Research on Microreactors in Supercritical Fluid Chemistry
SEP 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microreactor Technology Background and Objectives
Microreactors represent a revolutionary approach in chemical engineering, characterized by their miniaturized reaction channels with dimensions typically ranging from tens to hundreds of micrometers. The concept emerged in the late 1990s as part of the broader field of microfluidics, gaining significant momentum in the early 2000s with pioneering work from research institutions in Germany, Japan, and the United States. The evolution of this technology has been driven by the increasing demand for more efficient, sustainable, and precise chemical processes across various industries.
The integration of microreactor technology with supercritical fluid chemistry presents a particularly promising frontier. Supercritical fluids—substances at temperatures and pressures above their critical points—exhibit unique properties that combine aspects of both liquids and gases. These properties include enhanced diffusivity, low viscosity, and tunable solvent characteristics, making them ideal media for various chemical transformations. The historical trajectory of this integration began around 2005, with significant advancements occurring in the past decade.
The primary technical objectives in this field encompass several dimensions. First, researchers aim to optimize microreactor designs specifically for supercritical fluid conditions, addressing challenges related to high-pressure containment, material compatibility, and thermal management. Second, there is a focus on enhancing mass and heat transfer efficiencies, which are critical advantages of microreactor systems but require special consideration under supercritical conditions.
Another key objective involves developing robust control systems for precise manipulation of reaction parameters in these extreme environments. This includes real-time monitoring capabilities and feedback mechanisms to maintain optimal conditions throughout the reaction process. Additionally, researchers are working toward scalable manufacturing approaches that would enable industrial implementation while preserving the inherent advantages of microreactor technology.
The technological trajectory suggests a convergence with other emerging fields, including advanced materials science, artificial intelligence for process optimization, and sustainable chemistry principles. This convergence is expected to accelerate innovation and expand application possibilities. Current research indicates that microreactors in supercritical fluid chemistry could revolutionize pharmaceutical manufacturing, fine chemical synthesis, and biomass conversion processes.
Looking forward, the field is moving toward multi-functional microreactor systems capable of performing sequential reactions in supercritical media, potentially transforming traditional batch processes into continuous, intensified operations. The ultimate goal remains developing commercially viable technologies that offer significant advantages in terms of efficiency, selectivity, and environmental impact compared to conventional approaches.
The integration of microreactor technology with supercritical fluid chemistry presents a particularly promising frontier. Supercritical fluids—substances at temperatures and pressures above their critical points—exhibit unique properties that combine aspects of both liquids and gases. These properties include enhanced diffusivity, low viscosity, and tunable solvent characteristics, making them ideal media for various chemical transformations. The historical trajectory of this integration began around 2005, with significant advancements occurring in the past decade.
The primary technical objectives in this field encompass several dimensions. First, researchers aim to optimize microreactor designs specifically for supercritical fluid conditions, addressing challenges related to high-pressure containment, material compatibility, and thermal management. Second, there is a focus on enhancing mass and heat transfer efficiencies, which are critical advantages of microreactor systems but require special consideration under supercritical conditions.
Another key objective involves developing robust control systems for precise manipulation of reaction parameters in these extreme environments. This includes real-time monitoring capabilities and feedback mechanisms to maintain optimal conditions throughout the reaction process. Additionally, researchers are working toward scalable manufacturing approaches that would enable industrial implementation while preserving the inherent advantages of microreactor technology.
The technological trajectory suggests a convergence with other emerging fields, including advanced materials science, artificial intelligence for process optimization, and sustainable chemistry principles. This convergence is expected to accelerate innovation and expand application possibilities. Current research indicates that microreactors in supercritical fluid chemistry could revolutionize pharmaceutical manufacturing, fine chemical synthesis, and biomass conversion processes.
Looking forward, the field is moving toward multi-functional microreactor systems capable of performing sequential reactions in supercritical media, potentially transforming traditional batch processes into continuous, intensified operations. The ultimate goal remains developing commercially viable technologies that offer significant advantages in terms of efficiency, selectivity, and environmental impact compared to conventional approaches.
Market Analysis for Supercritical Fluid Applications
The global market for supercritical fluid applications has experienced significant growth over the past decade, driven by increasing demand for environmentally friendly processing technologies across various industries. The current market size is estimated at approximately 6.2 billion USD, with projections indicating a compound annual growth rate of 8.3% through 2028. This growth trajectory is primarily fueled by expanding applications in pharmaceutical manufacturing, food processing, and materials science.
Supercritical CO2 (scCO2) dominates the market, accounting for nearly 70% of all supercritical fluid applications due to its relatively mild critical conditions, non-toxicity, and status as a green solvent. The pharmaceutical sector represents the largest end-user segment, where supercritical fluid extraction and chromatography have become essential techniques for producing high-purity compounds and reducing solvent waste.
Regional analysis reveals that North America currently leads the market with approximately 35% share, followed closely by Europe at 30%. However, the Asia-Pacific region is experiencing the fastest growth rate, particularly in China, Japan, and South Korea, where significant investments in green chemistry infrastructure are being made. This regional shift is expected to reshape market dynamics over the next five years.
The integration of microreactor technology with supercritical fluids represents a high-growth niche within this broader market. This segment is currently valued at approximately 850 million USD and is projected to grow at 12.7% annually, outpacing the overall supercritical fluid market. The enhanced control over reaction parameters and improved safety profiles offered by microreactors are driving adoption in high-value applications.
Key market drivers include increasingly stringent environmental regulations limiting the use of conventional organic solvents, growing consumer demand for "clean label" products in food and cosmetics, and the pharmaceutical industry's push toward continuous manufacturing processes. The cost-effectiveness of supercritical fluid processes at scale has also improved significantly, reducing barriers to commercial adoption.
Market challenges persist, including high initial capital investment requirements for supercritical fluid equipment, technical expertise needed for operation, and competition from established conventional technologies. Additionally, the specialized nature of microreactor design for supercritical conditions presents both a market opportunity and an adoption hurdle.
Emerging application areas showing promising growth include nanomaterial synthesis, biofuel processing, and advanced polymer production, where the unique properties of supercritical fluids combined with microreactor technology offer significant advantages over batch processing methods.
Supercritical CO2 (scCO2) dominates the market, accounting for nearly 70% of all supercritical fluid applications due to its relatively mild critical conditions, non-toxicity, and status as a green solvent. The pharmaceutical sector represents the largest end-user segment, where supercritical fluid extraction and chromatography have become essential techniques for producing high-purity compounds and reducing solvent waste.
Regional analysis reveals that North America currently leads the market with approximately 35% share, followed closely by Europe at 30%. However, the Asia-Pacific region is experiencing the fastest growth rate, particularly in China, Japan, and South Korea, where significant investments in green chemistry infrastructure are being made. This regional shift is expected to reshape market dynamics over the next five years.
The integration of microreactor technology with supercritical fluids represents a high-growth niche within this broader market. This segment is currently valued at approximately 850 million USD and is projected to grow at 12.7% annually, outpacing the overall supercritical fluid market. The enhanced control over reaction parameters and improved safety profiles offered by microreactors are driving adoption in high-value applications.
Key market drivers include increasingly stringent environmental regulations limiting the use of conventional organic solvents, growing consumer demand for "clean label" products in food and cosmetics, and the pharmaceutical industry's push toward continuous manufacturing processes. The cost-effectiveness of supercritical fluid processes at scale has also improved significantly, reducing barriers to commercial adoption.
Market challenges persist, including high initial capital investment requirements for supercritical fluid equipment, technical expertise needed for operation, and competition from established conventional technologies. Additionally, the specialized nature of microreactor design for supercritical conditions presents both a market opportunity and an adoption hurdle.
Emerging application areas showing promising growth include nanomaterial synthesis, biofuel processing, and advanced polymer production, where the unique properties of supercritical fluids combined with microreactor technology offer significant advantages over batch processing methods.
Current Challenges in Supercritical Fluid Microreactors
Despite the promising potential of supercritical fluid (SCF) microreactors in chemical synthesis and processing, several significant challenges currently impede their widespread industrial adoption and optimal performance. The primary challenge lies in the design and fabrication of microreactors capable of withstanding the extreme pressure conditions required for supercritical fluids. Most SCFs operate at pressures exceeding 73 bar (for CO2), necessitating specialized materials and robust engineering solutions that can maintain structural integrity while allowing for efficient heat transfer and chemical reactions.
Material compatibility presents another substantial hurdle. The combination of high pressure, potentially corrosive reaction media, and temperature fluctuations creates a demanding environment for reactor materials. Conventional materials often suffer from degradation, leading to reduced operational lifetimes and potential safety hazards. While specialized alloys and ceramics offer improved resistance, they significantly increase manufacturing costs and may introduce fabrication complexities.
Temperature control and heat management remain persistent challenges in SCF microreactor systems. The unique heat transfer properties of supercritical fluids, which differ substantially from conventional liquids and gases, complicate precise temperature regulation. This is particularly problematic for reactions with narrow temperature windows or those requiring specific thermal gradients for optimal selectivity and yield.
Flow control and mixing efficiency represent critical operational challenges. The distinctive fluid dynamics of supercritical media—characterized by lower viscosity and higher diffusivity than liquids but higher density than gases—create complex flow patterns within microchannels. Achieving uniform mixing while maintaining controlled residence times requires sophisticated channel designs and flow management strategies that are still being optimized.
Scaling issues continue to limit industrial implementation. While laboratory-scale demonstrations have shown promising results, translating these successes to production-relevant throughput remains problematic. The challenge lies in maintaining the advantages of microreactors (enhanced mass and heat transfer, precise control) while increasing production capacity through numbering-up or scaling-out approaches.
Monitoring and analytical techniques for real-time process control present significant technical barriers. The high-pressure environment complicates the integration of sensors and analytical tools, making it difficult to implement process analytical technology (PAT) for continuous monitoring and feedback control. This limitation hinders both research advancement and quality assurance in potential industrial applications.
Regulatory and safety concerns further complicate development efforts. The combination of high pressures, potentially hazardous chemicals, and novel technology creates a complex regulatory landscape that lacks standardized protocols and safety guidelines specific to SCF microreactor technology.
Material compatibility presents another substantial hurdle. The combination of high pressure, potentially corrosive reaction media, and temperature fluctuations creates a demanding environment for reactor materials. Conventional materials often suffer from degradation, leading to reduced operational lifetimes and potential safety hazards. While specialized alloys and ceramics offer improved resistance, they significantly increase manufacturing costs and may introduce fabrication complexities.
Temperature control and heat management remain persistent challenges in SCF microreactor systems. The unique heat transfer properties of supercritical fluids, which differ substantially from conventional liquids and gases, complicate precise temperature regulation. This is particularly problematic for reactions with narrow temperature windows or those requiring specific thermal gradients for optimal selectivity and yield.
Flow control and mixing efficiency represent critical operational challenges. The distinctive fluid dynamics of supercritical media—characterized by lower viscosity and higher diffusivity than liquids but higher density than gases—create complex flow patterns within microchannels. Achieving uniform mixing while maintaining controlled residence times requires sophisticated channel designs and flow management strategies that are still being optimized.
Scaling issues continue to limit industrial implementation. While laboratory-scale demonstrations have shown promising results, translating these successes to production-relevant throughput remains problematic. The challenge lies in maintaining the advantages of microreactors (enhanced mass and heat transfer, precise control) while increasing production capacity through numbering-up or scaling-out approaches.
Monitoring and analytical techniques for real-time process control present significant technical barriers. The high-pressure environment complicates the integration of sensors and analytical tools, making it difficult to implement process analytical technology (PAT) for continuous monitoring and feedback control. This limitation hinders both research advancement and quality assurance in potential industrial applications.
Regulatory and safety concerns further complicate development efforts. The combination of high pressures, potentially hazardous chemicals, and novel technology creates a complex regulatory landscape that lacks standardized protocols and safety guidelines specific to SCF microreactor technology.
Current Microreactor Designs for Supercritical Conditions
01 Design and fabrication of microreactors for supercritical fluid applications
Specialized microreactors are designed and fabricated to withstand the high pressures and temperatures required for supercritical fluid chemistry. These microreactors often incorporate materials like silicon, glass, or metals with high pressure tolerance. The fabrication techniques include micromachining, etching, and bonding processes to create channels and chambers that can safely contain supercritical fluids while facilitating efficient heat and mass transfer.- Microreactor design for supercritical fluid applications: Specialized microreactor designs that can withstand high pressure and temperature conditions required for supercritical fluid chemistry. These designs incorporate robust materials, precise flow control mechanisms, and specialized sealing technologies to maintain supercritical conditions while enabling efficient heat and mass transfer. The compact nature of these reactors allows for precise control of reaction parameters and improved safety when working with supercritical fluids.
- Continuous flow synthesis in supercritical media: Continuous flow processes using microreactors for chemical synthesis in supercritical fluids offer advantages over batch processes, including better control of reaction conditions, improved heat transfer, and enhanced mass transfer. These systems enable rapid mixing, precise residence time control, and efficient scale-up possibilities. The combination of microreactor technology with supercritical fluids allows for greener chemistry approaches with reduced solvent use and improved reaction efficiency.
- Nanomaterial synthesis using supercritical fluid microreactors: Microreactors operating with supercritical fluids provide unique environments for synthesizing nanomaterials with controlled size, morphology, and composition. The unique properties of supercritical fluids, including high diffusivity, low viscosity, and tunable solvent properties, enable precise control over nucleation and growth processes. These systems are particularly valuable for producing high-quality nanoparticles, thin films, and other nanomaterials for applications in electronics, catalysis, and biomedicine.
- Extraction and separation processes using supercritical fluid microreactors: Microreactors designed for supercritical fluid extraction and separation processes offer enhanced efficiency compared to conventional methods. These systems utilize the unique solvent properties of supercritical fluids that can be precisely tuned by adjusting temperature and pressure. The high surface-to-volume ratio in microreactors improves mass transfer rates and extraction efficiency, making them suitable for applications in pharmaceutical processing, natural product isolation, and environmental remediation.
- Catalytic reactions in supercritical fluid microreactors: Microreactors provide an ideal platform for conducting catalytic reactions in supercritical fluid environments. The enhanced mass transfer properties of supercritical fluids combined with the high surface area and precise temperature control of microreactors result in improved catalytic performance. These systems allow for efficient catalyst immobilization, reduced catalyst deactivation, and simplified product separation. Applications include hydrogenation, oxidation, and various organic transformations with improved yields and selectivity.
02 Reaction enhancement using supercritical fluids in microreactors
Supercritical fluids in microreactors offer unique properties that enhance chemical reactions, including increased diffusion rates, reduced mass transfer limitations, and tunable solvent properties. These characteristics lead to faster reaction kinetics, higher yields, and improved selectivity compared to conventional solvents. The combination of supercritical conditions with the precise control offered by microreactors enables novel reaction pathways and more efficient chemical transformations.Expand Specific Solutions03 Flow control and pressure management in supercritical microreactors
Effective flow control and pressure management are critical in supercritical fluid microreactors. These systems incorporate specialized pumps, valves, and pressure regulators to maintain stable supercritical conditions. Advanced monitoring systems provide real-time feedback for precise control of flow rates, residence times, and pressure profiles throughout the microreactor. These control mechanisms ensure safe operation while optimizing reaction conditions for desired chemical transformations.Expand Specific Solutions04 Analytical and monitoring techniques for supercritical microreactor systems
Specialized analytical and monitoring techniques are integrated with supercritical fluid microreactors to enable real-time analysis of reaction progress and product formation. These include in-line spectroscopic methods, microscale sensors, and advanced imaging techniques that can operate under high-pressure conditions. The integration of these analytical tools with microreactor technology allows for rapid optimization of reaction parameters and facilitates process intensification in supercritical fluid chemistry.Expand Specific Solutions05 Applications of supercritical fluid microreactors in synthesis and processing
Supercritical fluid microreactors find diverse applications in chemical synthesis, materials processing, and green chemistry. They are particularly valuable for nanoparticle synthesis, pharmaceutical production, and environmentally friendly chemical transformations. The unique combination of microreactor technology with supercritical fluids enables continuous processing, reduces waste generation, improves product quality, and facilitates scale-up of laboratory processes to industrial production while maintaining precise control over reaction conditions.Expand Specific Solutions
Leading Organizations in Supercritical Microreactor Development
The microreactor technology in supercritical fluid chemistry is currently in a growth phase, with an estimated global market size of $300-500 million and expanding at 15-20% annually. The competitive landscape features established industrial players like Corning, Lonza, and China Petroleum & Chemical Corp developing commercial applications, while academic institutions such as Xi'an Jiaotong University, Zhejiang University, and California Institute of Technology drive fundamental research innovations. The technology maturity varies across sectors, with pharmaceutical applications (led by FUJIFILM and Lonza) being most advanced, while chemical synthesis applications are still evolving. Research collaborations between industry leaders and academic institutions are accelerating development, particularly in green chemistry applications and process intensification methodologies.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute of Chemical Physics (DICP) has pioneered microreactor technology for supercritical fluid chemistry, developing integrated continuous flow systems that operate under high pressure (up to 30 MPa) and temperature conditions (up to 450°C). Their microreactor designs feature specialized microchannel configurations with enhanced mass and heat transfer capabilities, allowing for precise control of reaction parameters in supercritical CO2 and water environments. DICP has successfully implemented these systems for various applications including catalytic oxidation reactions, biomass conversion, and green synthesis of nanomaterials. Their microreactors incorporate in-situ monitoring capabilities through integrated sensors and spectroscopic techniques, enabling real-time reaction optimization and kinetic studies. The institute has also developed novel catalyst immobilization techniques specifically designed for supercritical fluid conditions, enhancing reaction efficiency while maintaining catalyst stability under extreme operating conditions.
Strengths: Superior expertise in high-pressure microreactor engineering; extensive experience with catalyst systems optimized for supercritical conditions; strong integration of monitoring technologies. Weaknesses: Some systems require specialized materials that increase manufacturing costs; scaling up from laboratory to industrial applications presents engineering challenges.
China Petroleum & Chemical Corp.
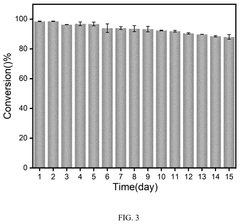
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed proprietary microreactor technology for supercritical fluid applications focused on petrochemical processing and green chemistry initiatives. Their microreactor systems utilize specialized corrosion-resistant alloys capable of withstanding supercritical water conditions (>374°C, >22.1 MPa) for extended operational periods. Sinopec's technology incorporates multi-channel parallel microreactor arrays that significantly enhance throughput while maintaining the benefits of microscale processing. The company has implemented these systems for supercritical water oxidation of industrial waste streams, achieving organic compound removal efficiencies exceeding 99.9% with residence times under 30 seconds. Their microreactors feature modular designs that allow for flexible configuration and scaling, with integrated heat recovery systems that improve energy efficiency by up to 40% compared to conventional batch processes. Sinopec has also pioneered the use of these microreactors for supercritical fluid extraction of high-value compounds from petroleum residues.
Strengths: Robust engineering suitable for industrial-scale implementation; excellent integration with existing petrochemical infrastructure; proven long-term durability under extreme conditions. Weaknesses: Systems are primarily optimized for petrochemical applications rather than fine chemicals; high initial capital investment requirements.
Key Patents and Innovations in SCF Microreactor Technology
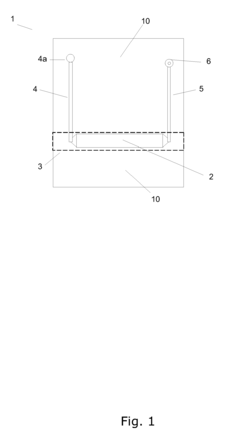
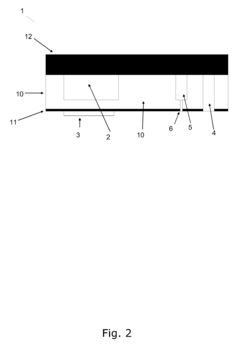
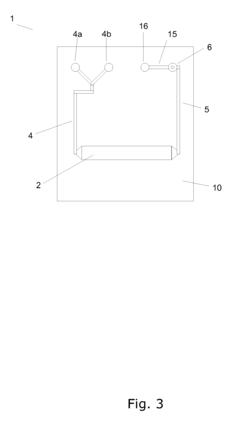
A microreactor
PatentInactiveEP1897612A1
Innovation
- A microreactor design where the reaction chamber, heating element, and capillary hole for pressure reduction are integrally formed in a single monolithic element, allowing for efficient heating and cooling without the need for forced cooling or high power consumption, enabling fast real-time access to chemical reactions.
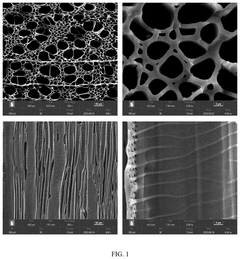
Carbon-based microreactor, and preparation method and application thereof
PatentActiveUS20250128233A1
Innovation
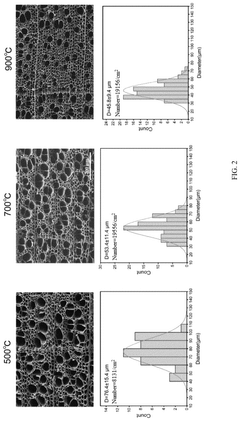
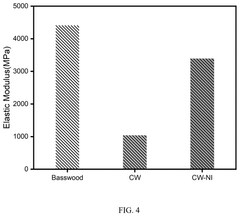
- A carbon-based microreactor is developed using microchannel structures of natural wood, which undergoes carbonization and catalyst immobilization, resulting in a reactor with regular channels, adjustable size, high stability, and low production costs.
Materials Science Advancements for High-Pressure Systems
The development of microreactors for supercritical fluid chemistry has necessitated significant advancements in materials science to withstand extreme operating conditions. Traditional materials often fail under the high pressures (typically 73-300 bar) and temperatures required for supercritical fluid processes, driving innovation in this specialized field.
High-strength alloys have emerged as critical components in microreactor construction, with nickel-based superalloys like Inconel 718 and Hastelloy C-276 demonstrating exceptional resistance to both mechanical stress and chemical corrosion. These materials maintain structural integrity at pressures exceeding 200 bar while resisting the oxidative effects of supercritical water and CO2, the most commonly used supercritical fluids in chemical processing.
Surface engineering techniques have revolutionized microreactor performance through the application of specialized coatings. Silicon carbide (SiC) and titanium nitride (TiN) coatings provide enhanced chemical resistance while minimizing catalytic side reactions that can compromise product purity. Recent developments in atomic layer deposition methods allow for nanometer-precision coating application, ensuring uniform protection even in complex microreactor channel geometries.
Composite materials represent another frontier, with carbon fiber reinforced polymers (CFRPs) offering an optimal balance of weight reduction and pressure resistance. For laboratory-scale applications, these materials enable the development of portable supercritical fluid systems while maintaining safety standards. Ceramic-metal composites (cermets) combine the thermal stability of ceramics with the toughness of metals, addressing the thermal cycling challenges inherent in supercritical processes.
Advanced manufacturing techniques have transformed microreactor fabrication capabilities. Selective laser melting (SLM) and direct metal laser sintering (DMLS) enable the production of complex internal geometries with integrated cooling channels that were previously impossible to manufacture. These techniques allow for optimized heat transfer while maintaining structural integrity under high-pressure conditions.
Monitoring and sensing materials have also evolved to meet the demands of supercritical environments. Sapphire windows resistant to pressures above 300 bar now enable real-time optical monitoring of reactions. Similarly, specialized pressure transducers incorporating silicon carbide sensing elements provide accurate measurements in corrosive supercritical environments, enhancing both safety and process control capabilities.
The integration of these material advances has enabled a new generation of microreactors capable of operating reliably in supercritical conditions, opening new possibilities for green chemistry applications, pharmaceutical synthesis, and nanomaterial production under previously inaccessible reaction conditions.
High-strength alloys have emerged as critical components in microreactor construction, with nickel-based superalloys like Inconel 718 and Hastelloy C-276 demonstrating exceptional resistance to both mechanical stress and chemical corrosion. These materials maintain structural integrity at pressures exceeding 200 bar while resisting the oxidative effects of supercritical water and CO2, the most commonly used supercritical fluids in chemical processing.
Surface engineering techniques have revolutionized microreactor performance through the application of specialized coatings. Silicon carbide (SiC) and titanium nitride (TiN) coatings provide enhanced chemical resistance while minimizing catalytic side reactions that can compromise product purity. Recent developments in atomic layer deposition methods allow for nanometer-precision coating application, ensuring uniform protection even in complex microreactor channel geometries.
Composite materials represent another frontier, with carbon fiber reinforced polymers (CFRPs) offering an optimal balance of weight reduction and pressure resistance. For laboratory-scale applications, these materials enable the development of portable supercritical fluid systems while maintaining safety standards. Ceramic-metal composites (cermets) combine the thermal stability of ceramics with the toughness of metals, addressing the thermal cycling challenges inherent in supercritical processes.
Advanced manufacturing techniques have transformed microreactor fabrication capabilities. Selective laser melting (SLM) and direct metal laser sintering (DMLS) enable the production of complex internal geometries with integrated cooling channels that were previously impossible to manufacture. These techniques allow for optimized heat transfer while maintaining structural integrity under high-pressure conditions.
Monitoring and sensing materials have also evolved to meet the demands of supercritical environments. Sapphire windows resistant to pressures above 300 bar now enable real-time optical monitoring of reactions. Similarly, specialized pressure transducers incorporating silicon carbide sensing elements provide accurate measurements in corrosive supercritical environments, enhancing both safety and process control capabilities.
The integration of these material advances has enabled a new generation of microreactors capable of operating reliably in supercritical conditions, opening new possibilities for green chemistry applications, pharmaceutical synthesis, and nanomaterial production under previously inaccessible reaction conditions.
Sustainability Impact of Microreactor SCF Processes
The integration of microreactor technology with supercritical fluid (SCF) processes represents a significant advancement in sustainable chemical processing. These systems offer substantial environmental benefits compared to conventional batch reactors, primarily through dramatic reductions in solvent usage. Microreactor SCF processes typically require only 10-15% of the solvent volume needed in traditional systems, directly reducing chemical waste and minimizing environmental contamination risks.
Energy efficiency stands as another critical sustainability advantage. The enhanced heat and mass transfer characteristics of microreactors, combined with the unique properties of supercritical fluids, result in energy consumption reductions of up to 40% compared to conventional methods. This efficiency derives from precise temperature control, reduced heating requirements for smaller volumes, and the elimination of energy-intensive separation steps often required in traditional processes.
Carbon footprint reduction represents a quantifiable sustainability impact of microreactor SCF technology. Studies indicate that implementing these systems can reduce greenhouse gas emissions by 30-60% across various chemical processes. This reduction stems from lower energy requirements, decreased transportation needs due to potential for distributed manufacturing, and the ability to operate continuous processes that eliminate energy-intensive startup and shutdown cycles.
From a resource conservation perspective, microreactor SCF processes demonstrate exceptional atom economy and yield improvements. The precise reaction control enables higher selectivity, reducing byproduct formation and increasing desired product yields by 15-25% in many applications. This efficiency translates directly to reduced raw material consumption and waste generation throughout the chemical value chain.
Water conservation benefits are particularly noteworthy in pharmaceutical and fine chemical applications. Traditional batch processes often require significant water volumes for cleaning and cooling, while microreactor SCF systems can reduce water usage by up to 80%. Additionally, the closed nature of these systems minimizes contaminated wastewater generation, further reducing environmental impact.
The life cycle assessment (LCA) of microreactor SCF technology reveals comprehensive sustainability advantages beyond immediate process benefits. These systems typically demonstrate 40-70% lower environmental impact scores across multiple categories including acidification potential, eutrophication potential, and human toxicity. Their compact footprint also reduces land use requirements for chemical processing facilities, supporting more sustainable industrial development patterns.
Energy efficiency stands as another critical sustainability advantage. The enhanced heat and mass transfer characteristics of microreactors, combined with the unique properties of supercritical fluids, result in energy consumption reductions of up to 40% compared to conventional methods. This efficiency derives from precise temperature control, reduced heating requirements for smaller volumes, and the elimination of energy-intensive separation steps often required in traditional processes.
Carbon footprint reduction represents a quantifiable sustainability impact of microreactor SCF technology. Studies indicate that implementing these systems can reduce greenhouse gas emissions by 30-60% across various chemical processes. This reduction stems from lower energy requirements, decreased transportation needs due to potential for distributed manufacturing, and the ability to operate continuous processes that eliminate energy-intensive startup and shutdown cycles.
From a resource conservation perspective, microreactor SCF processes demonstrate exceptional atom economy and yield improvements. The precise reaction control enables higher selectivity, reducing byproduct formation and increasing desired product yields by 15-25% in many applications. This efficiency translates directly to reduced raw material consumption and waste generation throughout the chemical value chain.
Water conservation benefits are particularly noteworthy in pharmaceutical and fine chemical applications. Traditional batch processes often require significant water volumes for cleaning and cooling, while microreactor SCF systems can reduce water usage by up to 80%. Additionally, the closed nature of these systems minimizes contaminated wastewater generation, further reducing environmental impact.
The life cycle assessment (LCA) of microreactor SCF technology reveals comprehensive sustainability advantages beyond immediate process benefits. These systems typically demonstrate 40-70% lower environmental impact scores across multiple categories including acidification potential, eutrophication potential, and human toxicity. Their compact footprint also reduces land use requirements for chemical processing facilities, supporting more sustainable industrial development patterns.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!