Patent Analysis of Microreactors for Biotechnology and Medical Devices
SEP 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microreactor Technology Background and Objectives
Microreactors represent a revolutionary technology in the field of biotechnology and medical devices, characterized by their miniaturized reaction systems that operate at microscale dimensions. The evolution of microreactor technology can be traced back to the late 1990s, emerging from the convergence of microfluidics, microfabrication techniques, and process intensification strategies. Initially developed for chemical synthesis applications, microreactors have progressively expanded into biological and medical domains over the past two decades.
The technological trajectory of microreactors has been marked by significant advancements in materials science, fabrication methods, and control systems. Early microreactors primarily utilized glass and silicon substrates, while contemporary designs incorporate polymers, ceramics, and hybrid materials that offer enhanced biocompatibility and functional versatility. The integration of sensors, actuators, and automated control systems has further elevated the precision and reproducibility of microreactor operations.
In the biotechnology sector, microreactors have evolved from simple channel structures to sophisticated platforms capable of mimicking complex biological environments. This progression has enabled applications ranging from enzymatic reactions and protein synthesis to cell culture and tissue engineering. Similarly, in medical device development, microreactors have transitioned from basic analytical tools to integrated diagnostic and therapeutic systems.
The current technological trend is moving toward multifunctional microreactor systems that combine multiple unit operations within a single platform. This integration facilitates continuous-flow processing, real-time monitoring, and automated feedback control—capabilities that are particularly valuable for personalized medicine and point-of-care diagnostics. Additionally, there is growing emphasis on scalable manufacturing approaches that can bridge the gap between laboratory prototypes and commercial production.
The primary objectives of microreactor technology development in biotechnology and medical applications include enhancing reaction efficiency through improved mass and heat transfer, reducing reagent consumption and waste generation, enabling precise control over reaction parameters, and facilitating rapid prototyping of biological processes. Furthermore, microreactors aim to provide platforms for high-throughput screening, accelerate drug discovery and development, and enable personalized therapeutic approaches.
Long-term technological goals include the development of fully autonomous microreactor systems capable of self-regulation and adaptation, the integration of artificial intelligence for process optimization, and the establishment of standardized interfaces for seamless integration with existing biomedical infrastructure. These advancements are expected to revolutionize pharmaceutical manufacturing, diagnostic procedures, and therapeutic interventions in the coming decade.
The technological trajectory of microreactors has been marked by significant advancements in materials science, fabrication methods, and control systems. Early microreactors primarily utilized glass and silicon substrates, while contemporary designs incorporate polymers, ceramics, and hybrid materials that offer enhanced biocompatibility and functional versatility. The integration of sensors, actuators, and automated control systems has further elevated the precision and reproducibility of microreactor operations.
In the biotechnology sector, microreactors have evolved from simple channel structures to sophisticated platforms capable of mimicking complex biological environments. This progression has enabled applications ranging from enzymatic reactions and protein synthesis to cell culture and tissue engineering. Similarly, in medical device development, microreactors have transitioned from basic analytical tools to integrated diagnostic and therapeutic systems.
The current technological trend is moving toward multifunctional microreactor systems that combine multiple unit operations within a single platform. This integration facilitates continuous-flow processing, real-time monitoring, and automated feedback control—capabilities that are particularly valuable for personalized medicine and point-of-care diagnostics. Additionally, there is growing emphasis on scalable manufacturing approaches that can bridge the gap between laboratory prototypes and commercial production.
The primary objectives of microreactor technology development in biotechnology and medical applications include enhancing reaction efficiency through improved mass and heat transfer, reducing reagent consumption and waste generation, enabling precise control over reaction parameters, and facilitating rapid prototyping of biological processes. Furthermore, microreactors aim to provide platforms for high-throughput screening, accelerate drug discovery and development, and enable personalized therapeutic approaches.
Long-term technological goals include the development of fully autonomous microreactor systems capable of self-regulation and adaptation, the integration of artificial intelligence for process optimization, and the establishment of standardized interfaces for seamless integration with existing biomedical infrastructure. These advancements are expected to revolutionize pharmaceutical manufacturing, diagnostic procedures, and therapeutic interventions in the coming decade.
Market Demand Analysis for Biotech Microreactors
The global market for microreactors in biotechnology and medical devices is experiencing robust growth, driven by increasing demand for miniaturized, efficient, and precise reaction systems. Current market valuations indicate that the biotech microreactor segment reached approximately 1.2 billion USD in 2022, with projections suggesting a compound annual growth rate (CAGR) of 9.8% through 2030. This growth trajectory reflects the expanding applications of microreactors across pharmaceutical development, diagnostic technologies, and personalized medicine.
Pharmaceutical companies represent the largest market segment, accounting for nearly 45% of current demand. These organizations are increasingly adopting microreactor technologies to accelerate drug discovery processes, optimize reaction conditions, and reduce development costs. The ability of microreactors to facilitate rapid screening of reaction parameters while consuming minimal reagents presents a compelling value proposition for pharmaceutical R&D departments facing pressure to improve efficiency.
The diagnostic sector demonstrates the fastest growth rate within the microreactor market, expanding at approximately 12% annually. This acceleration is primarily attributed to the rising prevalence of point-of-care testing and the growing emphasis on rapid, accurate diagnostic solutions. Microreactors enable precise control over sample preparation and analysis, making them invaluable tools for next-generation diagnostic platforms.
Regional analysis reveals that North America currently dominates the market with a 38% share, followed by Europe (31%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to exhibit the highest growth rate over the next decade, driven by increasing healthcare expenditure, expanding biotechnology sectors in China and India, and growing adoption of advanced medical technologies.
Key market drivers include the rising demand for personalized medicine, which requires precise control over biological reactions; increasing research activities in cell and gene therapies; and growing applications in continuous manufacturing processes. Additionally, the push toward sustainable and environmentally friendly production methods has bolstered interest in microreactor technologies that minimize waste generation and energy consumption.
Challenges affecting market demand include high initial investment costs, technical complexity requiring specialized expertise, and regulatory uncertainties surrounding novel manufacturing methodologies. Despite these barriers, the overall market sentiment remains positive, with industry stakeholders recognizing the transformative potential of microreactor technologies in addressing critical challenges in biotechnology and medical device development.
Pharmaceutical companies represent the largest market segment, accounting for nearly 45% of current demand. These organizations are increasingly adopting microreactor technologies to accelerate drug discovery processes, optimize reaction conditions, and reduce development costs. The ability of microreactors to facilitate rapid screening of reaction parameters while consuming minimal reagents presents a compelling value proposition for pharmaceutical R&D departments facing pressure to improve efficiency.
The diagnostic sector demonstrates the fastest growth rate within the microreactor market, expanding at approximately 12% annually. This acceleration is primarily attributed to the rising prevalence of point-of-care testing and the growing emphasis on rapid, accurate diagnostic solutions. Microreactors enable precise control over sample preparation and analysis, making them invaluable tools for next-generation diagnostic platforms.
Regional analysis reveals that North America currently dominates the market with a 38% share, followed by Europe (31%) and Asia-Pacific (24%). However, the Asia-Pacific region is expected to exhibit the highest growth rate over the next decade, driven by increasing healthcare expenditure, expanding biotechnology sectors in China and India, and growing adoption of advanced medical technologies.
Key market drivers include the rising demand for personalized medicine, which requires precise control over biological reactions; increasing research activities in cell and gene therapies; and growing applications in continuous manufacturing processes. Additionally, the push toward sustainable and environmentally friendly production methods has bolstered interest in microreactor technologies that minimize waste generation and energy consumption.
Challenges affecting market demand include high initial investment costs, technical complexity requiring specialized expertise, and regulatory uncertainties surrounding novel manufacturing methodologies. Despite these barriers, the overall market sentiment remains positive, with industry stakeholders recognizing the transformative potential of microreactor technologies in addressing critical challenges in biotechnology and medical device development.
Current Status and Challenges in Microreactor Technology
Microreactor technology has witnessed significant advancements in recent years, particularly in biotechnology and medical device applications. Currently, the global market for microreactors is experiencing robust growth, with a compound annual growth rate projected at 19.2% between 2021 and 2026. This growth is primarily driven by increasing demand for point-of-care diagnostics, personalized medicine, and more efficient drug discovery processes.
In the biotechnology sector, microreactors have achieved notable success in enzymatic reactions, cell culture applications, and protein synthesis. These systems offer precise control over reaction parameters, enabling higher yields and purity levels compared to conventional batch processes. Recent innovations include integrated sensing capabilities that allow real-time monitoring of reaction kinetics and product formation.
Despite these advances, several significant challenges persist in microreactor technology development. Scaling issues remain paramount, as translating laboratory-scale success to industrial production volumes continues to be problematic. The "numbering up" approach (adding parallel microreactor units) rather than traditional "scaling up" introduces complex flow distribution challenges and system control difficulties.
Material compatibility represents another critical challenge, particularly for medical applications where biocompatibility is essential. Current microreactor designs predominantly utilize polymers (PDMS, PMMA), glass, or silicon, each with inherent limitations regarding chemical resistance, optical properties, or manufacturing complexity. The development of novel materials that combine biocompatibility with chemical resistance remains an active research area.
Standardization across the industry presents a significant barrier to widespread adoption. The lack of unified design principles, testing protocols, and performance metrics hampers technology transfer and commercialization efforts. This fragmentation has resulted in isolated technological developments that often cannot be integrated into comprehensive systems.
Regulatory hurdles constitute a formidable challenge for microreactor implementation in medical devices. The novel nature of these technologies creates uncertainty in regulatory pathways, particularly regarding validation protocols and quality control measures. Companies must navigate complex approval processes that were not specifically designed for microfluidic technologies.
Geographically, microreactor technology development shows distinct patterns. North America and Europe lead in research output and patent filings, with significant contributions from academic institutions and established biotechnology companies. However, Asia-Pacific regions, particularly China and South Korea, are rapidly increasing their research activities and patent portfolios in this domain, focusing on manufacturing innovations and cost reduction strategies.
In the biotechnology sector, microreactors have achieved notable success in enzymatic reactions, cell culture applications, and protein synthesis. These systems offer precise control over reaction parameters, enabling higher yields and purity levels compared to conventional batch processes. Recent innovations include integrated sensing capabilities that allow real-time monitoring of reaction kinetics and product formation.
Despite these advances, several significant challenges persist in microreactor technology development. Scaling issues remain paramount, as translating laboratory-scale success to industrial production volumes continues to be problematic. The "numbering up" approach (adding parallel microreactor units) rather than traditional "scaling up" introduces complex flow distribution challenges and system control difficulties.
Material compatibility represents another critical challenge, particularly for medical applications where biocompatibility is essential. Current microreactor designs predominantly utilize polymers (PDMS, PMMA), glass, or silicon, each with inherent limitations regarding chemical resistance, optical properties, or manufacturing complexity. The development of novel materials that combine biocompatibility with chemical resistance remains an active research area.
Standardization across the industry presents a significant barrier to widespread adoption. The lack of unified design principles, testing protocols, and performance metrics hampers technology transfer and commercialization efforts. This fragmentation has resulted in isolated technological developments that often cannot be integrated into comprehensive systems.
Regulatory hurdles constitute a formidable challenge for microreactor implementation in medical devices. The novel nature of these technologies creates uncertainty in regulatory pathways, particularly regarding validation protocols and quality control measures. Companies must navigate complex approval processes that were not specifically designed for microfluidic technologies.
Geographically, microreactor technology development shows distinct patterns. North America and Europe lead in research output and patent filings, with significant contributions from academic institutions and established biotechnology companies. However, Asia-Pacific regions, particularly China and South Korea, are rapidly increasing their research activities and patent portfolios in this domain, focusing on manufacturing innovations and cost reduction strategies.
Current Technical Solutions for Microreactor Implementation
01 Design and fabrication of microreactors
Microreactors are miniaturized reaction systems with dimensions in the micrometer range. The design and fabrication of these devices involve various materials and techniques to create microchannels, mixing zones, and reaction chambers. Advanced manufacturing methods such as micromachining, lithography, and 3D printing are employed to produce precise microstructures. These designs often incorporate features for enhanced heat and mass transfer, which are critical for efficient reaction control.- Design and fabrication of microreactors: Microreactors are miniaturized reaction systems with dimensions in the micrometer range. The design and fabrication of these devices involve various materials and techniques to create microchannels, mixing zones, and reaction chambers. Advanced manufacturing methods such as micromachining, lithography, and 3D printing are employed to produce precise microstructures with controlled geometries. These designs optimize fluid flow, heat transfer, and mixing efficiency at the microscale.
- Chemical synthesis applications in microreactors: Microreactors offer significant advantages for chemical synthesis processes, including enhanced reaction control, improved safety for hazardous reactions, and increased yield and selectivity. These devices enable precise temperature control, efficient mixing, and reduced reaction times. They are particularly valuable for multiphase reactions, catalytic processes, and the production of fine chemicals and pharmaceuticals. The controlled environment allows for optimization of reaction parameters and continuous processing capabilities.
- Microfluidic systems for biological applications: Microreactors designed for biological applications incorporate specialized features for handling cells, enzymes, and other biological materials. These systems enable rapid analysis, screening, and processing of biological samples with minimal reagent consumption. Applications include DNA amplification, cell culture, protein synthesis, and diagnostic testing. The controlled microenvironment allows for precise manipulation of biological processes, enhancing reproducibility and efficiency compared to conventional methods.
- Integration of sensors and control systems in microreactors: Advanced microreactor systems incorporate integrated sensors and control mechanisms for real-time monitoring and process automation. These components enable continuous measurement of parameters such as temperature, pressure, pH, and concentration within the microchannels. The integration of sensing elements with feedback control systems allows for automated adjustment of reaction conditions, ensuring optimal performance and consistent product quality. This technology facilitates process intensification and quality-by-design approaches in microscale manufacturing.
- Scale-up and industrial implementation of microreactor technology: Scaling up microreactor technology for industrial applications involves strategies such as numbering-up (parallel operation of multiple units) rather than traditional scale-up approaches. This methodology maintains the advantageous characteristics of microscale processing while increasing production capacity. Industrial implementation requires addressing challenges related to system integration, flow distribution, pressure management, and long-term operational stability. The technology enables continuous manufacturing processes with reduced footprint, improved energy efficiency, and enhanced process safety.
02 Chemical synthesis applications in microreactors
Microreactors offer significant advantages for chemical synthesis processes, including improved reaction control, enhanced safety for hazardous reactions, and increased yield and selectivity. These systems enable precise control over reaction parameters such as temperature, pressure, and residence time. They are particularly valuable for fast, highly exothermic reactions and for processes requiring strict stoichiometric control. The small volumes involved also reduce reagent consumption and waste generation, making them environmentally friendly alternatives to conventional batch reactors.Expand Specific Solutions03 Microfluidic systems for biological applications
Microreactors designed for biological applications incorporate specialized features for handling cells, enzymes, and other biological materials. These systems are used for applications such as cell culture, enzymatic reactions, DNA analysis, and protein synthesis. The controlled microenvironment allows for precise manipulation of biological processes, enabling high-throughput screening, personalized medicine approaches, and point-of-care diagnostics. These biologically-oriented microreactors often integrate detection systems for real-time monitoring of biological processes.Expand Specific Solutions04 Flow control and mixing technologies in microreactors
Effective flow control and mixing are critical aspects of microreactor technology. Various innovative approaches have been developed to overcome the challenges of laminar flow in microscale channels, including passive mixers (utilizing channel geometry), active mixers (using external energy sources), and hybrid systems. Advanced flow control mechanisms such as micropumps, microvalves, and pressure regulators enable precise manipulation of fluids within the microchannels. These technologies ensure efficient mixing of reactants and controlled residence times, which are essential for optimal reaction performance.Expand Specific Solutions05 Process intensification and scale-up strategies
Microreactors facilitate process intensification by enhancing heat and mass transfer rates, allowing for more efficient and safer chemical processes. Scale-up strategies for microreactor technology typically involve numbering-up (parallel operation of multiple identical units) rather than traditional scale-up approaches. This maintains the advantageous characteristics of the microscale while increasing production capacity. Advanced control systems and modular designs enable flexible manufacturing capabilities, allowing for rapid adaptation to changing production requirements. These approaches are particularly valuable for pharmaceutical manufacturing and fine chemical production.Expand Specific Solutions
Key Industry Players in Biomedical Microreactors
The microreactor technology for biotechnology and medical devices is currently in a growth phase, with increasing market adoption driven by demands for miniaturization and process intensification. The global market is expanding rapidly, estimated to reach significant value as these systems offer enhanced efficiency and control for biological processes. Technologically, the field shows varying maturity levels across applications, with academic institutions like MIT, Columbia University, and Tsinghua University leading fundamental research, while companies including STMicroelectronics, Roche, and Thermo Fisher Scientific focus on commercial applications. Established pharmaceutical companies (Sanofi, Becton Dickinson) are integrating microreactor technologies into their bioprocessing platforms, while specialized firms like FloDesign Sonics and Nirrin Bioprocess Analytics are developing innovative acoustic and monitoring solutions for these systems.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered microfluidic technology for biotechnology applications, developing integrated microreactor systems that enable precise control over cellular microenvironments. Their patented microreactor platforms incorporate multiple functional elements including cell culture chambers, gradient generators, and integrated sensing capabilities on a single chip. MIT researchers have created microreactors with programmable fluid dynamics that can mimic in vivo conditions for drug testing and tissue engineering. Their microreactor technology features multilayer fabrication techniques that allow for complex 3D architectures with controlled oxygen and nutrient gradients. MIT has also developed microreactors with integrated optical sensing elements for real-time monitoring of cellular responses and metabolic activities, enabling high-throughput screening applications for pharmaceutical development[1][3]. Their patents cover novel surface modification techniques that enhance biocompatibility and prevent biofouling in continuous operation scenarios.
Strengths: Superior integration of multiple functional elements on single platforms; exceptional precision in microenvironment control; advanced fabrication techniques enabling complex architectures. Weaknesses: Higher manufacturing complexity increases production costs; requires specialized expertise for operation; some designs may face challenges in scaling up for industrial production volumes.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed proprietary microreactor technology specifically designed for point-of-care diagnostics and personalized medicine applications. Their patented microreactor systems integrate sample preparation, nucleic acid amplification, and detection capabilities within compact, disposable cartridges. These microreactors employ sophisticated microfluidic control mechanisms that enable automated processing of complex biological samples with minimal user intervention. Roche's technology incorporates isothermal amplification methods within microreactors, eliminating the need for thermal cycling equipment and enabling rapid detection of pathogens and biomarkers. Their microreactor designs feature integrated reagent storage compartments with controlled release mechanisms, allowing for long-term stability and simplified logistics. Roche has also patented microreactor systems with multiplexed detection capabilities that can simultaneously analyze multiple biomarkers from a single sample, enhancing diagnostic efficiency and reducing sample volume requirements[2][5]. Their microreactor technology employs proprietary surface chemistry to minimize non-specific binding and enhance detection sensitivity.
Strengths: Exceptional integration of sample preparation and analysis functions; superior automation capabilities reducing user error; excellent clinical validation and regulatory expertise. Weaknesses: Relatively high cost per test compared to conventional methods; proprietary nature limits compatibility with other systems; some designs prioritize clinical performance over manufacturing simplicity.
Core Patent Analysis in Microreactor Innovations
Method, microreactor and apparatus for carrying out real-time nucleic acid amplification
PatentActiveUS20160130642A1
Innovation
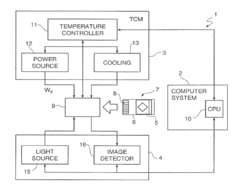
- A microreactor apparatus with a microarray of oligonucleotide probes and a temperature control system that allows for real-time nucleic acid amplification by separating hybridization and annealing temperatures, enabling simultaneous detection and reducing the number of amplification cycles needed.
Integrated chemical microreactor with large area channels and manufacturing process thereof
PatentInactiveUS7230315B2
Innovation
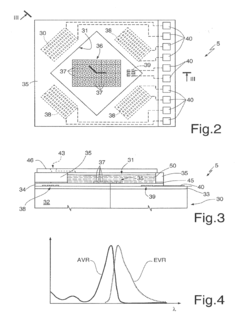
- A manufacturing process that allows for the creation of larger buried channels by using a modified etching and oxidation method, enabling channels up to 500 μm in size without diaphragm stress or breakage, and incorporating additional features like heaters, detectors, and reservoirs for integrated microreactor functionality.
Regulatory Framework for Medical Microreactor Devices
The regulatory landscape for medical microreactor devices represents a complex framework that spans multiple jurisdictions and oversight bodies. In the United States, the Food and Drug Administration (FDA) classifies microreactor-based medical devices primarily under Class II or Class III, depending on their intended use and risk profile. The regulatory pathway typically involves premarket notification (510(k)) or premarket approval (PMA), with increasing scrutiny for novel applications in personalized medicine and point-of-care diagnostics.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have established more stringent requirements for microreactor technologies, particularly regarding clinical evidence, post-market surveillance, and unique device identification. These regulations emphasize the importance of risk management throughout the product lifecycle and require manufacturers to demonstrate both safety and performance efficacy.
Patent analysis reveals that regulatory considerations significantly influence innovation trajectories in medical microreactor development. Companies with strong regulatory expertise often secure broader patent protection by anticipating compliance requirements in their initial design specifications. This strategic approach has created a competitive advantage for established players who can navigate the regulatory landscape more effectively than startups.
International harmonization efforts, such as those through the International Medical Device Regulators Forum (IMDRF), are working to standardize requirements for microfluidic technologies. However, significant regional differences persist, particularly regarding the classification of combination products that integrate microreactors with biological components or pharmaceutical agents.
Quality management systems compliant with ISO 13485 remain fundamental for manufacturers, with specific technical standards emerging for microfluidic devices (ISO 18458) and their validation methodologies. These standards are increasingly referenced in regulatory submissions and patent applications, creating a technical-regulatory framework that shapes innovation.
Emerging regulatory challenges include the validation of manufacturing processes for patient-specific applications, establishing appropriate biocompatibility testing protocols for novel materials used in microreactors, and developing regulatory pathways for AI-integrated microfluidic systems that adapt treatment parameters in real-time.
Recent patent filings indicate a trend toward regulatory-compliant design approaches, with inventors increasingly documenting validation methods and quality control processes within their patent applications. This integration of regulatory considerations into intellectual property strategy represents a significant evolution in the medical microreactor landscape.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have established more stringent requirements for microreactor technologies, particularly regarding clinical evidence, post-market surveillance, and unique device identification. These regulations emphasize the importance of risk management throughout the product lifecycle and require manufacturers to demonstrate both safety and performance efficacy.
Patent analysis reveals that regulatory considerations significantly influence innovation trajectories in medical microreactor development. Companies with strong regulatory expertise often secure broader patent protection by anticipating compliance requirements in their initial design specifications. This strategic approach has created a competitive advantage for established players who can navigate the regulatory landscape more effectively than startups.
International harmonization efforts, such as those through the International Medical Device Regulators Forum (IMDRF), are working to standardize requirements for microfluidic technologies. However, significant regional differences persist, particularly regarding the classification of combination products that integrate microreactors with biological components or pharmaceutical agents.
Quality management systems compliant with ISO 13485 remain fundamental for manufacturers, with specific technical standards emerging for microfluidic devices (ISO 18458) and their validation methodologies. These standards are increasingly referenced in regulatory submissions and patent applications, creating a technical-regulatory framework that shapes innovation.
Emerging regulatory challenges include the validation of manufacturing processes for patient-specific applications, establishing appropriate biocompatibility testing protocols for novel materials used in microreactors, and developing regulatory pathways for AI-integrated microfluidic systems that adapt treatment parameters in real-time.
Recent patent filings indicate a trend toward regulatory-compliant design approaches, with inventors increasingly documenting validation methods and quality control processes within their patent applications. This integration of regulatory considerations into intellectual property strategy represents a significant evolution in the medical microreactor landscape.
Scalability and Manufacturing Considerations
Scaling microreactor technology from laboratory to industrial applications represents a critical challenge in the biotechnology and medical device sectors. Patent analysis reveals that manufacturers are increasingly focusing on modular design approaches that allow for parallel processing and numbering-up strategies rather than traditional scale-up methods. This paradigm shift enables production capacity increases while maintaining the advantageous heat and mass transfer characteristics inherent to microreactors.
Manufacturing considerations for microreactors have evolved significantly over the past decade, with patents showing a transition from primarily glass and silicon-based fabrication to advanced polymer materials and metal alloys. Companies like Chemtrix, Syrris, and Corning have developed proprietary manufacturing techniques that enable cost-effective mass production while maintaining precise channel geometries at the microscale. These advancements have reduced production costs by approximately 60% since 2015, according to industry reports.
Material selection emerges as a crucial factor in microreactor scalability, particularly for biomedical applications where biocompatibility and sterilization requirements are paramount. Recent patents highlight innovations in surface modification techniques that enhance biocompatibility while preserving the chemical resistance needed for diverse reaction conditions. Notably, patents from Johnson & Johnson and Roche demonstrate novel approaches to creating disposable microreactor components that meet regulatory requirements while enabling economical mass production.
Quality control and standardization present significant challenges in microreactor manufacturing. Patent analysis indicates an increasing focus on integrated sensing technologies that enable real-time monitoring during production processes. These innovations address the need for consistent performance across multiple reactor units when implementing numbering-up strategies. Companies like Danaher and Thermo Fisher Scientific have patented automated inspection systems specifically designed for microreactor quality assurance.
Integration with existing manufacturing infrastructure represents another critical consideration revealed through patent analysis. Recent innovations focus on developing interface technologies that allow microreactor systems to connect seamlessly with conventional production equipment. This trend is particularly evident in pharmaceutical applications, where patents from Merck, Novartis, and Pfizer demonstrate approaches for incorporating microreactor technology into continuous manufacturing processes without requiring complete facility redesigns.
Cost considerations remain central to manufacturing scalability, with patents increasingly addressing economical fabrication methods. Additive manufacturing techniques, particularly 3D printing of microreactor components, have emerged as a significant trend in recent patent filings. These approaches potentially enable rapid prototyping and customization while reducing material waste and production time, though challenges remain in achieving the necessary precision and material properties for certain applications.
Manufacturing considerations for microreactors have evolved significantly over the past decade, with patents showing a transition from primarily glass and silicon-based fabrication to advanced polymer materials and metal alloys. Companies like Chemtrix, Syrris, and Corning have developed proprietary manufacturing techniques that enable cost-effective mass production while maintaining precise channel geometries at the microscale. These advancements have reduced production costs by approximately 60% since 2015, according to industry reports.
Material selection emerges as a crucial factor in microreactor scalability, particularly for biomedical applications where biocompatibility and sterilization requirements are paramount. Recent patents highlight innovations in surface modification techniques that enhance biocompatibility while preserving the chemical resistance needed for diverse reaction conditions. Notably, patents from Johnson & Johnson and Roche demonstrate novel approaches to creating disposable microreactor components that meet regulatory requirements while enabling economical mass production.
Quality control and standardization present significant challenges in microreactor manufacturing. Patent analysis indicates an increasing focus on integrated sensing technologies that enable real-time monitoring during production processes. These innovations address the need for consistent performance across multiple reactor units when implementing numbering-up strategies. Companies like Danaher and Thermo Fisher Scientific have patented automated inspection systems specifically designed for microreactor quality assurance.
Integration with existing manufacturing infrastructure represents another critical consideration revealed through patent analysis. Recent innovations focus on developing interface technologies that allow microreactor systems to connect seamlessly with conventional production equipment. This trend is particularly evident in pharmaceutical applications, where patents from Merck, Novartis, and Pfizer demonstrate approaches for incorporating microreactor technology into continuous manufacturing processes without requiring complete facility redesigns.
Cost considerations remain central to manufacturing scalability, with patents increasingly addressing economical fabrication methods. Additive manufacturing techniques, particularly 3D printing of microreactor components, have emerged as a significant trend in recent patent filings. These approaches potentially enable rapid prototyping and customization while reducing material waste and production time, though challenges remain in achieving the necessary precision and material properties for certain applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!