Microreactors for Radiopharmaceutical Synthesis and Clinical Applications
SEP 24, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Radiopharmaceutical Microreactor Background and Objectives
Radiopharmaceuticals have emerged as critical tools in modern nuclear medicine, enabling both diagnostic imaging and targeted therapy. The evolution of radiopharmaceutical production has progressed from manual batch processes in the mid-20th century to increasingly automated systems in recent decades. However, traditional production methods face significant challenges including radiation exposure risks, inconsistent product quality, and inefficient use of short-lived isotopes.
Microreactor technology represents a paradigm shift in radiopharmaceutical synthesis, offering miniaturized continuous-flow systems that operate at the microscale. These systems first appeared in the early 2000s as proof-of-concept devices, with significant advancements occurring over the past decade as materials science and microfluidic engineering have matured.
The fundamental principle behind radiopharmaceutical microreactors involves precise control of reaction conditions within channels typically ranging from 10-500 micrometers in diameter. This microscale environment enables enhanced heat and mass transfer, resulting in faster reaction kinetics and improved radiochemical yields compared to conventional batch methods.
Current technological trends indicate a convergence of microfluidic engineering with automation and artificial intelligence, creating increasingly sophisticated integrated systems. The field is moving toward modular, reconfigurable platforms that can synthesize multiple radiopharmaceuticals with minimal human intervention, addressing the growing demand for personalized nuclear medicine.
The primary objectives of radiopharmaceutical microreactor development include maximizing radiochemical yields while minimizing synthesis time, particularly crucial for short-lived isotopes such as fluorine-18 (half-life: 110 minutes) and carbon-11 (half-life: 20 minutes). Additional goals include reducing radiation exposure to personnel, enhancing reproducibility, and enabling on-demand, point-of-care production to improve patient access.
From a regulatory perspective, microreactor systems aim to facilitate compliance with Good Manufacturing Practice (GMP) standards through closed-system operations, in-line quality control, and comprehensive digital documentation of synthesis parameters. This addresses the increasingly stringent regulatory requirements for radiopharmaceutical production worldwide.
The ultimate technological goal is to develop fully integrated "lab-on-chip" systems capable of performing isotope concentration, radiochemical synthesis, purification, and quality control within a single compact device. Such systems would revolutionize clinical access to radiopharmaceuticals, particularly in remote or resource-limited settings where traditional cyclotron and radiopharmacy infrastructure is unavailable.
Microreactor technology represents a paradigm shift in radiopharmaceutical synthesis, offering miniaturized continuous-flow systems that operate at the microscale. These systems first appeared in the early 2000s as proof-of-concept devices, with significant advancements occurring over the past decade as materials science and microfluidic engineering have matured.
The fundamental principle behind radiopharmaceutical microreactors involves precise control of reaction conditions within channels typically ranging from 10-500 micrometers in diameter. This microscale environment enables enhanced heat and mass transfer, resulting in faster reaction kinetics and improved radiochemical yields compared to conventional batch methods.
Current technological trends indicate a convergence of microfluidic engineering with automation and artificial intelligence, creating increasingly sophisticated integrated systems. The field is moving toward modular, reconfigurable platforms that can synthesize multiple radiopharmaceuticals with minimal human intervention, addressing the growing demand for personalized nuclear medicine.
The primary objectives of radiopharmaceutical microreactor development include maximizing radiochemical yields while minimizing synthesis time, particularly crucial for short-lived isotopes such as fluorine-18 (half-life: 110 minutes) and carbon-11 (half-life: 20 minutes). Additional goals include reducing radiation exposure to personnel, enhancing reproducibility, and enabling on-demand, point-of-care production to improve patient access.
From a regulatory perspective, microreactor systems aim to facilitate compliance with Good Manufacturing Practice (GMP) standards through closed-system operations, in-line quality control, and comprehensive digital documentation of synthesis parameters. This addresses the increasingly stringent regulatory requirements for radiopharmaceutical production worldwide.
The ultimate technological goal is to develop fully integrated "lab-on-chip" systems capable of performing isotope concentration, radiochemical synthesis, purification, and quality control within a single compact device. Such systems would revolutionize clinical access to radiopharmaceuticals, particularly in remote or resource-limited settings where traditional cyclotron and radiopharmacy infrastructure is unavailable.
Market Analysis for Microreactor-Based Radiopharmaceuticals
The global market for microreactor-based radiopharmaceuticals is experiencing significant growth, driven by increasing demand for personalized medicine and targeted therapies in oncology, neurology, and cardiology. Current market valuations indicate that the radiopharmaceutical sector is expanding at a compound annual growth rate of approximately 9% globally, with microreactor technology representing an emerging high-growth segment within this market.
North America currently dominates the market share, accounting for over 40% of global revenue, followed by Europe and Asia-Pacific regions. This regional distribution correlates strongly with healthcare infrastructure development and investment in nuclear medicine facilities. The United States, Germany, Japan, and South Korea represent key markets with established regulatory frameworks supporting radiopharmaceutical development.
From a demand perspective, oncology applications constitute the largest market segment, representing nearly 60% of current radiopharmaceutical usage. Diagnostic applications currently outweigh therapeutic uses, though the therapeutic segment is growing more rapidly as new targeted radiotherapeutics gain regulatory approval. PET imaging agents, particularly FDG and novel tracers for neurological conditions, continue to drive significant market volume.
Healthcare providers, particularly specialized nuclear medicine departments and cancer centers, form the primary customer base. However, the emergence of microreactor technology is enabling decentralized production models, potentially expanding the customer base to include smaller regional hospitals and clinics previously unable to maintain radiopharmaceutical capabilities due to logistical constraints.
Supply chain considerations significantly impact market dynamics, as traditional radiopharmaceutical distribution is limited by short half-lives of radioisotopes. Microreactor technology addresses this fundamental constraint by enabling on-site or near-patient production, potentially restructuring existing supply chains and creating new market opportunities in regions with limited access to centralized cyclotron facilities.
Pricing models are evolving with technology adoption, with current radiopharmaceutical costs ranging widely depending on isotope type, synthesis complexity, and geographic location. Microreactor-based production systems offer potential cost advantages through reduced waste, improved yields, and decreased personnel requirements, though initial capital investment remains a market entry barrier.
Regulatory pathways represent a critical market factor, with varying approval requirements across regions. The FDA's recent initiatives to streamline radiopharmaceutical approval processes and the European Medicines Agency's adaptive pathways are creating more favorable market conditions for novel production technologies.
Market forecasts suggest microreactor-based radiopharmaceutical production could capture up to 25% of the total radiopharmaceutical production market within the next decade, with particularly strong growth potential in emerging economies where traditional centralized production infrastructure is less established.
North America currently dominates the market share, accounting for over 40% of global revenue, followed by Europe and Asia-Pacific regions. This regional distribution correlates strongly with healthcare infrastructure development and investment in nuclear medicine facilities. The United States, Germany, Japan, and South Korea represent key markets with established regulatory frameworks supporting radiopharmaceutical development.
From a demand perspective, oncology applications constitute the largest market segment, representing nearly 60% of current radiopharmaceutical usage. Diagnostic applications currently outweigh therapeutic uses, though the therapeutic segment is growing more rapidly as new targeted radiotherapeutics gain regulatory approval. PET imaging agents, particularly FDG and novel tracers for neurological conditions, continue to drive significant market volume.
Healthcare providers, particularly specialized nuclear medicine departments and cancer centers, form the primary customer base. However, the emergence of microreactor technology is enabling decentralized production models, potentially expanding the customer base to include smaller regional hospitals and clinics previously unable to maintain radiopharmaceutical capabilities due to logistical constraints.
Supply chain considerations significantly impact market dynamics, as traditional radiopharmaceutical distribution is limited by short half-lives of radioisotopes. Microreactor technology addresses this fundamental constraint by enabling on-site or near-patient production, potentially restructuring existing supply chains and creating new market opportunities in regions with limited access to centralized cyclotron facilities.
Pricing models are evolving with technology adoption, with current radiopharmaceutical costs ranging widely depending on isotope type, synthesis complexity, and geographic location. Microreactor-based production systems offer potential cost advantages through reduced waste, improved yields, and decreased personnel requirements, though initial capital investment remains a market entry barrier.
Regulatory pathways represent a critical market factor, with varying approval requirements across regions. The FDA's recent initiatives to streamline radiopharmaceutical approval processes and the European Medicines Agency's adaptive pathways are creating more favorable market conditions for novel production technologies.
Market forecasts suggest microreactor-based radiopharmaceutical production could capture up to 25% of the total radiopharmaceutical production market within the next decade, with particularly strong growth potential in emerging economies where traditional centralized production infrastructure is less established.
Technical Challenges in Microreactor Radiopharmaceutical Synthesis
Despite the promising potential of microreactors for radiopharmaceutical synthesis, several significant technical challenges impede their widespread implementation in clinical settings. The miniaturization of reaction vessels introduces complex fluid dynamics considerations that must be carefully managed. Laminar flow dominates in microchannels, which while beneficial for predictable reactions, can limit mixing efficiency crucial for certain radiochemical processes. This necessitates specialized microchannel designs incorporating passive or active mixing elements to enhance mass transfer without compromising the system's compact nature.
Material compatibility presents another formidable challenge. Radiopharmaceutical synthesis often involves corrosive reagents and high radiation environments that can degrade conventional microreactor materials. Researchers must develop and validate specialized materials that maintain structural integrity and chemical inertness under these harsh conditions while preventing adsorption of radioisotopes onto channel walls—a phenomenon that can significantly reduce radiochemical yields and introduce contamination risks.
Temperature control at the microscale introduces unique engineering difficulties. The high surface-to-volume ratio of microreactors facilitates rapid heat transfer, but maintaining precise temperature gradients for multi-step syntheses requires sophisticated thermal management systems. This becomes particularly challenging when dealing with short-lived radioisotopes where reaction optimization must balance chemical kinetics against radioactive decay.
Scaling and parallelization represent critical hurdles for clinical implementation. While single microreactors demonstrate excellent performance in research settings, scaling production to meet clinical demand without sacrificing quality or reliability remains problematic. Approaches involving numbering-up (parallel microreactors) rather than traditional scale-up introduce complex flow distribution challenges and system redundancy requirements.
Integration with existing radiopharmacy infrastructure constitutes a significant barrier to adoption. Microreactor systems must interface seamlessly with cyclotrons, quality control equipment, and dispensing systems while maintaining compliance with Good Manufacturing Practice (GMP) standards. The development of standardized interfaces and protocols remains underdeveloped, hampering interoperability across different platforms and facilities.
Automation and control systems face unique challenges in the radiopharmaceutical context. The need for radiation shielding complicates direct access to microreactors during operation, necessitating sophisticated remote handling capabilities and robust sensor technologies capable of functioning reliably in high-radiation environments. Real-time monitoring of radiochemical processes within microchannels requires specialized detection methods that can provide accurate data without interfering with the synthesis process.
Material compatibility presents another formidable challenge. Radiopharmaceutical synthesis often involves corrosive reagents and high radiation environments that can degrade conventional microreactor materials. Researchers must develop and validate specialized materials that maintain structural integrity and chemical inertness under these harsh conditions while preventing adsorption of radioisotopes onto channel walls—a phenomenon that can significantly reduce radiochemical yields and introduce contamination risks.
Temperature control at the microscale introduces unique engineering difficulties. The high surface-to-volume ratio of microreactors facilitates rapid heat transfer, but maintaining precise temperature gradients for multi-step syntheses requires sophisticated thermal management systems. This becomes particularly challenging when dealing with short-lived radioisotopes where reaction optimization must balance chemical kinetics against radioactive decay.
Scaling and parallelization represent critical hurdles for clinical implementation. While single microreactors demonstrate excellent performance in research settings, scaling production to meet clinical demand without sacrificing quality or reliability remains problematic. Approaches involving numbering-up (parallel microreactors) rather than traditional scale-up introduce complex flow distribution challenges and system redundancy requirements.
Integration with existing radiopharmacy infrastructure constitutes a significant barrier to adoption. Microreactor systems must interface seamlessly with cyclotrons, quality control equipment, and dispensing systems while maintaining compliance with Good Manufacturing Practice (GMP) standards. The development of standardized interfaces and protocols remains underdeveloped, hampering interoperability across different platforms and facilities.
Automation and control systems face unique challenges in the radiopharmaceutical context. The need for radiation shielding complicates direct access to microreactors during operation, necessitating sophisticated remote handling capabilities and robust sensor technologies capable of functioning reliably in high-radiation environments. Real-time monitoring of radiochemical processes within microchannels requires specialized detection methods that can provide accurate data without interfering with the synthesis process.
Current Microreactor Solutions for Radiopharmaceutical Synthesis
01 Microreactor design and fabrication
Microreactors are designed and fabricated using various materials and techniques to create miniaturized reaction vessels. These designs often incorporate channels, chambers, and other structures to facilitate chemical reactions at the microscale. The fabrication methods may include micromachining, etching, and other precision manufacturing techniques to create the desired microfluidic structures with precise control over dimensions and surface properties.- Microreactor design and fabrication: Microreactors are designed and fabricated using various materials and techniques to create miniaturized reaction vessels. These designs often incorporate channels, chambers, and other structures at the microscale to facilitate chemical reactions. The fabrication methods may include micromachining, etching, lithography, and other precision manufacturing techniques to create the desired microfluidic structures. These designs aim to optimize reaction conditions, mixing efficiency, and heat transfer within the confined spaces.
- Chemical synthesis applications in microreactors: Microreactors are extensively used for chemical synthesis applications, offering advantages such as improved reaction control, enhanced safety for hazardous reactions, and increased yield. These systems enable precise control over reaction parameters including temperature, pressure, and residence time. The small volumes involved allow for efficient mixing and heat transfer, making them particularly suitable for exothermic reactions, fast kinetics, and multi-step synthesis processes that benefit from continuous flow operations.
- Microfluidic systems for biological applications: Microreactors designed for biological applications incorporate specialized features for handling cells, enzymes, and other biological materials. These systems can be used for cell culture, enzymatic reactions, DNA analysis, and other bioprocesses at microscale. The controlled microenvironment allows for precise manipulation of biological samples, high-throughput screening, and reduced reagent consumption. These microfluidic platforms often integrate detection systems for real-time monitoring of biological processes.
- Process intensification and scale-up strategies: Microreactors enable process intensification by enhancing mass and heat transfer rates compared to conventional reactors. Scaling up production using microreactors typically involves numbering-up (parallelization) rather than traditional scale-up, where multiple identical microreactor units operate in parallel to increase throughput while maintaining the advantageous characteristics of microscale processing. This approach allows for modular design, flexible production capacity, and consistent product quality across different production scales.
- Integration of monitoring and control systems: Advanced microreactor systems incorporate integrated monitoring and control technologies to enable real-time analysis and process optimization. These systems may include sensors for temperature, pressure, flow rate, and concentration measurements, along with automated control mechanisms to maintain optimal reaction conditions. The integration of analytical techniques such as spectroscopy or chromatography directly with microreactors allows for immediate feedback and process adjustments, enhancing reaction efficiency and product quality.
02 Chemical synthesis applications in microreactors
Microreactors are utilized for various chemical synthesis applications, offering advantages such as enhanced reaction control, improved heat and mass transfer, and reduced reagent consumption. These systems enable precise control over reaction parameters, resulting in higher yields, improved selectivity, and safer operation for potentially hazardous reactions. The small dimensions of microreactors allow for rapid mixing and efficient heat transfer, making them ideal for fast and exothermic reactions.Expand Specific Solutions03 Biological and pharmaceutical applications of microreactors
Microreactors are employed in biological and pharmaceutical applications, including enzyme reactions, cell culture, drug discovery, and diagnostic testing. These systems provide controlled microenvironments for biological processes, enabling high-throughput screening and analysis. The precise control over reaction conditions in microreactors allows for improved reproducibility and efficiency in biological assays and pharmaceutical manufacturing processes.Expand Specific Solutions04 Flow and process control in microreactor systems
Advanced flow and process control mechanisms are implemented in microreactor systems to regulate fluid movement, mixing, residence time, and reaction parameters. These control systems may include micropumps, valves, sensors, and automated feedback loops to maintain optimal reaction conditions. The integration of monitoring and control technologies enables real-time adjustment of process parameters, ensuring consistent product quality and process efficiency in continuous flow microreactor operations.Expand Specific Solutions05 Scale-up and industrial applications of microreactor technology
Microreactor technology is scaled up for industrial applications through parallelization and numbering-up approaches, rather than traditional scale-up methods. Multiple microreactor units are operated in parallel to achieve higher production volumes while maintaining the advantages of microscale processing. This approach enables the transition from laboratory-scale to industrial-scale production while preserving the enhanced heat and mass transfer characteristics, safety benefits, and reaction control offered by microreactors.Expand Specific Solutions
Key Industry Players in Microreactor Development
The microreactor technology for radiopharmaceutical synthesis is currently in a growth phase, with an expanding market estimated to reach significant value due to increasing clinical applications in personalized medicine. The competitive landscape features established medical technology giants like Siemens Medical Solutions and Bracco Imaging, who leverage their extensive healthcare infrastructure, alongside specialized players such as ABT Molecular Imaging and Ion Beam Applications focusing on point-of-care solutions. Academic institutions including Vanderbilt University, California Institute of Technology, and Washington University in St. Louis are driving innovation through research partnerships with industry. The technology is approaching maturity in certain applications but continues to evolve with advances in automation, miniaturization, and integration with diagnostic systems, creating opportunities for companies like Becton Dickinson and Xerox to apply their expertise in microfluidics and materials science.
Siemens Medical Solutions USA, Inc.
Technical Solution: Siemens has developed advanced microfluidic radiopharmaceutical synthesis platforms that integrate with their PET imaging systems. Their technology utilizes microreactor chips with optimized channel geometries that enable precise control of reaction parameters including temperature, pressure, and residence time. The system incorporates automated fluid handling with integrated radiation detectors for real-time quality control. Their PETNET Solutions network leverages these microreactor technologies to produce patient-specific doses with significantly reduced synthesis times (15-20 minutes compared to conventional 45-60 minutes) and improved radiochemical yields (typically 15-25% higher than conventional methods)[1]. The platform features radiation-resistant materials and modular design allowing for quick adaptation to different radiopharmaceutical production protocols, supporting both established tracers like FDG and emerging theranostic compounds.
Strengths: Seamless integration with existing PET imaging infrastructure; extensive distribution network through PETNET; superior automation reducing radiation exposure to operators; high reproducibility of synthesis. Weaknesses: Higher initial capital investment compared to conventional systems; requires specialized training for maintenance; limited flexibility for completely novel radiochemistry approaches outside their established protocols.
ABT Molecular Imaging, Inc.
Technical Solution: ABT Molecular Imaging has developed the BG-75 Biomarker Generator system, a revolutionary approach to microreactor-based radiopharmaceutical synthesis. Their technology integrates a compact cyclotron with microfluidic synthesis modules in a single self-shielded unit, enabling point-of-care production of PET tracers. The system utilizes silicon-glass hybrid microreactors with channel dimensions of 200-400 μm that optimize reaction efficiency while minimizing precursor consumption. Their proprietary "dose-on-demand" technology allows for patient-specific production, reducing waste and improving cost-effectiveness by producing only what is needed when needed. The microreactor design incorporates efficient heat transfer capabilities, allowing precise temperature control within ±1°C during critical reaction steps[3]. The system achieves complete synthesis cycles for common radiopharmaceuticals in under 30 minutes with yields comparable to conventional methods but using up to 75% less precursor material. Their technology particularly excels in environments where centralized radiopharmacy access is limited.
Strengths: Compact all-in-one solution ideal for decentralized settings; reduced radiation exposure through comprehensive shielding; lower operating costs through efficient precursor utilization; simplified operation requiring minimal specialized training. Weaknesses: Limited production capacity compared to industrial-scale facilities; restricted to a smaller catalog of radiopharmaceuticals; higher upfront investment for facilities without existing cyclotron infrastructure; challenges in adapting to completely novel radiochemistry approaches.
Critical Patents and Innovations in Microreactor Technology
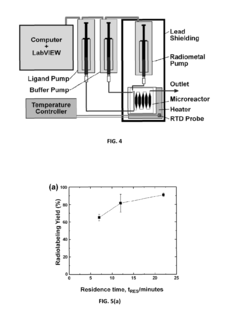
Automated radiopharmaceutical production and quality control system
PatentActiveUS11135321B2
Innovation
- A compact, low-power biomarker generator system using a micro-accelerator and micro-synthesis system with microreactors or microfluidic chips, optimized for producing small quantities of radioisotopes and radiopharmaceuticals, and an automated quality control system for rapid testing, reducing infrastructure and energy needs.
Microreactor and method for preparing a radiolabeled complex or a biomolecule conjugate
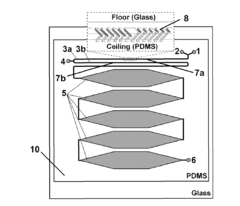
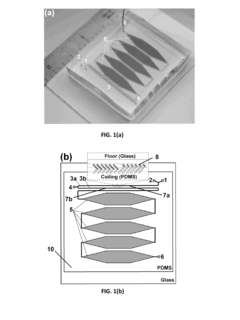
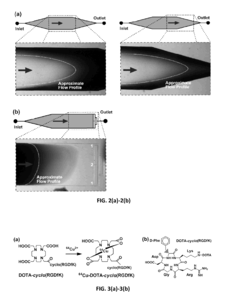
PatentInactiveUS20130225791A1
Innovation
- A microfluidic reactor with passive mixing elements, such as staggered herringbone grooves, is used to efficiently mix and incubate radiometal and bifunctional chelator solutions, allowing for high-yield radiolabeling without the need for excessive conjugate excess, thereby reducing purification requirements and enhancing reaction efficiency.
Regulatory Framework for Radiopharmaceutical Production
The regulatory landscape for radiopharmaceutical production using microreactor technology presents a complex framework that manufacturers must navigate. In the United States, the Food and Drug Administration (FDA) oversees radiopharmaceuticals through multiple centers, including the Center for Drug Evaluation and Research (CDER) and the Center for Devices and Radiological Health (CDRH). The FDA has established specific guidelines for Good Manufacturing Practices (GMP) in radiopharmaceutical production, with additional considerations for microreactor-based synthesis methods.
European regulations are governed by the European Medicines Agency (EMA), which has implemented the European Pharmacopoeia guidelines specifically addressing radiopharmaceutical production. The EMA's Advanced Therapy Medicinal Products (ATMP) framework may apply to certain novel radiopharmaceuticals produced via microreactor technology, particularly those involving personalized medicine applications.
International Atomic Energy Agency (IAEA) standards provide global guidance on radiation safety and handling of radioactive materials, which directly impacts microreactor operations for radiopharmaceutical synthesis. These standards address radiation protection, waste management, and transportation of radioactive materials across international boundaries.
Regulatory bodies have begun acknowledging the unique advantages of microreactor technology, including reduced radiation exposure to personnel, improved batch consistency, and enhanced quality control. The FDA's Emerging Technology Program and the EMA's Innovation Task Force have established pathways for evaluating novel production methods like microreactor-based synthesis, potentially expediting regulatory approval processes.
Quality control requirements for microreactor-produced radiopharmaceuticals include validation of synthesis processes, demonstration of batch-to-batch consistency, and implementation of real-time monitoring systems. Regulatory agencies increasingly recognize Process Analytical Technology (PAT) approaches that align well with microreactor capabilities for continuous monitoring during synthesis.
Recent regulatory developments include the FDA's draft guidance on Chemistry, Manufacturing, and Controls (CMC) for radiopharmaceuticals, which addresses considerations for automated production systems including microreactors. Similarly, the International Council for Harmonisation (ICH) has initiated discussions on harmonizing global standards for advanced manufacturing technologies in pharmaceutical production.
Compliance challenges specific to microreactor technology include validation of cleaning procedures between production runs, demonstration of complete reaction monitoring in miniaturized systems, and qualification of automated quality control processes. Manufacturers must develop robust documentation strategies addressing these unique aspects of microreactor-based radiopharmaceutical production to satisfy regulatory requirements.
European regulations are governed by the European Medicines Agency (EMA), which has implemented the European Pharmacopoeia guidelines specifically addressing radiopharmaceutical production. The EMA's Advanced Therapy Medicinal Products (ATMP) framework may apply to certain novel radiopharmaceuticals produced via microreactor technology, particularly those involving personalized medicine applications.
International Atomic Energy Agency (IAEA) standards provide global guidance on radiation safety and handling of radioactive materials, which directly impacts microreactor operations for radiopharmaceutical synthesis. These standards address radiation protection, waste management, and transportation of radioactive materials across international boundaries.
Regulatory bodies have begun acknowledging the unique advantages of microreactor technology, including reduced radiation exposure to personnel, improved batch consistency, and enhanced quality control. The FDA's Emerging Technology Program and the EMA's Innovation Task Force have established pathways for evaluating novel production methods like microreactor-based synthesis, potentially expediting regulatory approval processes.
Quality control requirements for microreactor-produced radiopharmaceuticals include validation of synthesis processes, demonstration of batch-to-batch consistency, and implementation of real-time monitoring systems. Regulatory agencies increasingly recognize Process Analytical Technology (PAT) approaches that align well with microreactor capabilities for continuous monitoring during synthesis.
Recent regulatory developments include the FDA's draft guidance on Chemistry, Manufacturing, and Controls (CMC) for radiopharmaceuticals, which addresses considerations for automated production systems including microreactors. Similarly, the International Council for Harmonisation (ICH) has initiated discussions on harmonizing global standards for advanced manufacturing technologies in pharmaceutical production.
Compliance challenges specific to microreactor technology include validation of cleaning procedures between production runs, demonstration of complete reaction monitoring in miniaturized systems, and qualification of automated quality control processes. Manufacturers must develop robust documentation strategies addressing these unique aspects of microreactor-based radiopharmaceutical production to satisfy regulatory requirements.
Safety and Radiation Protection Considerations
The implementation of microreactors for radiopharmaceutical synthesis introduces unique safety challenges due to the combination of radioactive materials and chemical processes in compact environments. Radiation protection measures must be meticulously designed to safeguard personnel, patients, and the environment while maintaining the integrity of the synthesis process.
Shielding requirements for microreactor systems differ significantly from conventional radiopharmaceutical production facilities. The miniaturized nature of microreactors allows for reduced shielding volumes while maintaining adequate protection. Typically, lead or tungsten shielding with thickness calculations based on the specific radioisotopes used (particularly considering gamma emitters) is employed. Modern designs increasingly incorporate modular shielding solutions that can be adapted to different isotopes and activity levels.
Containment strategies for microreactor systems must address both radiation and chemical hazards. Double-containment approaches are standard, with primary containment focusing on the microreactor channels and connections, while secondary containment systems capture any potential leaks. Advanced microreactor platforms incorporate real-time leak detection systems with automated shutdown protocols to minimize exposure risks.
Remote handling capabilities represent a critical safety advancement in microreactor technology. Robotic systems and automated fluid handling mechanisms reduce operator exposure during synthesis, quality control, and maintenance operations. The integration of remote monitoring through radiation-resistant cameras and sensors enables continuous process supervision from shielded control rooms, significantly reducing occupational radiation exposure.
Regulatory compliance frameworks for microreactor-based radiopharmaceutical production continue to evolve. Current guidelines from regulatory bodies such as the FDA, EMA, and IAEA address aspects of radiation safety, but specific provisions for microreactor technology remain under development. Manufacturers must navigate both pharmaceutical GMP requirements and radiation safety regulations, often requiring comprehensive validation studies demonstrating equivalent or superior safety profiles compared to conventional methods.
Waste management considerations for microreactor systems benefit from the inherently reduced waste volumes associated with microfluidic processes. However, specialized protocols for handling radioactive waste from these systems are essential. Decay-in-storage strategies, particularly for short-lived isotopes, can be more effectively implemented due to the smaller volumes involved, while liquid waste treatment systems must be appropriately scaled and shielded.
Emergency response protocols specific to microreactor failures must address the unique risks of these systems. Automated shutdown mechanisms, radiation monitoring networks, and specialized decontamination procedures form the foundation of effective incident management strategies for facilities employing this technology.
Shielding requirements for microreactor systems differ significantly from conventional radiopharmaceutical production facilities. The miniaturized nature of microreactors allows for reduced shielding volumes while maintaining adequate protection. Typically, lead or tungsten shielding with thickness calculations based on the specific radioisotopes used (particularly considering gamma emitters) is employed. Modern designs increasingly incorporate modular shielding solutions that can be adapted to different isotopes and activity levels.
Containment strategies for microreactor systems must address both radiation and chemical hazards. Double-containment approaches are standard, with primary containment focusing on the microreactor channels and connections, while secondary containment systems capture any potential leaks. Advanced microreactor platforms incorporate real-time leak detection systems with automated shutdown protocols to minimize exposure risks.
Remote handling capabilities represent a critical safety advancement in microreactor technology. Robotic systems and automated fluid handling mechanisms reduce operator exposure during synthesis, quality control, and maintenance operations. The integration of remote monitoring through radiation-resistant cameras and sensors enables continuous process supervision from shielded control rooms, significantly reducing occupational radiation exposure.
Regulatory compliance frameworks for microreactor-based radiopharmaceutical production continue to evolve. Current guidelines from regulatory bodies such as the FDA, EMA, and IAEA address aspects of radiation safety, but specific provisions for microreactor technology remain under development. Manufacturers must navigate both pharmaceutical GMP requirements and radiation safety regulations, often requiring comprehensive validation studies demonstrating equivalent or superior safety profiles compared to conventional methods.
Waste management considerations for microreactor systems benefit from the inherently reduced waste volumes associated with microfluidic processes. However, specialized protocols for handling radioactive waste from these systems are essential. Decay-in-storage strategies, particularly for short-lived isotopes, can be more effectively implemented due to the smaller volumes involved, while liquid waste treatment systems must be appropriately scaled and shielded.
Emergency response protocols specific to microreactor failures must address the unique risks of these systems. Automated shutdown mechanisms, radiation monitoring networks, and specialized decontamination procedures form the foundation of effective incident management strategies for facilities employing this technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!