Regulations and Standards for Microreactors in Pharmaceutical Production
SEP 24, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microreactor Technology Background and Objectives
Microreactor technology represents a paradigm shift in pharmaceutical manufacturing, evolving from traditional batch processing to continuous flow chemistry. This evolution began in the early 2000s when researchers recognized the potential benefits of miniaturized reaction systems for pharmaceutical applications. The technology leverages microscale channels (typically 10-500 micrometers) to facilitate precise control over reaction parameters, enabling unprecedented efficiency in chemical synthesis.
The historical trajectory of microreactor development has been characterized by progressive refinements in materials science, engineering design, and process integration. Initially confined to academic laboratories, microreactor technology has gradually transitioned into industrial settings, with pharmaceutical companies increasingly adopting these systems for both R&D and production purposes. This transition has been accelerated by regulatory initiatives promoting continuous manufacturing approaches, particularly from the FDA's Quality by Design (QbD) framework introduced in the mid-2000s.
Current technological objectives for microreactors in pharmaceutical production center on several key dimensions. Primary among these is the establishment of robust regulatory frameworks that can accommodate the unique characteristics of continuous flow processes while maintaining stringent quality and safety standards. This includes developing standardized validation protocols, in-line analytical methods, and quality control procedures specifically tailored to microreactor operations.
Another critical objective involves scaling capabilities—developing modular systems that can seamlessly transition from laboratory development to commercial production without compromising process parameters or product quality. This "scale-out" rather than "scale-up" approach represents a fundamental reconceptualization of pharmaceutical manufacturing capacity expansion.
The integration of advanced monitoring technologies constitutes a third major objective, with real-time process analytical technology (PAT) emerging as essential for ensuring consistent product quality and regulatory compliance. These systems enable continuous verification of critical quality attributes throughout the production process, facilitating rapid intervention when deviations occur.
Looking forward, the technological trajectory points toward increasingly automated and integrated microreactor systems capable of multi-step synthesis with minimal human intervention. The ultimate goal is to establish comprehensive regulatory standards that specifically address microreactor technology, providing clear guidance for implementation, validation, and compliance across the pharmaceutical industry while accommodating the inherent advantages of continuous processing in terms of efficiency, sustainability, and product quality.
The historical trajectory of microreactor development has been characterized by progressive refinements in materials science, engineering design, and process integration. Initially confined to academic laboratories, microreactor technology has gradually transitioned into industrial settings, with pharmaceutical companies increasingly adopting these systems for both R&D and production purposes. This transition has been accelerated by regulatory initiatives promoting continuous manufacturing approaches, particularly from the FDA's Quality by Design (QbD) framework introduced in the mid-2000s.
Current technological objectives for microreactors in pharmaceutical production center on several key dimensions. Primary among these is the establishment of robust regulatory frameworks that can accommodate the unique characteristics of continuous flow processes while maintaining stringent quality and safety standards. This includes developing standardized validation protocols, in-line analytical methods, and quality control procedures specifically tailored to microreactor operations.
Another critical objective involves scaling capabilities—developing modular systems that can seamlessly transition from laboratory development to commercial production without compromising process parameters or product quality. This "scale-out" rather than "scale-up" approach represents a fundamental reconceptualization of pharmaceutical manufacturing capacity expansion.
The integration of advanced monitoring technologies constitutes a third major objective, with real-time process analytical technology (PAT) emerging as essential for ensuring consistent product quality and regulatory compliance. These systems enable continuous verification of critical quality attributes throughout the production process, facilitating rapid intervention when deviations occur.
Looking forward, the technological trajectory points toward increasingly automated and integrated microreactor systems capable of multi-step synthesis with minimal human intervention. The ultimate goal is to establish comprehensive regulatory standards that specifically address microreactor technology, providing clear guidance for implementation, validation, and compliance across the pharmaceutical industry while accommodating the inherent advantages of continuous processing in terms of efficiency, sustainability, and product quality.
Pharmaceutical Market Demand for Microreactor Systems
The pharmaceutical industry is experiencing a significant shift towards continuous manufacturing processes, with microreactor technology emerging as a key enabler of this transformation. Market analysis indicates that the global pharmaceutical continuous manufacturing market is projected to reach $2.4 billion by 2027, growing at a CAGR of 13.8% from 2022. Within this broader trend, microreactor systems represent one of the fastest-growing segments due to their unique advantages in process intensification and quality control.
Primary market drivers for pharmaceutical microreactor adoption include increasing regulatory pressure for quality-by-design approaches, rising competition from generics necessitating more efficient production methods, and growing demand for personalized medicine requiring flexible manufacturing capabilities. The FDA's Emerging Technology Program has specifically highlighted continuous manufacturing technologies, including microreactors, as innovations that can address drug shortages and quality issues.
Market segmentation reveals distinct demand patterns across different pharmaceutical sectors. Large pharmaceutical companies primarily seek microreactor systems for high-value active pharmaceutical ingredient (API) synthesis, particularly for complex molecules where traditional batch processes face yield or selectivity challenges. Contract manufacturing organizations (CMOs) represent another significant market segment, valuing microreactors for their versatility across multiple product lines and reduced facility footprint.
Geographic analysis shows North America currently leading market demand with approximately 40% share, followed by Europe at 35% and Asia-Pacific at 20%. However, the Asia-Pacific region demonstrates the highest growth rate, driven by expanding pharmaceutical manufacturing capacity in China and India, coupled with government initiatives promoting advanced manufacturing technologies.
From a product perspective, demand is strongest for modular microreactor systems that can be integrated into existing production lines, allowing pharmaceutical manufacturers to gradually transition from batch to continuous processing. Systems offering comprehensive process analytical technology (PAT) integration command premium pricing, reflecting the industry's focus on real-time quality assurance.
Market research indicates that pharmaceutical companies are increasingly seeking microreactor solutions for specific challenging reaction types, particularly hazardous reactions involving unstable intermediates, highly exothermic processes, and photochemical transformations. This specialized demand is creating opportunities for technology providers with expertise in these niche applications.
Customer surveys reveal that return on investment considerations heavily influence purchasing decisions, with pharmaceutical manufacturers expecting microreactor implementations to deliver manufacturing cost reductions of 15-30% through improved yields, reduced waste, and lower energy consumption. Additionally, the potential for microreactors to enable "on-demand" manufacturing models is gaining attention as a strategy to optimize supply chain resilience in response to recent global disruptions.
Primary market drivers for pharmaceutical microreactor adoption include increasing regulatory pressure for quality-by-design approaches, rising competition from generics necessitating more efficient production methods, and growing demand for personalized medicine requiring flexible manufacturing capabilities. The FDA's Emerging Technology Program has specifically highlighted continuous manufacturing technologies, including microreactors, as innovations that can address drug shortages and quality issues.
Market segmentation reveals distinct demand patterns across different pharmaceutical sectors. Large pharmaceutical companies primarily seek microreactor systems for high-value active pharmaceutical ingredient (API) synthesis, particularly for complex molecules where traditional batch processes face yield or selectivity challenges. Contract manufacturing organizations (CMOs) represent another significant market segment, valuing microreactors for their versatility across multiple product lines and reduced facility footprint.
Geographic analysis shows North America currently leading market demand with approximately 40% share, followed by Europe at 35% and Asia-Pacific at 20%. However, the Asia-Pacific region demonstrates the highest growth rate, driven by expanding pharmaceutical manufacturing capacity in China and India, coupled with government initiatives promoting advanced manufacturing technologies.
From a product perspective, demand is strongest for modular microreactor systems that can be integrated into existing production lines, allowing pharmaceutical manufacturers to gradually transition from batch to continuous processing. Systems offering comprehensive process analytical technology (PAT) integration command premium pricing, reflecting the industry's focus on real-time quality assurance.
Market research indicates that pharmaceutical companies are increasingly seeking microreactor solutions for specific challenging reaction types, particularly hazardous reactions involving unstable intermediates, highly exothermic processes, and photochemical transformations. This specialized demand is creating opportunities for technology providers with expertise in these niche applications.
Customer surveys reveal that return on investment considerations heavily influence purchasing decisions, with pharmaceutical manufacturers expecting microreactor implementations to deliver manufacturing cost reductions of 15-30% through improved yields, reduced waste, and lower energy consumption. Additionally, the potential for microreactors to enable "on-demand" manufacturing models is gaining attention as a strategy to optimize supply chain resilience in response to recent global disruptions.
Current Regulatory Landscape and Technical Challenges
The pharmaceutical industry is currently navigating a complex regulatory landscape regarding microreactor technology implementation. Major regulatory bodies including the FDA, EMA, and ICH have established frameworks that impact continuous manufacturing processes, though specific guidelines for microreactors remain limited. The FDA's Emerging Technology Program and Quality by Design (QbD) initiatives provide some direction, but comprehensive standards tailored to pharmaceutical microreactors are still evolving.
Current Good Manufacturing Practice (cGMP) regulations present significant challenges for microreactor adoption. Traditional batch-oriented validation protocols are often incompatible with continuous flow processes, creating regulatory uncertainty. Real-time release testing requirements and process analytical technology (PAT) integration standards are particularly underdeveloped for microreactor systems, leaving manufacturers to navigate ambiguous compliance pathways.
Technical standardization represents another major hurdle. The industry lacks consensus on microreactor design specifications, materials compatibility standards, and scaling parameters. This absence of standardization complicates technology transfer between development and production environments and hinders regulatory approval processes. Furthermore, the miniaturized nature of microreactors introduces unique challenges in implementing effective cleaning validation protocols and cross-contamination prevention measures.
Process control and monitoring systems face substantial technical barriers. The rapid reaction kinetics and reduced residence times in microreactors demand ultra-fast analytical methods and sophisticated control algorithms that exceed current technological capabilities. Regulatory expectations for process monitoring granularity often outpace available sensor technology for microfluidic environments.
Safety standards for microreactor operations present additional challenges. While microreactors inherently reduce certain hazards through smaller reaction volumes, they introduce new risks related to high pressure operations, potential blockages, and thermal management. Current safety guidelines inadequately address these unique operational characteristics, creating compliance uncertainties.
International harmonization of microreactor regulations remains fragmented. Significant regional variations in acceptance criteria and validation requirements create barriers to global implementation. Japanese and European regulatory frameworks have shown greater flexibility toward continuous manufacturing technologies compared to other regions, resulting in uneven global adoption patterns.
Data integrity and digital infrastructure requirements present further complications. The continuous nature of microreactor processes generates substantial data streams requiring robust management systems. Regulatory expectations for data traceability, electronic records compliance (21 CFR Part 11), and process verification create significant technical and administrative burdens for implementation teams.
Current Good Manufacturing Practice (cGMP) regulations present significant challenges for microreactor adoption. Traditional batch-oriented validation protocols are often incompatible with continuous flow processes, creating regulatory uncertainty. Real-time release testing requirements and process analytical technology (PAT) integration standards are particularly underdeveloped for microreactor systems, leaving manufacturers to navigate ambiguous compliance pathways.
Technical standardization represents another major hurdle. The industry lacks consensus on microreactor design specifications, materials compatibility standards, and scaling parameters. This absence of standardization complicates technology transfer between development and production environments and hinders regulatory approval processes. Furthermore, the miniaturized nature of microreactors introduces unique challenges in implementing effective cleaning validation protocols and cross-contamination prevention measures.
Process control and monitoring systems face substantial technical barriers. The rapid reaction kinetics and reduced residence times in microreactors demand ultra-fast analytical methods and sophisticated control algorithms that exceed current technological capabilities. Regulatory expectations for process monitoring granularity often outpace available sensor technology for microfluidic environments.
Safety standards for microreactor operations present additional challenges. While microreactors inherently reduce certain hazards through smaller reaction volumes, they introduce new risks related to high pressure operations, potential blockages, and thermal management. Current safety guidelines inadequately address these unique operational characteristics, creating compliance uncertainties.
International harmonization of microreactor regulations remains fragmented. Significant regional variations in acceptance criteria and validation requirements create barriers to global implementation. Japanese and European regulatory frameworks have shown greater flexibility toward continuous manufacturing technologies compared to other regions, resulting in uneven global adoption patterns.
Data integrity and digital infrastructure requirements present further complications. The continuous nature of microreactor processes generates substantial data streams requiring robust management systems. Regulatory expectations for data traceability, electronic records compliance (21 CFR Part 11), and process verification create significant technical and administrative burdens for implementation teams.
Current Compliance Solutions and Implementation Strategies
01 Design and fabrication of microreactors
Microreactors are miniaturized reaction systems with channels or chambers typically in the micrometer range. The design and fabrication of these devices involve various materials such as glass, silicon, or polymers, and techniques like photolithography, etching, and bonding. These microdevices offer advantages including improved heat and mass transfer, precise control over reaction conditions, and enhanced safety for hazardous reactions due to their small volumes.- Design and fabrication of microreactors: Microreactors are miniaturized reaction systems with channels typically in the micrometer range. The design and fabrication of these devices involve various materials and techniques such as micromachining, etching, and 3D printing. These small-scale reactors offer advantages including improved heat and mass transfer, precise control of reaction parameters, and enhanced safety for hazardous reactions. The fabrication methods determine key characteristics like channel geometry, surface properties, and overall reactor performance.
- Chemical synthesis applications in microreactors: Microreactors enable efficient chemical synthesis with improved yield, selectivity, and purity compared to conventional batch processes. They are particularly valuable for reactions requiring precise temperature control, handling of hazardous intermediates, or fast mixing. The controlled environment allows for optimization of reaction conditions, reduction of side products, and continuous production of chemicals. These systems are used for pharmaceutical compounds, fine chemicals, and specialty materials synthesis where quality and consistency are critical.
- Microfluidic systems for biological applications: Microreactors designed for biological applications incorporate specialized features for cell culture, enzymatic reactions, and biomolecular analysis. These systems can mimic physiological conditions, provide controlled microenvironments for cell growth, and enable high-throughput screening of biological samples. Applications include drug discovery, personalized medicine, diagnostic testing, and tissue engineering. The integration of sensors allows real-time monitoring of biological processes at microscale levels with minimal sample consumption.
- Process intensification and scale-up strategies: Microreactors facilitate process intensification by enhancing reaction efficiency and reducing equipment footprint. Scale-up strategies include numbering-up (parallel operation of multiple identical units) rather than traditional size increase, maintaining the advantageous characteristics of microscale processing. This approach enables flexible production capacity while preserving reaction performance. The modular nature of microreactor systems allows for distributed manufacturing and on-demand production, particularly beneficial for decentralized chemical processing and point-of-use applications.
- Integration of monitoring and control systems: Advanced microreactor systems incorporate integrated sensors, actuators, and control systems for real-time monitoring and process automation. These features enable precise regulation of reaction parameters, automated sampling, and feedback control loops. The integration of analytical techniques such as spectroscopy, chromatography, and electrochemical detection allows for continuous quality monitoring and process optimization. Smart microreactor systems can adjust operating conditions dynamically in response to measured parameters, enhancing process robustness and product consistency.
02 Chemical synthesis applications in microreactors
Microreactors enable efficient chemical synthesis with improved yield and selectivity compared to conventional batch processes. They are particularly valuable for reactions requiring precise temperature control, handling of hazardous intermediates, or fast mixing. The continuous flow nature of many microreactor systems allows for easier scale-up through numbering-up rather than traditional scaling methods, making them suitable for both laboratory research and industrial production of fine chemicals and pharmaceuticals.Expand Specific Solutions03 Microreactors for biological and biochemical applications
Microreactors are increasingly used in biological and biochemical applications, including enzymatic reactions, cell culture, and diagnostic assays. These systems provide controlled microenvironments for biological processes, enabling high-throughput screening, rapid analysis, and reduced reagent consumption. Specialized microreactors can mimic physiological conditions, making them valuable tools for drug development, personalized medicine, and point-of-care diagnostics.Expand Specific Solutions04 Integration of analytical techniques with microreactors
Modern microreactor systems often integrate various analytical techniques for real-time monitoring and analysis of reactions. These integrated systems may incorporate spectroscopic methods, electrochemical sensors, or microscopy techniques directly into the microreactor platform. This integration enables immediate feedback on reaction progress, product formation, and process parameters, facilitating process optimization and quality control in continuous flow synthesis.Expand Specific Solutions05 Advanced microreactor technologies and materials
Recent advances in microreactor technology include the development of novel materials and fabrication methods to enhance performance and functionality. These innovations include 3D-printed microreactors, microreactors with integrated catalysts, multiphase flow handling capabilities, and smart materials that respond to external stimuli. Additionally, modular microreactor systems allow for flexible configuration and easy adaptation to different reaction requirements, making them versatile tools for various applications in research and industry.Expand Specific Solutions
Key Industry Players and Regulatory Bodies
The pharmaceutical microreactor technology landscape is evolving rapidly, currently positioned in the growth phase with an estimated market size of $300-500 million and projected annual growth of 15-20%. Technical maturity varies across applications, with leading academic institutions (MIT, Zhejiang University of Technology, Nanjing Tech University) driving fundamental research while pharmaceutical companies (Janssen Biotech, Amgen, Lonza) focus on implementation. Equipment manufacturers (Corning, Hitachi, Finesse Solutions) are developing standardized platforms, though regulatory frameworks remain under development. The competitive landscape features collaboration between technology providers and pharmaceutical manufacturers, with increasing interest from regulatory bodies in establishing standards for this emerging technology.
Lonza Ltd.
Technical Solution: Lonza has developed comprehensive microreactor technology platforms specifically designed for pharmaceutical production that comply with GMP standards. Their FlowPlate® microreactor systems incorporate modular designs that enable continuous flow chemistry while maintaining regulatory compliance. These systems feature integrated Process Analytical Technology (PAT) for real-time monitoring and control, ensuring consistent product quality. Lonza has worked closely with regulatory bodies to establish validation protocols for their microreactor platforms, addressing key concerns such as residence time distribution, mixing efficiency, and scale-up parameters. Their approach includes detailed risk assessment methodologies aligned with ICH Q9 guidelines and implementation of electronic batch records that meet 21 CFR Part 11 requirements for data integrity[1]. Lonza's regulatory framework incorporates cleaning validation protocols specifically adapted for microreactor channels and surfaces, addressing concerns about cross-contamination in multi-product facilities.
Strengths: Extensive experience in GMP implementation for continuous manufacturing, established relationships with regulatory authorities, and integrated quality systems. Weaknesses: Higher initial capital investment compared to traditional batch processes and requires specialized operator training for microreactor technology.
Corning, Inc.
Technical Solution: Corning has pioneered the Advanced-Flow™ Reactor (AFR) technology specifically designed to meet pharmaceutical regulatory requirements. Their microreactor systems incorporate materials of construction that comply with USP Class VI standards and feature designs that facilitate cleaning validation according to FDA guidelines. Corning's regulatory approach includes comprehensive documentation packages that address installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) requirements for continuous flow processes. Their microreactors are engineered with transparent sections that enable visual inspection and validation of cleaning procedures, addressing a key regulatory concern[2]. Corning has developed standardized protocols for process characterization studies that demonstrate process understanding and control as required by QbD (Quality by Design) principles. Their systems include sampling ports strategically positioned to enable process verification and material testing in compliance with pharmacopeial standards, while their documentation systems support electronic records that meet data integrity requirements.
Strengths: Robust glass-based microreactor technology with excellent chemical compatibility, transparent design allowing visual process verification, and established regulatory compliance packages. Weaknesses: Limited flexibility for certain reaction types requiring specialized materials and relatively higher costs for smaller-scale pharmaceutical applications.
Critical Regulatory Standards and Compliance Frameworks
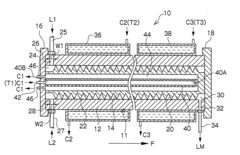
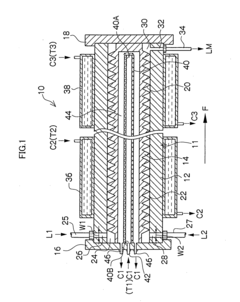
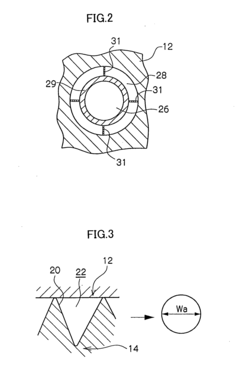
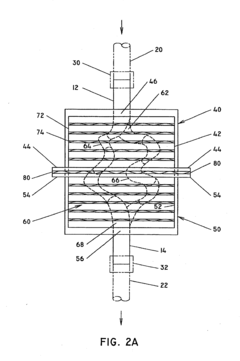
Microreactor
PatentInactiveUS20050031507A1
Innovation
- A microreactor with a spiral reaction channel formed by cutting a spiral screw thread on a round-bar-shaped core member or an outer cylindrical member, allowing for easy and robust manufacturing using general machining technologies, eliminating the need for special precision micro processing techniques.
Processing Apparatus Fabrication
PatentInactiveUS20140079608A1
Innovation
- A modular processing apparatus with a design that allows for customizable components and passageways to achieve desired flow profiles, residence times, and heat transfer, using metal layers connected by brazing or adhesives, enabling efficient and precise control of chemical reactions while allowing for easy scalability and catalyst recovery.
Risk Assessment and Quality Assurance Methodologies
Risk assessment in microreactor pharmaceutical production requires a systematic approach that differs significantly from traditional batch processing methodologies. The miniaturized nature of microreactors introduces unique quality assurance challenges that must be addressed through specialized frameworks. Current methodologies typically employ Failure Mode and Effects Analysis (FMEA) adapted specifically for continuous flow processes, with particular emphasis on flow rate stability, mixing efficiency, and reaction kinetics.
Process Analytical Technology (PAT) plays a crucial role in microreactor quality assurance, enabling real-time monitoring of critical process parameters. Advanced spectroscopic techniques such as Raman, near-infrared (NIR), and ultraviolet-visible (UV-Vis) spectroscopy have been successfully integrated with microreactor systems to provide continuous quality verification. These technologies allow for immediate detection of deviations and facilitate rapid corrective actions, significantly reducing quality risks.
Statistical process control (SPC) methodologies have been adapted for the continuous nature of microreactor operations. Unlike batch processes where sampling occurs at discrete intervals, microreactor SPC employs continuous sampling strategies with advanced statistical models that account for the dynamic nature of flow chemistry. Time-series analysis and multivariate statistical techniques have proven particularly valuable in identifying process drift and ensuring consistent product quality.
Quality by Design (QbD) principles are increasingly applied to microreactor implementations, with emphasis on establishing a thorough understanding of the design space. This approach involves comprehensive risk mapping of process parameters against critical quality attributes, followed by validation of control strategies. The narrow process windows typical in microreactor operations necessitate more precise definition of acceptable operating ranges compared to traditional batch processes.
Validation protocols for microreactor systems have evolved to address their unique characteristics. Continuous process verification (CPV) has largely replaced traditional three-batch validation approaches, focusing instead on demonstrating consistent control over extended production periods. Regulatory authorities increasingly accept this approach when supported by robust risk assessment documentation and comprehensive monitoring data.
Cross-contamination risk management presents unique challenges in microreactor systems. While the closed nature of these systems offers inherent advantages, cleaning validation requires specialized approaches. Time-based sampling during extended production runs and material compatibility studies are essential components of microreactor cleaning validation protocols, ensuring product quality across manufacturing campaigns.
Process Analytical Technology (PAT) plays a crucial role in microreactor quality assurance, enabling real-time monitoring of critical process parameters. Advanced spectroscopic techniques such as Raman, near-infrared (NIR), and ultraviolet-visible (UV-Vis) spectroscopy have been successfully integrated with microreactor systems to provide continuous quality verification. These technologies allow for immediate detection of deviations and facilitate rapid corrective actions, significantly reducing quality risks.
Statistical process control (SPC) methodologies have been adapted for the continuous nature of microreactor operations. Unlike batch processes where sampling occurs at discrete intervals, microreactor SPC employs continuous sampling strategies with advanced statistical models that account for the dynamic nature of flow chemistry. Time-series analysis and multivariate statistical techniques have proven particularly valuable in identifying process drift and ensuring consistent product quality.
Quality by Design (QbD) principles are increasingly applied to microreactor implementations, with emphasis on establishing a thorough understanding of the design space. This approach involves comprehensive risk mapping of process parameters against critical quality attributes, followed by validation of control strategies. The narrow process windows typical in microreactor operations necessitate more precise definition of acceptable operating ranges compared to traditional batch processes.
Validation protocols for microreactor systems have evolved to address their unique characteristics. Continuous process verification (CPV) has largely replaced traditional three-batch validation approaches, focusing instead on demonstrating consistent control over extended production periods. Regulatory authorities increasingly accept this approach when supported by robust risk assessment documentation and comprehensive monitoring data.
Cross-contamination risk management presents unique challenges in microreactor systems. While the closed nature of these systems offers inherent advantages, cleaning validation requires specialized approaches. Time-based sampling during extended production runs and material compatibility studies are essential components of microreactor cleaning validation protocols, ensuring product quality across manufacturing campaigns.
International Harmonization of Microreactor Standards
The global nature of pharmaceutical manufacturing necessitates a coordinated approach to microreactor standards across different regulatory jurisdictions. Currently, significant disparities exist between regulatory frameworks in major pharmaceutical markets including the United States (FDA), European Union (EMA), Japan (PMDA), and China (NMPA). These differences create compliance challenges for pharmaceutical companies operating internationally and potentially impede the broader adoption of microreactor technology.
Efforts toward international harmonization have gained momentum through initiatives like the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). The ICH Q13 guideline on continuous manufacturing represents a significant step forward, though it addresses microreactors only as a subset of continuous manufacturing technologies rather than as a distinct category requiring specialized standards.
The International Organization for Standardization (ISO) has established working groups focused on microfluidic systems standardization, with ISO/TC 48/WG 3 specifically addressing microprocess engineering devices. These efforts aim to create universally accepted terminology, testing methods, and performance criteria that can be referenced by regulatory bodies worldwide.
Industry consortia like the Microreactor Technology Pharmaceutical Consortium (MTPC) have emerged as important drivers of harmonization, bringing together pharmaceutical companies, equipment manufacturers, and regulatory experts to develop consensus-based recommendations for standardization. Their white papers on microreactor validation protocols have influenced regulatory thinking across multiple jurisdictions.
Key areas requiring harmonization include materials compatibility standards, cleaning validation protocols, process analytical technology (PAT) implementation, and scale-out validation approaches. The development of standardized connection interfaces between microreactor modules from different manufacturers represents another critical area where international consensus would significantly benefit the industry.
Regulatory acceptance of real-time release testing (RTRT) methodologies for microreactor-produced pharmaceuticals varies considerably between regions, creating a significant barrier to global implementation. Harmonized standards for RTRT validation would substantially accelerate microreactor adoption in pharmaceutical manufacturing.
The path toward comprehensive international harmonization will likely require a phased approach, beginning with agreement on fundamental definitions and testing methodologies, followed by alignment on validation requirements and eventually moving toward mutual recognition of inspection results between regulatory authorities. This process may take 5-10 years to fully implement but would ultimately create a more predictable regulatory environment that encourages innovation while maintaining stringent quality and safety standards.
Efforts toward international harmonization have gained momentum through initiatives like the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). The ICH Q13 guideline on continuous manufacturing represents a significant step forward, though it addresses microreactors only as a subset of continuous manufacturing technologies rather than as a distinct category requiring specialized standards.
The International Organization for Standardization (ISO) has established working groups focused on microfluidic systems standardization, with ISO/TC 48/WG 3 specifically addressing microprocess engineering devices. These efforts aim to create universally accepted terminology, testing methods, and performance criteria that can be referenced by regulatory bodies worldwide.
Industry consortia like the Microreactor Technology Pharmaceutical Consortium (MTPC) have emerged as important drivers of harmonization, bringing together pharmaceutical companies, equipment manufacturers, and regulatory experts to develop consensus-based recommendations for standardization. Their white papers on microreactor validation protocols have influenced regulatory thinking across multiple jurisdictions.
Key areas requiring harmonization include materials compatibility standards, cleaning validation protocols, process analytical technology (PAT) implementation, and scale-out validation approaches. The development of standardized connection interfaces between microreactor modules from different manufacturers represents another critical area where international consensus would significantly benefit the industry.
Regulatory acceptance of real-time release testing (RTRT) methodologies for microreactor-produced pharmaceuticals varies considerably between regions, creating a significant barrier to global implementation. Harmonized standards for RTRT validation would substantially accelerate microreactor adoption in pharmaceutical manufacturing.
The path toward comprehensive international harmonization will likely require a phased approach, beginning with agreement on fundamental definitions and testing methodologies, followed by alignment on validation requirements and eventually moving toward mutual recognition of inspection results between regulatory authorities. This process may take 5-10 years to fully implement but would ultimately create a more predictable regulatory environment that encourages innovation while maintaining stringent quality and safety standards.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!