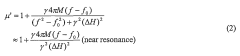Microreactors in High-Throughput Screening of Catalysts and Enzymes
SEP 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Microreactor Technology Evolution and Objectives
Microreactor technology has evolved significantly over the past three decades, transforming from laboratory curiosities to powerful tools for chemical and biochemical process intensification. The initial development in the 1990s focused primarily on proof-of-concept demonstrations, with simple channel structures etched in glass or silicon substrates. These early microreactors offered limited functionality but established the fundamental principles of miniaturized reaction systems.
The early 2000s marked a pivotal transition as microreactor technology began incorporating advanced fabrication techniques, including soft lithography, 3D printing, and precision micromachining. This period saw the emergence of more sophisticated designs with integrated sensors, mixers, and temperature control systems, significantly enhancing their utility for screening applications.
By the 2010s, microreactor technology had matured considerably, with commercial systems becoming available for specific applications in pharmaceutical development, fine chemicals synthesis, and biocatalysis. The integration of automation and high-throughput capabilities transformed these devices into powerful platforms for parallel experimentation, capable of generating vast datasets under precisely controlled conditions.
Recent technological advances have focused on creating modular, reconfigurable microreactor systems that can be rapidly adapted to different reaction conditions and catalyst/enzyme screening protocols. The incorporation of advanced analytical techniques, including in-line spectroscopy, mass spectrometry, and microscopy, has further enhanced the information density obtainable from microreactor experiments.
The primary objective of microreactor technology in high-throughput screening applications is to accelerate the discovery and optimization of catalysts and enzymes by enabling rapid, parallel evaluation of multiple candidates under precisely controlled conditions. This approach aims to overcome the limitations of traditional batch screening methods, which are often time-consuming, material-intensive, and prone to inconsistencies.
Additional objectives include minimizing reagent consumption through microscale operation, enhancing safety by containing potentially hazardous reactions within enclosed microchannels, and improving reproducibility through precise control of reaction parameters such as temperature, residence time, and mixing efficiency.
Looking forward, the field is moving toward fully integrated "lab-on-a-chip" systems that combine reaction, separation, and analysis functions within a single device. The convergence of microreactor technology with artificial intelligence and machine learning approaches represents a particularly promising direction, potentially enabling autonomous experimental design and execution for catalyst and enzyme discovery.
The early 2000s marked a pivotal transition as microreactor technology began incorporating advanced fabrication techniques, including soft lithography, 3D printing, and precision micromachining. This period saw the emergence of more sophisticated designs with integrated sensors, mixers, and temperature control systems, significantly enhancing their utility for screening applications.
By the 2010s, microreactor technology had matured considerably, with commercial systems becoming available for specific applications in pharmaceutical development, fine chemicals synthesis, and biocatalysis. The integration of automation and high-throughput capabilities transformed these devices into powerful platforms for parallel experimentation, capable of generating vast datasets under precisely controlled conditions.
Recent technological advances have focused on creating modular, reconfigurable microreactor systems that can be rapidly adapted to different reaction conditions and catalyst/enzyme screening protocols. The incorporation of advanced analytical techniques, including in-line spectroscopy, mass spectrometry, and microscopy, has further enhanced the information density obtainable from microreactor experiments.
The primary objective of microreactor technology in high-throughput screening applications is to accelerate the discovery and optimization of catalysts and enzymes by enabling rapid, parallel evaluation of multiple candidates under precisely controlled conditions. This approach aims to overcome the limitations of traditional batch screening methods, which are often time-consuming, material-intensive, and prone to inconsistencies.
Additional objectives include minimizing reagent consumption through microscale operation, enhancing safety by containing potentially hazardous reactions within enclosed microchannels, and improving reproducibility through precise control of reaction parameters such as temperature, residence time, and mixing efficiency.
Looking forward, the field is moving toward fully integrated "lab-on-a-chip" systems that combine reaction, separation, and analysis functions within a single device. The convergence of microreactor technology with artificial intelligence and machine learning approaches represents a particularly promising direction, potentially enabling autonomous experimental design and execution for catalyst and enzyme discovery.
Market Analysis for High-Throughput Screening Solutions
The high-throughput screening (HTS) market for catalyst and enzyme discovery has experienced substantial growth over the past decade, driven by increasing demands in pharmaceutical, chemical, and biotechnology industries. The global market for HTS solutions reached approximately $19.5 billion in 2022, with a compound annual growth rate (CAGR) of 7.2% projected through 2028.
Microreactor-based HTS technologies represent a rapidly expanding segment within this market, currently valued at $3.2 billion and expected to grow at 9.8% annually. This accelerated growth stems from the superior efficiency, reduced reagent consumption, and enhanced control parameters offered by microreactor platforms compared to traditional screening methods.
Pharmaceutical companies remain the largest end-users, accounting for 42% of the market share, followed by academic research institutions (27%), biotechnology companies (18%), and chemical manufacturers (13%). Geographically, North America dominates with 38% market share, followed by Europe (32%), Asia-Pacific (24%), and rest of the world (6%). The Asia-Pacific region, particularly China and India, demonstrates the fastest growth trajectory at 11.3% annually.
Key market drivers include increasing R&D investments in biocatalysis, growing emphasis on sustainable chemical processes, and rising demand for personalized medicine requiring rapid screening capabilities. The integration of artificial intelligence and machine learning with microreactor HTS platforms has created a premium market segment growing at 14.2% annually.
Customer demand patterns reveal strong preference for modular, scalable systems that can transition seamlessly from laboratory screening to production-scale applications. End-users increasingly prioritize systems offering comprehensive data analytics capabilities, with 78% of survey respondents identifying integrated data management as a critical purchasing factor.
Market challenges include high initial capital investment requirements, technical expertise barriers for operation, and regulatory complexities for pharmaceutical applications. The average return on investment period for microreactor HTS systems ranges from 14-18 months, depending on application intensity and industry sector.
Subscription-based business models are gaining traction, with approximately 35% of new installations now utilizing pay-per-use or leasing arrangements rather than outright purchases. This trend particularly benefits small and medium enterprises seeking access to advanced screening capabilities without prohibitive capital expenditures.
Microreactor-based HTS technologies represent a rapidly expanding segment within this market, currently valued at $3.2 billion and expected to grow at 9.8% annually. This accelerated growth stems from the superior efficiency, reduced reagent consumption, and enhanced control parameters offered by microreactor platforms compared to traditional screening methods.
Pharmaceutical companies remain the largest end-users, accounting for 42% of the market share, followed by academic research institutions (27%), biotechnology companies (18%), and chemical manufacturers (13%). Geographically, North America dominates with 38% market share, followed by Europe (32%), Asia-Pacific (24%), and rest of the world (6%). The Asia-Pacific region, particularly China and India, demonstrates the fastest growth trajectory at 11.3% annually.
Key market drivers include increasing R&D investments in biocatalysis, growing emphasis on sustainable chemical processes, and rising demand for personalized medicine requiring rapid screening capabilities. The integration of artificial intelligence and machine learning with microreactor HTS platforms has created a premium market segment growing at 14.2% annually.
Customer demand patterns reveal strong preference for modular, scalable systems that can transition seamlessly from laboratory screening to production-scale applications. End-users increasingly prioritize systems offering comprehensive data analytics capabilities, with 78% of survey respondents identifying integrated data management as a critical purchasing factor.
Market challenges include high initial capital investment requirements, technical expertise barriers for operation, and regulatory complexities for pharmaceutical applications. The average return on investment period for microreactor HTS systems ranges from 14-18 months, depending on application intensity and industry sector.
Subscription-based business models are gaining traction, with approximately 35% of new installations now utilizing pay-per-use or leasing arrangements rather than outright purchases. This trend particularly benefits small and medium enterprises seeking access to advanced screening capabilities without prohibitive capital expenditures.
Current Limitations in Catalyst and Enzyme Screening
Despite significant advancements in microreactor technology for high-throughput screening of catalysts and enzymes, several critical limitations continue to challenge researchers and industry professionals. One of the primary constraints is the scalability gap between microreactor screening conditions and industrial-scale applications. Reactions that perform optimally in microreactors often exhibit different kinetics, selectivity, and conversion rates when scaled up to production levels, creating a significant translational challenge for researchers.
Sample preparation and handling remain labor-intensive processes that limit true high-throughput capabilities. While microreactors themselves enable rapid testing, the upstream preparation of diverse catalyst or enzyme libraries frequently becomes a bottleneck, particularly when dealing with complex formulations or immobilization requirements. This preparation bottleneck significantly reduces the effective throughput of the entire screening process.
Detection and analytical limitations present another significant challenge. Real-time monitoring of reactions within microreactors is technically difficult due to the miniaturized dimensions and rapid reaction rates. Many analytical techniques require sample extraction or have insufficient sensitivity for the small volumes involved, creating delays between experimentation and result analysis that impede truly high-throughput workflows.
The physical constraints of microreactor design also pose limitations. Current fabrication techniques struggle to create uniform catalyst or enzyme distribution within microchannels, leading to potential hotspots, uneven flow patterns, and mass transfer limitations. These physical constraints can produce misleading screening results that fail to predict actual catalyst or enzyme performance under real-world conditions.
Data management and interpretation challenges have emerged as the volume of screening data has grown exponentially. Researchers often lack sophisticated data analysis tools capable of identifying complex patterns across multidimensional parameter spaces. This limitation frequently results in suboptimal utilization of the vast datasets generated through high-throughput screening campaigns.
Cost considerations remain significant, particularly for specialized microreactor systems with integrated analytical capabilities. The high initial investment required for advanced microreactor platforms limits their adoption in smaller research institutions and companies, creating an innovation gap between well-funded organizations and those with more limited resources.
Standardization issues further complicate the field, with various research groups employing different microreactor designs, operating protocols, and data reporting formats. This lack of standardization makes cross-comparison of results challenging and hinders collaborative advancement of the technology across the scientific community.
Sample preparation and handling remain labor-intensive processes that limit true high-throughput capabilities. While microreactors themselves enable rapid testing, the upstream preparation of diverse catalyst or enzyme libraries frequently becomes a bottleneck, particularly when dealing with complex formulations or immobilization requirements. This preparation bottleneck significantly reduces the effective throughput of the entire screening process.
Detection and analytical limitations present another significant challenge. Real-time monitoring of reactions within microreactors is technically difficult due to the miniaturized dimensions and rapid reaction rates. Many analytical techniques require sample extraction or have insufficient sensitivity for the small volumes involved, creating delays between experimentation and result analysis that impede truly high-throughput workflows.
The physical constraints of microreactor design also pose limitations. Current fabrication techniques struggle to create uniform catalyst or enzyme distribution within microchannels, leading to potential hotspots, uneven flow patterns, and mass transfer limitations. These physical constraints can produce misleading screening results that fail to predict actual catalyst or enzyme performance under real-world conditions.
Data management and interpretation challenges have emerged as the volume of screening data has grown exponentially. Researchers often lack sophisticated data analysis tools capable of identifying complex patterns across multidimensional parameter spaces. This limitation frequently results in suboptimal utilization of the vast datasets generated through high-throughput screening campaigns.
Cost considerations remain significant, particularly for specialized microreactor systems with integrated analytical capabilities. The high initial investment required for advanced microreactor platforms limits their adoption in smaller research institutions and companies, creating an innovation gap between well-funded organizations and those with more limited resources.
Standardization issues further complicate the field, with various research groups employing different microreactor designs, operating protocols, and data reporting formats. This lack of standardization makes cross-comparison of results challenging and hinders collaborative advancement of the technology across the scientific community.
Contemporary Microreactor Designs for Screening Applications
01 Microfluidic devices for high-throughput screening
Microfluidic devices provide platforms for high-throughput screening by enabling the manipulation of small volumes of fluids in channels with dimensions of tens to hundreds of micrometers. These devices allow for parallel processing of multiple samples, reducing reagent consumption and analysis time. The integration of various functional components such as mixers, separators, and detectors within a single microfluidic chip enhances the efficiency of screening processes for applications in drug discovery, catalyst development, and biological assays.- Microfluidic systems for high-throughput screening: Microfluidic systems provide platforms for high-throughput screening by enabling the manipulation of small volumes of fluids in channels with dimensions of tens to hundreds of micrometers. These systems allow for parallel processing of multiple samples, rapid mixing of reagents, and real-time monitoring of reactions. The integration of pumps, valves, and sensors within microfluidic devices enhances their capability for automated screening applications in drug discovery, catalyst development, and biological assays.
- Microreactor design for enhanced reaction control: Specialized microreactor designs enable precise control over reaction parameters such as temperature, pressure, and residence time. These designs incorporate features like mixing zones, heat exchangers, and residence time distribution control elements to optimize reaction conditions. Advanced microreactors may include integrated sensors for real-time monitoring and feedback control, allowing for rapid optimization of reaction conditions during high-throughput screening campaigns.
- Parallel processing techniques in microreactor arrays: Microreactor arrays enable parallel processing of multiple reactions simultaneously, significantly increasing screening throughput. These arrays can be configured in various formats including plate-based systems, droplet arrays, or continuous flow parallel reactors. Integration with automated liquid handling systems and robotic platforms further enhances throughput capabilities. Parallel processing techniques in microreactor arrays allow researchers to explore large parameter spaces efficiently, accelerating the discovery and optimization of chemical and biological processes.
- Detection and analysis methods for microreactor screening: Advanced detection and analysis methods are crucial for extracting meaningful data from microreactor high-throughput screening. These include optical techniques such as fluorescence, absorbance, and Raman spectroscopy, as well as mass spectrometry and electrochemical detection. Integration of these analytical methods directly with microreactor platforms enables real-time monitoring of reaction progress and outcomes. Machine learning and data analysis algorithms help process the large datasets generated during screening campaigns to identify trends and optimal conditions.
- Applications of microreactor high-throughput screening in drug discovery and materials science: Microreactor high-throughput screening has diverse applications in drug discovery, catalyst development, materials science, and biochemical research. In drug discovery, these systems enable rapid screening of compound libraries against biological targets. For materials science, microreactors allow for systematic exploration of synthesis conditions to develop novel materials with tailored properties. The ability to precisely control reaction conditions while minimizing reagent consumption makes microreactor screening particularly valuable for expensive or hazardous reactions.
02 Continuous flow microreactors for reaction optimization
Continuous flow microreactors enable rapid optimization of reaction conditions by allowing precise control over reaction parameters such as temperature, pressure, and residence time. These systems facilitate high-throughput screening of reaction conditions by operating multiple parallel reactors simultaneously or by rapidly changing conditions in sequence. The continuous nature of these systems provides advantages in terms of reproducibility, scalability, and safety, particularly for reactions involving hazardous materials or requiring precise control over reaction environments.Expand Specific Solutions03 Integration of detection systems with microreactors
Advanced detection systems integrated with microreactors enhance high-throughput screening capabilities by enabling real-time monitoring of reactions. These systems incorporate spectroscopic techniques (such as UV-Vis, fluorescence, or Raman), mass spectrometry, or electrochemical sensors directly into the microreactor platform. The integration allows for immediate analysis of reaction products without the need for sample transfer, reducing analysis time and increasing throughput while providing valuable kinetic data for process optimization.Expand Specific Solutions04 Automated sample handling and data processing systems
Automated systems for sample preparation, injection, and data processing significantly enhance the efficiency of high-throughput screening using microreactors. These systems incorporate robotic sample handlers, automated liquid dispensers, and sophisticated software for experimental design and data analysis. The automation reduces human error, increases reproducibility, and allows for continuous operation, thereby maximizing the throughput potential of microreactor platforms while generating large datasets that can be analyzed using machine learning approaches.Expand Specific Solutions05 Droplet-based microreactors for compartmentalized reactions
Droplet-based microreactors create isolated reaction compartments by generating discrete droplets within an immiscible carrier fluid. This approach enables the screening of thousands to millions of individual reactions in a high-throughput manner. Each droplet functions as a miniature reaction vessel with volumes in the picoliter to nanoliter range, dramatically reducing reagent consumption while increasing statistical significance through large numbers of replicates. The technology is particularly valuable for screening enzyme variants, cell-based assays, and combinatorial chemistry applications.Expand Specific Solutions
Leading Organizations in Microreactor Technology
Microreactors in high-throughput screening of catalysts and enzymes are currently in a growth phase, with the market expanding rapidly due to increasing demand for efficient drug discovery and chemical process optimization. The global market size is estimated to reach $2.5 billion by 2025, driven by pharmaceutical and petrochemical industries seeking cost-effective research solutions. Technologically, the field is advancing from early adoption to mainstream implementation, with companies demonstrating varying levels of maturity. Industry leaders include ExxonMobil Technology & Engineering and Sinopec Research Institute, which have developed proprietary microreactor platforms, while Agilent Technologies and Unchained Labs offer commercial solutions. Academic institutions like California Institute of Technology and Technical University of Denmark are pioneering next-generation microfluidic systems, creating a competitive landscape balanced between established corporations and innovative research organizations.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced microreactor technology for high-throughput catalyst screening in petroleum refining processes. Their platform integrates microfluidic channels with in-situ spectroscopic analysis capabilities, allowing simultaneous evaluation of multiple catalyst formulations under identical reaction conditions. The system employs parallel microreactor arrays with integrated temperature and pressure sensors that enable precise control of reaction parameters across hundreds of catalyst samples[1]. Sinopec's technology incorporates automated sampling systems and real-time analytics to rapidly assess catalyst performance metrics including conversion efficiency, selectivity, and deactivation rates. Their microreactor design features specialized coating materials that prevent catalyst poisoning and fouling during extended screening operations[3].
Strengths: Superior throughput capacity allowing evaluation of hundreds of catalyst formulations simultaneously; excellent reproducibility through precise parameter control; reduced catalyst consumption (milligram quantities) compared to conventional methods. Weaknesses: Limited scalability from micro to industrial scale; potential mass transfer limitations that may not accurately represent industrial reactor conditions; higher initial capital investment compared to conventional screening methods.
ExxonMobil Technology & Engineering Co.
Technical Solution: ExxonMobil has pioneered a proprietary high-throughput microreactor screening platform called Parallel Catalyst Evaluation (PCE) technology for accelerated discovery of novel catalytic materials. Their system features an array of individually controlled microreactors operating in parallel, each containing milligram quantities of different catalyst formulations. The technology incorporates advanced microfluidic control systems that precisely regulate reactant flow rates, pressure, and temperature across all channels simultaneously[2]. ExxonMobil's platform includes integrated analytical capabilities with rapid gas chromatography and mass spectrometry for real-time performance assessment. Their microreactor design incorporates specialized materials resistant to high temperatures (up to 800°C) and pressures (up to 100 bar), enabling screening under industrially relevant conditions[4]. The system also features automated catalyst loading and unloading mechanisms to minimize human error and increase throughput.
Strengths: Exceptional temperature and pressure range capabilities allowing testing under realistic industrial conditions; sophisticated data analytics for rapid identification of promising catalyst candidates; excellent reproducibility between micro-scale and commercial-scale performance. Weaknesses: Complex system requiring specialized expertise to operate; higher maintenance requirements due to sophisticated components; potential for channel clogging during long-duration testing of certain catalyst types.
Critical Patents in Microfluidic Screening Technologies
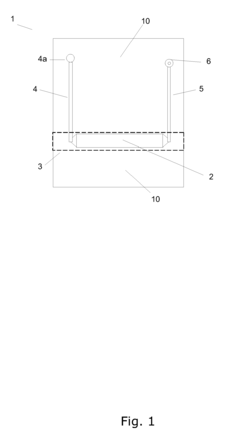
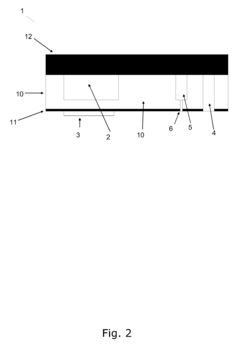
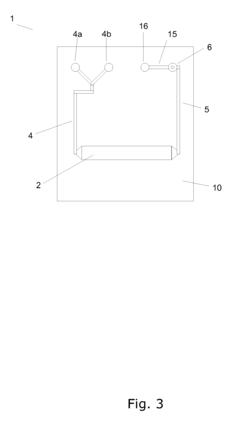
A microreactor
PatentInactiveEP1897612A1
Innovation
- A microreactor design where the reaction chamber, heating element, and capillary hole for pressure reduction are integrally formed in a single monolithic element, allowing for efficient heating and cooling without the need for forced cooling or high power consumption, enabling fast real-time access to chemical reactions.
High throughput screening of catalysts using spin resonance
PatentWO2006063205A2
Innovation
- The development of an evanescent wave probe capable of performing high throughput screening using either electron spin resonance (ESR) or nuclear magnetic resonance (NMR) techniques, allowing for localized detection of spin resonance in catalyst libraries or micro-reactor arrays, enabling the characterization of catalytic reactions with high sensitivity and spatial resolution.
Scale-up Challenges from Lab to Industrial Implementation
The transition from laboratory-scale microreactor systems to industrial implementation presents significant challenges that must be addressed for successful commercialization. Laboratory microreactors typically operate with volumes in the microliter to milliliter range, while industrial applications require throughput in the liter to cubic meter scale. This dramatic increase in scale introduces complex engineering problems related to fluid dynamics, heat transfer, and mixing efficiency.
One primary challenge is maintaining the advantageous characteristics of microreactors—such as enhanced mass and heat transfer, precise reaction control, and safety—when scaling up. The surface-to-volume ratio decreases with increasing reactor dimensions, potentially compromising the heat and mass transfer benefits that make microreactors attractive for catalyst and enzyme screening in the first place.
Process control complexity increases exponentially during scale-up. Laboratory systems typically employ precise syringe pumps and temperature controllers that cannot be directly translated to industrial settings. Industrial implementation requires robust control systems capable of handling variations in feedstock quality, environmental conditions, and maintaining consistent performance over extended operation periods.
Manufacturing challenges also emerge when moving from lab to industrial scale. Fabrication techniques suitable for producing small numbers of laboratory devices, such as photolithography or 3D printing, may be impractical or prohibitively expensive for mass production of industrial-scale units. Materials selection becomes critical, as industrial systems must withstand harsh chemical environments, high pressures, and temperatures for years rather than days or weeks.
Numbering-up (parallelization) versus traditional scale-up represents a fundamental strategic decision. While numbering-up preserves the beneficial characteristics of microreactors, it introduces challenges in flow distribution, ensuring uniform performance across parallel channels, and managing the complexity of thousands of identical units operating simultaneously.
Economic considerations ultimately determine commercial viability. The capital expenditure for microreactor technology must be justified by improved yield, selectivity, safety, or other performance metrics. Additionally, operational costs including maintenance, energy consumption, and specialized training for personnel must be factored into implementation decisions.
Regulatory hurdles present another significant barrier, particularly in highly regulated industries like pharmaceuticals. New manufacturing technologies require extensive validation and may face conservative resistance from regulatory bodies unfamiliar with continuous microreactor processing paradigms compared to traditional batch methods.
One primary challenge is maintaining the advantageous characteristics of microreactors—such as enhanced mass and heat transfer, precise reaction control, and safety—when scaling up. The surface-to-volume ratio decreases with increasing reactor dimensions, potentially compromising the heat and mass transfer benefits that make microreactors attractive for catalyst and enzyme screening in the first place.
Process control complexity increases exponentially during scale-up. Laboratory systems typically employ precise syringe pumps and temperature controllers that cannot be directly translated to industrial settings. Industrial implementation requires robust control systems capable of handling variations in feedstock quality, environmental conditions, and maintaining consistent performance over extended operation periods.
Manufacturing challenges also emerge when moving from lab to industrial scale. Fabrication techniques suitable for producing small numbers of laboratory devices, such as photolithography or 3D printing, may be impractical or prohibitively expensive for mass production of industrial-scale units. Materials selection becomes critical, as industrial systems must withstand harsh chemical environments, high pressures, and temperatures for years rather than days or weeks.
Numbering-up (parallelization) versus traditional scale-up represents a fundamental strategic decision. While numbering-up preserves the beneficial characteristics of microreactors, it introduces challenges in flow distribution, ensuring uniform performance across parallel channels, and managing the complexity of thousands of identical units operating simultaneously.
Economic considerations ultimately determine commercial viability. The capital expenditure for microreactor technology must be justified by improved yield, selectivity, safety, or other performance metrics. Additionally, operational costs including maintenance, energy consumption, and specialized training for personnel must be factored into implementation decisions.
Regulatory hurdles present another significant barrier, particularly in highly regulated industries like pharmaceuticals. New manufacturing technologies require extensive validation and may face conservative resistance from regulatory bodies unfamiliar with continuous microreactor processing paradigms compared to traditional batch methods.
Data Management Systems for High-Throughput Results
The high-throughput screening (HTS) of catalysts and enzymes using microreactors generates vast amounts of experimental data that require sophisticated management systems. Current data management solutions for microreactor-based HTS have evolved significantly to address the complex challenges of storing, processing, and analyzing large datasets generated during catalyst and enzyme screening campaigns.
Modern data management systems for high-throughput results typically incorporate relational databases with specialized schemas designed to capture the multidimensional nature of catalyst and enzyme performance data. These systems enable researchers to store detailed information about reaction conditions, catalyst compositions, conversion rates, selectivity profiles, and kinetic parameters in structured formats that facilitate subsequent analysis.
Cloud-based platforms have emerged as the preferred infrastructure for managing high-throughput screening data, offering scalable storage solutions and computational resources that can adapt to fluctuating research demands. These platforms typically implement robust security protocols to protect proprietary catalyst formulations and experimental methodologies while enabling controlled collaboration among research teams across different geographical locations.
Machine learning integration represents a significant advancement in data management systems for microreactor-based screening. Advanced algorithms can now automatically identify patterns in catalyst performance data, predict optimal reaction conditions, and suggest promising catalyst candidates for further investigation. This capability has dramatically accelerated the discovery process by reducing the number of experimental iterations required to optimize catalyst formulations.
Visualization tools have become essential components of modern data management systems, allowing researchers to generate interactive plots, heat maps, and multidimensional representations of catalyst performance landscapes. These tools enable scientists to quickly identify trends and relationships that might otherwise remain hidden in complex datasets, facilitating more informed decision-making during the catalyst development process.
Standardized data formats and ontologies have been developed to ensure interoperability between different experimental platforms and analysis tools. These standards enable seamless data exchange between microreactor systems, analytical instruments, and computational modeling tools, creating integrated workflows that minimize manual data handling and reduce the risk of transcription errors.
Automated data validation and quality control mechanisms are now routinely implemented in high-throughput data management systems. These features automatically flag anomalous results, identify potential experimental artifacts, and ensure data integrity throughout the screening process, significantly improving the reliability of conclusions drawn from high-throughput experiments.
Modern data management systems for high-throughput results typically incorporate relational databases with specialized schemas designed to capture the multidimensional nature of catalyst and enzyme performance data. These systems enable researchers to store detailed information about reaction conditions, catalyst compositions, conversion rates, selectivity profiles, and kinetic parameters in structured formats that facilitate subsequent analysis.
Cloud-based platforms have emerged as the preferred infrastructure for managing high-throughput screening data, offering scalable storage solutions and computational resources that can adapt to fluctuating research demands. These platforms typically implement robust security protocols to protect proprietary catalyst formulations and experimental methodologies while enabling controlled collaboration among research teams across different geographical locations.
Machine learning integration represents a significant advancement in data management systems for microreactor-based screening. Advanced algorithms can now automatically identify patterns in catalyst performance data, predict optimal reaction conditions, and suggest promising catalyst candidates for further investigation. This capability has dramatically accelerated the discovery process by reducing the number of experimental iterations required to optimize catalyst formulations.
Visualization tools have become essential components of modern data management systems, allowing researchers to generate interactive plots, heat maps, and multidimensional representations of catalyst performance landscapes. These tools enable scientists to quickly identify trends and relationships that might otherwise remain hidden in complex datasets, facilitating more informed decision-making during the catalyst development process.
Standardized data formats and ontologies have been developed to ensure interoperability between different experimental platforms and analysis tools. These standards enable seamless data exchange between microreactor systems, analytical instruments, and computational modeling tools, creating integrated workflows that minimize manual data handling and reduce the risk of transcription errors.
Automated data validation and quality control mechanisms are now routinely implemented in high-throughput data management systems. These features automatically flag anomalous results, identify potential experimental artifacts, and ensure data integrity throughout the screening process, significantly improving the reliability of conclusions drawn from high-throughput experiments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!