of uniform 3D spheroids, mimicking in vivo tissue architecture more accurately than traditional 2D cultures. These microreactors are manufactured using proprietary optical-grade materials that provide exceptional clarity for high-resolution imaging while maintaining minimal autofluorescence. For personalized medicine applications, Corning's Transwell® organ-on-chip platform incorporates a permeable support membrane between two microfluidic chambers, enabling co-culture of different cell types to recreate tissue interfaces (such as blood-brain barrier or lung alveoli). Their newest microreactor system integrates automated media exchange capabilities that maintain precise control of nutrient and drug concentrations (±2% variation) throughout extended culture periods of up to 28 days. The platform's standardized 384-well format is compatible with existing high-throughput screening equipment, facilitating adoption in pharmaceutical research settings.
Strengths: Extensive materials science expertise ensuring consistent, high-quality microreactor surfaces; compatibility with existing laboratory automation infrastructure; comprehensive product ecosystem spanning multiple applications. Weaknesses: Less integration of analytical capabilities compared to some competitors; requires additional instrumentation for advanced functionality; primarily focused on providing platforms rather than complete screening solutions.
Key Patents in Microfluidic Drug Testing Systems
Drug screening device and method based on ion channel
PatentWO2023221347A1
Innovation
- A drug screening device based on ion channels is designed, including an automatic pipetting work platform and an atomic absorption spectrometer to achieve fully automatic sample sampling, injection and detection. It uses non-radioactive tracer ions such as rubidium ions and lithium ions to pass through the atoms. The absorption spectrometer detects the concentration of tracer ions in the cell lysate and draws an inhibition curve to analyze the effect of drugs on ion channels.
Precision drug screening for personalized cancer therapy
PatentPendingUS20210285054A1
Innovation
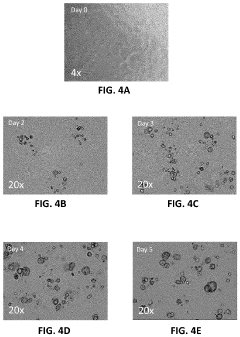
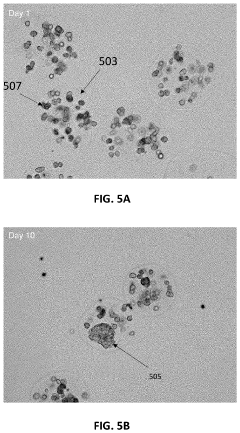
- The development of Patient-Derived Micro-Organospheres (PMOSs) that can be formed from a single biopsy within days, allowing for the rapid generation of hundreds to thousands of uniform, stable, and biologically relevant 3D cellular structures that mimic the tumor microenvironment, enabling high-throughput drug screening.
Regulatory Framework for Clinical Microreactor Applications
The regulatory landscape for microreactors in clinical applications represents a complex and evolving framework that significantly impacts their implementation in drug screening and personalized medicine. Currently, microreactor technologies fall under multiple regulatory jurisdictions, with the FDA in the United States, the EMA in Europe, and similar bodies in other regions establishing oversight mechanisms. These regulatory bodies typically classify microreactors as medical devices or laboratory developed tests (LDTs), depending on their specific application and claims.
The FDA has established the Laboratory Developed Test framework which applies to many microreactor applications, particularly those used for diagnostic purposes in personalized medicine. However, this framework is undergoing significant revision, creating uncertainty for developers. For drug screening applications, microreactors may fall under both pharmaceutical development regulations and medical device regulations, creating a complex compliance environment.
Quality control standards present another critical regulatory consideration. ISO 13485 for medical devices and Good Laboratory Practice (GLP) guidelines provide the foundation for quality management systems in microreactor development and operation. Additionally, the Clinical Laboratory Improvement Amendments (CLIA) in the US establish quality standards for laboratory testing, which apply to microreactor-based diagnostic applications.
Data security and patient privacy regulations, including HIPAA in the US and GDPR in Europe, impose strict requirements on the handling of patient data generated through microreactor-based personalized medicine applications. These regulations necessitate robust data management systems and protocols to ensure compliance.
Validation and verification requirements represent perhaps the most significant regulatory hurdle. Regulatory bodies require extensive evidence demonstrating the reliability, reproducibility, and clinical validity of microreactor-based tests. This typically involves comparative studies with established methodologies and clinical trials to demonstrate efficacy and safety.
The pathway to regulatory approval varies significantly based on the intended use and claims. Microreactors intended for research use only (RUO) face minimal regulatory barriers, while those making diagnostic claims require extensive clinical validation and regulatory review. The FDA's breakthrough device designation offers an accelerated pathway for novel technologies that address unmet clinical needs, which some microreactor technologies may qualify for.
International harmonization efforts, including the International Medical Device Regulators Forum (IMDRF), are working to standardize regulatory approaches across different regions, potentially simplifying the global deployment of microreactor technologies. However, significant regional differences in regulatory requirements persist, creating challenges for international implementation.
The FDA has established the Laboratory Developed Test framework which applies to many microreactor applications, particularly those used for diagnostic purposes in personalized medicine. However, this framework is undergoing significant revision, creating uncertainty for developers. For drug screening applications, microreactors may fall under both pharmaceutical development regulations and medical device regulations, creating a complex compliance environment.
Quality control standards present another critical regulatory consideration. ISO 13485 for medical devices and Good Laboratory Practice (GLP) guidelines provide the foundation for quality management systems in microreactor development and operation. Additionally, the Clinical Laboratory Improvement Amendments (CLIA) in the US establish quality standards for laboratory testing, which apply to microreactor-based diagnostic applications.
Data security and patient privacy regulations, including HIPAA in the US and GDPR in Europe, impose strict requirements on the handling of patient data generated through microreactor-based personalized medicine applications. These regulations necessitate robust data management systems and protocols to ensure compliance.
Validation and verification requirements represent perhaps the most significant regulatory hurdle. Regulatory bodies require extensive evidence demonstrating the reliability, reproducibility, and clinical validity of microreactor-based tests. This typically involves comparative studies with established methodologies and clinical trials to demonstrate efficacy and safety.
The pathway to regulatory approval varies significantly based on the intended use and claims. Microreactors intended for research use only (RUO) face minimal regulatory barriers, while those making diagnostic claims require extensive clinical validation and regulatory review. The FDA's breakthrough device designation offers an accelerated pathway for novel technologies that address unmet clinical needs, which some microreactor technologies may qualify for.
International harmonization efforts, including the International Medical Device Regulators Forum (IMDRF), are working to standardize regulatory approaches across different regions, potentially simplifying the global deployment of microreactor technologies. However, significant regional differences in regulatory requirements persist, creating challenges for international implementation.
Cost-Benefit Analysis of Microreactor Implementation
The implementation of microreactors for drug screening and personalized medicine applications requires careful cost-benefit analysis to justify investment decisions. Initial capital expenditure for microreactor systems typically ranges from $50,000 to $500,000 depending on complexity, automation level, and analytical capabilities. This represents a significant upfront investment compared to traditional batch processing methods, which may deter smaller laboratories or healthcare facilities.
However, operational cost savings become evident when examining reagent consumption. Microreactors typically reduce reagent usage by 80-95% compared to conventional methods, translating to annual savings of $10,000-$50,000 for medium-sized operations. Additionally, the miniaturization of reaction volumes significantly decreases waste management costs, estimated at 70-85% reduction compared to traditional methods.
Labor efficiency improvements present another substantial benefit. Studies indicate that automated microreactor systems can reduce hands-on laboratory time by 60-75%, allowing skilled personnel to focus on data analysis and interpretation rather than repetitive manual processes. This efficiency gain translates to approximately $40,000-$100,000 in annual labor cost savings for a typical clinical laboratory.
The accelerated drug development timeline enabled by microreactor technology delivers perhaps the most significant economic benefit. Parallel testing capabilities and rapid iteration cycles can reduce early-stage drug screening timelines by 40-60%. When considering that each day saved in bringing a drug to market can be worth $1-3 million in potential revenue for pharmaceutical companies, this acceleration creates substantial value.
For personalized medicine applications, microreactors enable point-of-care testing that reduces diagnostic turnaround times from days to hours. This improved efficiency can decrease hospital stays by 1-3 days per patient requiring personalized treatment, generating savings of $1,500-$5,000 per patient in healthcare costs.
Return on investment (ROI) analysis indicates that most microreactor implementations achieve breakeven within 12-36 months, depending on utilization rates and application scope. Healthcare institutions implementing microreactors for personalized medicine applications typically see faster ROI (12-18 months) compared to research-focused implementations (24-36 months) due to immediate clinical impact and reimbursement structures.
Long-term economic sustainability is further enhanced by the scalability and adaptability of microreactor platforms. As technology advances, modular systems can be upgraded incrementally rather than requiring complete replacement, extending the useful life of the initial investment and improving lifetime value by an estimated 30-40% compared to traditional laboratory equipment.
However, operational cost savings become evident when examining reagent consumption. Microreactors typically reduce reagent usage by 80-95% compared to conventional methods, translating to annual savings of $10,000-$50,000 for medium-sized operations. Additionally, the miniaturization of reaction volumes significantly decreases waste management costs, estimated at 70-85% reduction compared to traditional methods.
Labor efficiency improvements present another substantial benefit. Studies indicate that automated microreactor systems can reduce hands-on laboratory time by 60-75%, allowing skilled personnel to focus on data analysis and interpretation rather than repetitive manual processes. This efficiency gain translates to approximately $40,000-$100,000 in annual labor cost savings for a typical clinical laboratory.
The accelerated drug development timeline enabled by microreactor technology delivers perhaps the most significant economic benefit. Parallel testing capabilities and rapid iteration cycles can reduce early-stage drug screening timelines by 40-60%. When considering that each day saved in bringing a drug to market can be worth $1-3 million in potential revenue for pharmaceutical companies, this acceleration creates substantial value.
For personalized medicine applications, microreactors enable point-of-care testing that reduces diagnostic turnaround times from days to hours. This improved efficiency can decrease hospital stays by 1-3 days per patient requiring personalized treatment, generating savings of $1,500-$5,000 per patient in healthcare costs.
Return on investment (ROI) analysis indicates that most microreactor implementations achieve breakeven within 12-36 months, depending on utilization rates and application scope. Healthcare institutions implementing microreactors for personalized medicine applications typically see faster ROI (12-18 months) compared to research-focused implementations (24-36 months) due to immediate clinical impact and reimbursement structures.
Long-term economic sustainability is further enhanced by the scalability and adaptability of microreactor platforms. As technology advances, modular systems can be upgraded incrementally rather than requiring complete replacement, extending the useful life of the initial investment and improving lifetime value by an estimated 30-40% compared to traditional laboratory equipment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!