Microreactors for Pharmaceutical Synthesis under Continuous Flow Conditions
SEP 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microreactor Technology Evolution and Objectives
Microreactor technology has evolved significantly over the past three decades, transforming from academic curiosity to industrial application. The concept originated in the 1990s when researchers began exploring miniaturized reaction systems for chemical synthesis. By the early 2000s, the first commercial microreactors emerged, primarily serving analytical chemistry applications. The pharmaceutical industry's interest intensified around 2005-2010, when continuous flow chemistry principles were increasingly applied to address batch processing limitations.
The evolution accelerated with advances in materials science, particularly the development of chemical-resistant polymers and specialized glass and metal alloys that could withstand harsh reaction conditions. Concurrently, microfabrication techniques borrowed from the semiconductor industry enabled more precise and complex microreactor designs, facilitating better mixing, heat transfer, and reaction control at the microscale.
A significant technological milestone occurred around 2015 with the integration of real-time analytics and automation systems, enabling in-line monitoring and process control. This advancement transformed microreactors from simple flow devices to sophisticated reaction platforms capable of self-optimization and quality assurance during pharmaceutical synthesis operations.
The primary objective of microreactor technology in pharmaceutical synthesis is to overcome the limitations of traditional batch processes by enabling precise control over reaction parameters. This includes enhanced heat and mass transfer efficiency, improved mixing characteristics, and reduced reaction volumes. These advantages translate to higher yields, greater selectivity, and reduced waste generation—all critical factors in pharmaceutical manufacturing.
Another key objective is process intensification, whereby continuous flow conditions in microreactors allow for higher throughput despite smaller physical footprints. This addresses pharmaceutical industry challenges related to facility space constraints and production scaling. Additionally, microreactors aim to enable safer handling of hazardous or highly energetic intermediates through improved thermal management and reduced inventory of reactive materials.
Looking forward, the technology trajectory points toward fully integrated continuous manufacturing systems where microreactors serve as critical components in end-to-end pharmaceutical production lines. The ultimate goal is to establish microreactor platforms that can seamlessly transition from drug discovery to commercial manufacturing, supporting the industry's push toward more agile, efficient, and sustainable production methodologies. This evolution aligns with regulatory encouragement from agencies like the FDA, which has promoted continuous manufacturing adoption through various initiatives since 2015.
The evolution accelerated with advances in materials science, particularly the development of chemical-resistant polymers and specialized glass and metal alloys that could withstand harsh reaction conditions. Concurrently, microfabrication techniques borrowed from the semiconductor industry enabled more precise and complex microreactor designs, facilitating better mixing, heat transfer, and reaction control at the microscale.
A significant technological milestone occurred around 2015 with the integration of real-time analytics and automation systems, enabling in-line monitoring and process control. This advancement transformed microreactors from simple flow devices to sophisticated reaction platforms capable of self-optimization and quality assurance during pharmaceutical synthesis operations.
The primary objective of microreactor technology in pharmaceutical synthesis is to overcome the limitations of traditional batch processes by enabling precise control over reaction parameters. This includes enhanced heat and mass transfer efficiency, improved mixing characteristics, and reduced reaction volumes. These advantages translate to higher yields, greater selectivity, and reduced waste generation—all critical factors in pharmaceutical manufacturing.
Another key objective is process intensification, whereby continuous flow conditions in microreactors allow for higher throughput despite smaller physical footprints. This addresses pharmaceutical industry challenges related to facility space constraints and production scaling. Additionally, microreactors aim to enable safer handling of hazardous or highly energetic intermediates through improved thermal management and reduced inventory of reactive materials.
Looking forward, the technology trajectory points toward fully integrated continuous manufacturing systems where microreactors serve as critical components in end-to-end pharmaceutical production lines. The ultimate goal is to establish microreactor platforms that can seamlessly transition from drug discovery to commercial manufacturing, supporting the industry's push toward more agile, efficient, and sustainable production methodologies. This evolution aligns with regulatory encouragement from agencies like the FDA, which has promoted continuous manufacturing adoption through various initiatives since 2015.
Pharmaceutical Industry Demand for Continuous Flow Synthesis
The pharmaceutical industry is experiencing a paradigm shift towards continuous flow synthesis, driven by increasing demands for more efficient, sustainable, and cost-effective manufacturing processes. Traditional batch processing methods, while historically dominant, present numerous limitations including scalability challenges, quality inconsistencies, and significant waste generation. Continuous flow synthesis using microreactors addresses these pain points by offering precise control over reaction parameters, enhanced safety profiles, and improved product quality.
Market research indicates that pharmaceutical companies are actively seeking technologies that can reduce time-to-market for new drugs while maintaining stringent quality standards. The pressure to decrease development cycles has intensified as patent cliffs approach for many blockbuster drugs, compelling manufacturers to optimize their production processes. Continuous flow technology enables rapid process development and seamless scale-up, potentially reducing development timelines by 30-50% compared to traditional methods.
Regulatory agencies worldwide, including the FDA and EMA, have demonstrated support for continuous manufacturing approaches through various initiatives and guidance documents. This regulatory endorsement has further catalyzed industry adoption, as companies recognize the compliance advantages of real-time quality monitoring and process analytical technology (PAT) integration that continuous flow systems facilitate.
Economic factors also drive demand for microreactor technology. The pharmaceutical industry faces persistent pressure to reduce manufacturing costs while maintaining product quality. Continuous flow processes typically require smaller facility footprints, consume fewer raw materials, generate less waste, and utilize energy more efficiently than batch processes. These advantages translate to significant operational cost reductions over product lifecycles.
Personalized medicine trends and the rise of small-volume, high-potency active pharmaceutical ingredients (HPAPIs) create additional market pull for microreactor technology. These specialized therapeutics require manufacturing flexibility and containment capabilities that continuous flow systems naturally provide, allowing for safer handling of potent compounds and easier production of varying batch sizes.
Contract development and manufacturing organizations (CDMOs) represent another significant market segment embracing continuous flow technology. These organizations seek competitive advantages through technological differentiation, with advanced manufacturing capabilities becoming key selling points for securing partnerships with pharmaceutical innovators.
Environmental sustainability objectives further strengthen the case for continuous flow adoption. Pharmaceutical companies increasingly face pressure to reduce their environmental footprint, and continuous processing offers substantial improvements in green chemistry metrics, including reduced solvent usage, improved atom economy, and decreased energy consumption.
Market research indicates that pharmaceutical companies are actively seeking technologies that can reduce time-to-market for new drugs while maintaining stringent quality standards. The pressure to decrease development cycles has intensified as patent cliffs approach for many blockbuster drugs, compelling manufacturers to optimize their production processes. Continuous flow technology enables rapid process development and seamless scale-up, potentially reducing development timelines by 30-50% compared to traditional methods.
Regulatory agencies worldwide, including the FDA and EMA, have demonstrated support for continuous manufacturing approaches through various initiatives and guidance documents. This regulatory endorsement has further catalyzed industry adoption, as companies recognize the compliance advantages of real-time quality monitoring and process analytical technology (PAT) integration that continuous flow systems facilitate.
Economic factors also drive demand for microreactor technology. The pharmaceutical industry faces persistent pressure to reduce manufacturing costs while maintaining product quality. Continuous flow processes typically require smaller facility footprints, consume fewer raw materials, generate less waste, and utilize energy more efficiently than batch processes. These advantages translate to significant operational cost reductions over product lifecycles.
Personalized medicine trends and the rise of small-volume, high-potency active pharmaceutical ingredients (HPAPIs) create additional market pull for microreactor technology. These specialized therapeutics require manufacturing flexibility and containment capabilities that continuous flow systems naturally provide, allowing for safer handling of potent compounds and easier production of varying batch sizes.
Contract development and manufacturing organizations (CDMOs) represent another significant market segment embracing continuous flow technology. These organizations seek competitive advantages through technological differentiation, with advanced manufacturing capabilities becoming key selling points for securing partnerships with pharmaceutical innovators.
Environmental sustainability objectives further strengthen the case for continuous flow adoption. Pharmaceutical companies increasingly face pressure to reduce their environmental footprint, and continuous processing offers substantial improvements in green chemistry metrics, including reduced solvent usage, improved atom economy, and decreased energy consumption.
Current Microreactor Capabilities and Technical Barriers
Microreactors have revolutionized pharmaceutical synthesis by enabling continuous flow processes with unprecedented control over reaction parameters. Current microreactor technologies offer several advanced capabilities, including enhanced heat and mass transfer rates that significantly outperform traditional batch reactors. The high surface-to-volume ratio in microreactors allows for efficient temperature control, reducing side reactions and improving product quality.
Precise residence time control represents another significant advantage, enabling consistent reaction times and product uniformity across production runs. Modern microreactors can maintain residence time distributions with standard deviations below 5%, ensuring batch-to-batch consistency that is critical for pharmaceutical applications. Additionally, the small internal volumes facilitate rapid screening of reaction conditions, accelerating process development and optimization.
Despite these advantages, several technical barriers limit the widespread adoption of microreactor technology in pharmaceutical manufacturing. Clogging remains a persistent challenge, particularly when handling solid-forming reactions or working with particulate-containing streams. Current solutions involving ultrasonic integration or specialized surface treatments provide only partial remedies and often compromise other performance aspects.
Scaling up microreactor processes presents another significant hurdle. While "numbering up" (adding parallel reactors) theoretically preserves reaction conditions, practical implementation reveals challenges in maintaining uniform flow distribution across multiple channels. Flow maldistribution can reach up to 15% in complex multi-channel systems, undermining the precision that makes microreactors attractive.
Materials compatibility issues also constrain microreactor applications. Most commercial systems utilize glass, silicon, or stainless steel, which may not be suitable for highly corrosive reagents or extreme reaction conditions. Advanced materials like silicon carbide or specialized fluoropolymers offer improved chemical resistance but introduce manufacturing complexities and significantly higher costs.
Monitoring and control systems for microreactors remain underdeveloped compared to traditional batch processes. Real-time analytical techniques that can be integrated directly into microfluidic platforms are limited, with current inline spectroscopic methods often suffering from sensitivity issues due to the short optical path lengths inherent in microchannels.
The integration of multiphase reactions (gas-liquid, liquid-liquid) presents additional challenges, as achieving consistent phase distributions and interfacial areas requires sophisticated channel designs and precise flow control. Current microreactor systems struggle to maintain stable slug or annular flow patterns across varying flow rates, limiting operational flexibility.
Precise residence time control represents another significant advantage, enabling consistent reaction times and product uniformity across production runs. Modern microreactors can maintain residence time distributions with standard deviations below 5%, ensuring batch-to-batch consistency that is critical for pharmaceutical applications. Additionally, the small internal volumes facilitate rapid screening of reaction conditions, accelerating process development and optimization.
Despite these advantages, several technical barriers limit the widespread adoption of microreactor technology in pharmaceutical manufacturing. Clogging remains a persistent challenge, particularly when handling solid-forming reactions or working with particulate-containing streams. Current solutions involving ultrasonic integration or specialized surface treatments provide only partial remedies and often compromise other performance aspects.
Scaling up microreactor processes presents another significant hurdle. While "numbering up" (adding parallel reactors) theoretically preserves reaction conditions, practical implementation reveals challenges in maintaining uniform flow distribution across multiple channels. Flow maldistribution can reach up to 15% in complex multi-channel systems, undermining the precision that makes microreactors attractive.
Materials compatibility issues also constrain microreactor applications. Most commercial systems utilize glass, silicon, or stainless steel, which may not be suitable for highly corrosive reagents or extreme reaction conditions. Advanced materials like silicon carbide or specialized fluoropolymers offer improved chemical resistance but introduce manufacturing complexities and significantly higher costs.
Monitoring and control systems for microreactors remain underdeveloped compared to traditional batch processes. Real-time analytical techniques that can be integrated directly into microfluidic platforms are limited, with current inline spectroscopic methods often suffering from sensitivity issues due to the short optical path lengths inherent in microchannels.
The integration of multiphase reactions (gas-liquid, liquid-liquid) presents additional challenges, as achieving consistent phase distributions and interfacial areas requires sophisticated channel designs and precise flow control. Current microreactor systems struggle to maintain stable slug or annular flow patterns across varying flow rates, limiting operational flexibility.
Contemporary Microreactor Designs for Pharmaceutical Applications
01 Microreactor design for continuous flow processes
Microreactors are designed with specific channel geometries and structures to optimize continuous flow processes. These designs include features such as microchannel arrays, mixing zones, and heat exchange elements that enhance reaction efficiency. The small dimensions of microreactors provide high surface-to-volume ratios, allowing for improved heat and mass transfer compared to conventional reactors, which results in better reaction control and higher yields.- Microreactor design for continuous flow processes: Microreactors are designed with specific channel geometries and structures to optimize continuous flow processes. These designs include features such as mixing zones, heat exchange elements, and controlled residence time distributions. The small dimensions of microchannels provide high surface-to-volume ratios, enabling efficient heat and mass transfer, which is crucial for reaction control and process intensification in continuous flow operations.
- Continuous flow synthesis applications in microreactors: Microreactors enable continuous flow synthesis of various compounds with improved efficiency and selectivity compared to batch processes. Applications include pharmaceutical synthesis, fine chemicals production, and materials processing. The controlled environment within microreactors allows for precise reaction conditions, reduced side reactions, and enhanced product quality, making them valuable tools for developing sustainable and scalable chemical manufacturing processes.
- Process control and monitoring in microreactor systems: Advanced process control and monitoring techniques are implemented in microreactor systems to ensure consistent performance during continuous flow operations. These include real-time analytics, automated feedback control systems, and integrated sensors for monitoring parameters such as temperature, pressure, and concentration. Such capabilities enable precise reaction control, rapid optimization, and enhanced safety features that are particularly valuable for handling hazardous or highly exothermic reactions.
- Scale-up strategies for microreactor continuous flow processes: Various approaches are employed to scale up microreactor continuous flow processes from laboratory to industrial production. These include numbering-up (parallel operation of multiple identical units), smart scale-out methodologies, and modular designs that maintain the advantageous characteristics of microreactors while increasing throughput. These strategies help overcome traditional scale-up challenges by preserving mixing efficiency, heat transfer capabilities, and reaction kinetics across different production scales.
- Integration of separation and purification in continuous flow microreactors: Continuous flow microreactors can be integrated with downstream separation and purification processes to create complete end-to-end manufacturing systems. These integrated systems incorporate techniques such as membrane separation, extraction, crystallization, and chromatography directly within the microfluidic platform. This integration minimizes intermediate handling steps, reduces solvent usage, and enables continuous production of purified products, which is particularly valuable for pharmaceutical and fine chemical applications.
02 Continuous flow synthesis applications in microreactors
Microreactors enable continuous flow synthesis for various chemical and pharmaceutical applications. These systems allow for precise control of reaction parameters such as temperature, pressure, and residence time, resulting in improved product quality and consistency. Continuous flow synthesis in microreactors is particularly beneficial for reactions that are hazardous, highly exothermic, or require precise mixing, as the small volumes involved minimize risks and enhance process safety.Expand Specific Solutions03 Integration of monitoring and control systems in microreactors
Advanced monitoring and control systems are integrated into microreactor setups to enable real-time analysis and process optimization. These systems include sensors for temperature, pressure, flow rate, and concentration measurements, as well as automated feedback control mechanisms. The integration of analytical techniques such as spectroscopy and chromatography allows for continuous monitoring of reaction progress and product quality, facilitating process automation and quality control in continuous flow operations.Expand Specific Solutions04 Scale-up strategies for microreactor technology
Various strategies are employed to scale up microreactor technology for industrial production while maintaining the advantages of microscale processing. These approaches include numbering-up (parallel operation of multiple microreactor units), smart scale-out designs, and modular systems that can be easily expanded. Scale-up methodologies focus on preserving the high heat and mass transfer characteristics of microreactors while increasing throughput to meet production demands.Expand Specific Solutions05 Specialized microreactor materials and fabrication techniques
Specialized materials and fabrication techniques are used to create microreactors with enhanced performance characteristics for continuous flow applications. Materials such as glass, silicon, ceramics, and various metals and alloys are selected based on their chemical compatibility, thermal conductivity, and mechanical properties. Advanced fabrication methods including micromachining, etching, 3D printing, and laser ablation enable the creation of complex microstructures with precise dimensions and surface properties tailored for specific reaction requirements.Expand Specific Solutions
Leading Companies and Research Institutions in Microreactor Technology
The microreactor technology for pharmaceutical synthesis under continuous flow conditions is currently in a growth phase, with the market expanding rapidly due to increasing demand for efficient, sustainable manufacturing processes. The global market size for this technology is estimated to be over $1 billion, with projected annual growth of 8-10%. Technologically, the field shows varying maturity levels across players: established companies like Corning, Inc. and Siemens Corp. demonstrate advanced capabilities, while academic institutions such as MIT, Caltech, and Zhejiang University are driving fundamental innovations. Pharmaceutical companies including Jiangsu Hengrui and Jubilant Pharmova are increasingly adopting these technologies. Equipment manufacturers like YMC Co. and Hitachi Plant Services are developing specialized microreactor systems, indicating the technology's transition from research to commercial implementation.
Corning, Inc.
Technical Solution: Corning has developed the Advanced-Flow™ Reactor (AFR) technology specifically for pharmaceutical continuous flow synthesis. Their glass microreactors feature unique heart-shaped mixing chambers that create uniform mixing patterns through controlled vortex formation, enabling precise reaction control. The modular design allows for easy scale-up from lab to production without redesigning processes, with flow rates ranging from 1 mL/min to over 100 L/hr[1]. Corning's reactors incorporate advanced heat exchange capabilities, maintaining isothermal conditions even for highly exothermic reactions, with heat transfer coefficients up to 10 times higher than conventional batch reactors[2]. Their G4 reactor system integrates multiple reaction zones with real-time monitoring and control systems, allowing for multi-step synthesis in a continuous process. The glass construction provides superior chemical compatibility with aggressive reagents while allowing visual inspection of the reaction progress[3].
Strengths: Superior chemical resistance of glass materials; excellent heat transfer capabilities; visual reaction monitoring; proven scalability from lab to production; modular design for process flexibility. Weaknesses: Higher initial capital investment compared to traditional batch systems; requires specialized expertise for implementation; glass components may be more fragile than metal alternatives; limited pressure ratings compared to some metal-based systems.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered an integrated continuous manufacturing platform for pharmaceuticals that combines microreactor technology with downstream processing. Their system features silicon-based microreactors with precise temperature control (±0.1°C) and residence time distributions optimized for complex pharmaceutical reactions[1]. MIT researchers have developed novel reactor designs incorporating 3D-printed components that enable multiphase reactions with enhanced mass transfer rates up to 50 times greater than conventional systems[2]. Their platform integrates real-time analytical techniques including inline IR, UV, and Raman spectroscopy for process monitoring and quality control, enabling closed-loop feedback control systems. MIT has demonstrated complete end-to-end synthesis of pharmaceuticals including fluoxetine and diphenhydramine in a continuous process, reducing manufacturing footprint by up to 90% compared to batch processes[3]. Their technology incorporates advanced computational fluid dynamics modeling to optimize reactor geometry for specific reaction kinetics, minimizing side reactions and improving yield.
Strengths: Cutting-edge research in reactor design and process integration; strong focus on real-time analytics and process control; comprehensive approach combining reaction engineering with downstream processing; access to advanced computational modeling capabilities. Weaknesses: Some technologies remain at laboratory scale and require further development for industrial implementation; academic focus may prioritize innovation over commercial practicality; potential intellectual property complexities for commercial adoption.
Critical Patents and Breakthroughs in Continuous Flow Chemistry
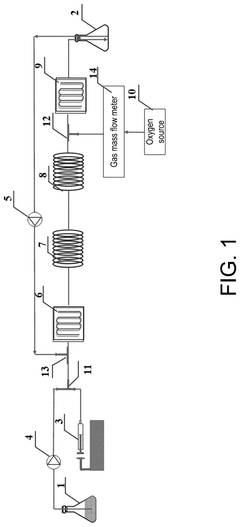
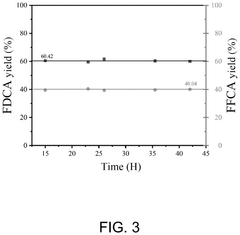
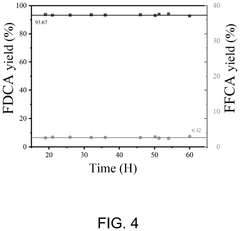
Microreactor and method for continuous flow synthesis of 2,5-furandicarboxylic acid
PatentPendingUS20250059149A1
Innovation
- A microreactor system for continuous flow synthesis of 2,5-furandicarboxylic acid, which includes a first and second microreactor substrate with S-shaped flow channels and baffles, ion exchange resin adsorption pipes, activated carbon adsorption pipes, and an oxygen source, allowing for precise control of reaction conditions and the removal of by-products.
Microreactor and method for continuous flow synthesis of 2,5-furandicarboxylic acid
PatentPendingUS20250059149A1
Innovation
- A microreactor system for continuous flow synthesis of 2,5-furandicarboxylic acid, which includes a first and second microreactor substrate with S-shaped flow channels and baffles, ion exchange resin adsorption pipes, activated carbon adsorption pipes, and an oxygen source, allowing for precise control of reaction conditions and the removal of by-products.
Scale-up Strategies and Manufacturing Integration
The transition from laboratory-scale microreactor systems to commercial pharmaceutical manufacturing presents significant challenges that require systematic approaches. Successful scale-up strategies typically follow either a "numbering-up" approach, where multiple microreactor units operate in parallel, or a dimensional scale-up, where reactor channels are enlarged while maintaining key performance parameters. The numbering-up approach preserves the advantageous heat and mass transfer characteristics of microreactors but introduces complexity in flow distribution and control systems.
Integration of microreactor technology into existing pharmaceutical manufacturing infrastructure requires careful consideration of upstream and downstream processes. Continuous crystallization, filtration, and drying operations must be adapted to handle the continuous output from microreactor systems. Companies like Lonza and Novartis have implemented hybrid approaches, where microreactors handle critical reaction steps while conventional equipment manages subsequent processing stages.
Real-time monitoring and process analytical technology (PAT) play crucial roles in scale-up success. Inline spectroscopic methods (Raman, NIR, UV) enable continuous quality verification and facilitate process control through feedback loops. These monitoring systems must be designed for robustness in manufacturing environments while maintaining the sensitivity required for pharmaceutical quality standards.
Regulatory considerations significantly impact scale-up strategies, with FDA's Quality by Design (QbD) framework providing guidance for continuous manufacturing implementation. Defining the design space for microreactor processes requires comprehensive understanding of critical process parameters and their acceptable ranges. Companies must demonstrate process consistency across scales to satisfy regulatory requirements.
Economic factors ultimately determine commercial viability of microreactor technology. Capital investment for microreactor systems typically exceeds conventional batch equipment costs, but can be offset by reduced facility footprint, lower energy consumption, and improved yield. Industry case studies from GSK and Johnson & Johnson demonstrate ROI periods of 2-3 years for continuous manufacturing implementations, with savings primarily derived from reduced waste generation and improved process efficiency.
Standardization efforts are emerging to facilitate wider adoption of microreactor technology. The Standardized Continuous-Flow Chemistry Platform (SCFCP) initiative aims to establish common interfaces and control protocols, enabling modular integration across equipment from different vendors. This standardization will be crucial for pharmaceutical companies seeking to implement microreactor technology without becoming dependent on proprietary systems.
Integration of microreactor technology into existing pharmaceutical manufacturing infrastructure requires careful consideration of upstream and downstream processes. Continuous crystallization, filtration, and drying operations must be adapted to handle the continuous output from microreactor systems. Companies like Lonza and Novartis have implemented hybrid approaches, where microreactors handle critical reaction steps while conventional equipment manages subsequent processing stages.
Real-time monitoring and process analytical technology (PAT) play crucial roles in scale-up success. Inline spectroscopic methods (Raman, NIR, UV) enable continuous quality verification and facilitate process control through feedback loops. These monitoring systems must be designed for robustness in manufacturing environments while maintaining the sensitivity required for pharmaceutical quality standards.
Regulatory considerations significantly impact scale-up strategies, with FDA's Quality by Design (QbD) framework providing guidance for continuous manufacturing implementation. Defining the design space for microreactor processes requires comprehensive understanding of critical process parameters and their acceptable ranges. Companies must demonstrate process consistency across scales to satisfy regulatory requirements.
Economic factors ultimately determine commercial viability of microreactor technology. Capital investment for microreactor systems typically exceeds conventional batch equipment costs, but can be offset by reduced facility footprint, lower energy consumption, and improved yield. Industry case studies from GSK and Johnson & Johnson demonstrate ROI periods of 2-3 years for continuous manufacturing implementations, with savings primarily derived from reduced waste generation and improved process efficiency.
Standardization efforts are emerging to facilitate wider adoption of microreactor technology. The Standardized Continuous-Flow Chemistry Platform (SCFCP) initiative aims to establish common interfaces and control protocols, enabling modular integration across equipment from different vendors. This standardization will be crucial for pharmaceutical companies seeking to implement microreactor technology without becoming dependent on proprietary systems.
Regulatory Compliance and Quality Assurance for Continuous Manufacturing
The pharmaceutical industry's transition to continuous manufacturing processes using microreactors necessitates robust regulatory frameworks and quality assurance protocols. The FDA and EMA have developed specific guidelines for continuous manufacturing, emphasizing real-time release testing (RTRT) and process analytical technology (PAT) implementation. These regulatory bodies require manufacturers to demonstrate consistent product quality through validated process control strategies and comprehensive risk assessments.
Quality by Design (QbD) principles form the cornerstone of regulatory compliance in continuous flow synthesis, requiring manufacturers to establish critical quality attributes (CQAs) and critical process parameters (CPPs). The identification of these parameters enables the development of control strategies that ensure consistent product quality throughout the manufacturing process. Design space approaches, as outlined in ICH Q8, allow for operational flexibility while maintaining regulatory compliance.
Process validation for microreactor systems presents unique challenges compared to batch processing. Manufacturers must validate equipment cleaning procedures, demonstrate consistent performance across extended production runs, and establish appropriate sampling strategies. The validation approach typically involves three stages: process design, process qualification, and continued process verification, with emphasis on statistical process control methods to monitor system performance.
Real-time monitoring technologies, including inline spectroscopic methods (NIR, Raman, UV-Vis), represent essential components of quality assurance systems for continuous manufacturing. These technologies enable immediate detection of process deviations and facilitate rapid corrective actions. Regulatory agencies increasingly expect manufacturers to implement PAT tools that provide continuous verification of product quality rather than relying solely on end-product testing.
Data integrity and management systems present significant regulatory considerations for continuous manufacturing operations. Electronic records must comply with 21 CFR Part 11 requirements, ensuring appropriate audit trails, data security, and system validation. The continuous nature of the process generates substantial data volumes, necessitating robust data management strategies and advanced analytics capabilities to identify trends and potential quality issues.
Scale-up and technology transfer considerations require specific regulatory approaches for continuous flow processes. Unlike traditional batch manufacturing, where scale-up often involves larger equipment, continuous processes typically achieve increased production through extended run times or equipment numbering-up. Regulatory submissions must address how process parameters are maintained during these transitions to ensure consistent product quality across different production scales and facilities.
Quality by Design (QbD) principles form the cornerstone of regulatory compliance in continuous flow synthesis, requiring manufacturers to establish critical quality attributes (CQAs) and critical process parameters (CPPs). The identification of these parameters enables the development of control strategies that ensure consistent product quality throughout the manufacturing process. Design space approaches, as outlined in ICH Q8, allow for operational flexibility while maintaining regulatory compliance.
Process validation for microreactor systems presents unique challenges compared to batch processing. Manufacturers must validate equipment cleaning procedures, demonstrate consistent performance across extended production runs, and establish appropriate sampling strategies. The validation approach typically involves three stages: process design, process qualification, and continued process verification, with emphasis on statistical process control methods to monitor system performance.
Real-time monitoring technologies, including inline spectroscopic methods (NIR, Raman, UV-Vis), represent essential components of quality assurance systems for continuous manufacturing. These technologies enable immediate detection of process deviations and facilitate rapid corrective actions. Regulatory agencies increasingly expect manufacturers to implement PAT tools that provide continuous verification of product quality rather than relying solely on end-product testing.
Data integrity and management systems present significant regulatory considerations for continuous manufacturing operations. Electronic records must comply with 21 CFR Part 11 requirements, ensuring appropriate audit trails, data security, and system validation. The continuous nature of the process generates substantial data volumes, necessitating robust data management strategies and advanced analytics capabilities to identify trends and potential quality issues.
Scale-up and technology transfer considerations require specific regulatory approaches for continuous flow processes. Unlike traditional batch manufacturing, where scale-up often involves larger equipment, continuous processes typically achieve increased production through extended run times or equipment numbering-up. Regulatory submissions must address how process parameters are maintained during these transitions to ensure consistent product quality across different production scales and facilities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!