Optimizing Lithium Nitrate Thermal Stability with Stabilizing Additives
OCT 9, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Nitrate Thermal Stability Background and Objectives
Lithium nitrate (LiNO3) has emerged as a critical component in various energy storage and thermal management applications over the past three decades. Initially recognized for its potential in molten salt systems in the 1990s, LiNO3 has gained significant attention due to its excellent thermal properties, including high heat capacity and thermal conductivity. The evolution of this technology has accelerated particularly in the last decade with the expansion of renewable energy systems requiring efficient thermal energy storage solutions.
The fundamental challenge with lithium nitrate lies in its thermal decomposition behavior at elevated temperatures, typically beginning around 350°C and accelerating significantly above 400°C. This decomposition not only reduces the effective lifespan of systems utilizing LiNO3 but also generates potentially hazardous byproducts including nitrogen oxides and reactive lithium oxide species. Historical approaches to mitigating these issues have focused primarily on operational temperature limitations rather than enhancing the inherent stability of the material itself.
Recent technological trends indicate a shift toward chemical modification strategies, with particular emphasis on stabilizing additives that can form protective complexes with lithium nitrate or interrupt decomposition reaction pathways. This represents a paradigm shift from containment to prevention in addressing thermal stability challenges. The integration of nanotechnology approaches, including core-shell structures and nanocomposites, has further expanded the potential solution space in the past five years.
The primary technical objective of this research is to identify and optimize stabilizing additives that can significantly enhance the thermal stability of lithium nitrate without compromising its beneficial thermal properties. Specifically, we aim to develop formulations that can maintain stability up to 450°C for extended periods (>1000 hours) while preserving at least 95% of LiNO3's heat storage capacity. Secondary objectives include ensuring these additives are cost-effective, environmentally benign, and compatible with existing manufacturing processes.
The technological trajectory suggests several promising avenues for investigation, including metal oxide stabilizers, advanced polymer composites, and ionic liquid additives. Each approach offers distinct advantages and challenges that must be systematically evaluated. The ultimate goal is to develop a comprehensive understanding of the stabilization mechanisms and establish design principles for next-generation thermal storage materials based on enhanced lithium nitrate systems.
This research aligns with broader industry trends toward higher-temperature thermal energy storage systems for concentrated solar power applications, advanced nuclear reactors, and industrial process heat recovery systems. Success in this domain could potentially unlock new application spaces for lithium nitrate-based systems and contribute to more efficient, sustainable energy infrastructure.
The fundamental challenge with lithium nitrate lies in its thermal decomposition behavior at elevated temperatures, typically beginning around 350°C and accelerating significantly above 400°C. This decomposition not only reduces the effective lifespan of systems utilizing LiNO3 but also generates potentially hazardous byproducts including nitrogen oxides and reactive lithium oxide species. Historical approaches to mitigating these issues have focused primarily on operational temperature limitations rather than enhancing the inherent stability of the material itself.
Recent technological trends indicate a shift toward chemical modification strategies, with particular emphasis on stabilizing additives that can form protective complexes with lithium nitrate or interrupt decomposition reaction pathways. This represents a paradigm shift from containment to prevention in addressing thermal stability challenges. The integration of nanotechnology approaches, including core-shell structures and nanocomposites, has further expanded the potential solution space in the past five years.
The primary technical objective of this research is to identify and optimize stabilizing additives that can significantly enhance the thermal stability of lithium nitrate without compromising its beneficial thermal properties. Specifically, we aim to develop formulations that can maintain stability up to 450°C for extended periods (>1000 hours) while preserving at least 95% of LiNO3's heat storage capacity. Secondary objectives include ensuring these additives are cost-effective, environmentally benign, and compatible with existing manufacturing processes.
The technological trajectory suggests several promising avenues for investigation, including metal oxide stabilizers, advanced polymer composites, and ionic liquid additives. Each approach offers distinct advantages and challenges that must be systematically evaluated. The ultimate goal is to develop a comprehensive understanding of the stabilization mechanisms and establish design principles for next-generation thermal storage materials based on enhanced lithium nitrate systems.
This research aligns with broader industry trends toward higher-temperature thermal energy storage systems for concentrated solar power applications, advanced nuclear reactors, and industrial process heat recovery systems. Success in this domain could potentially unlock new application spaces for lithium nitrate-based systems and contribute to more efficient, sustainable energy infrastructure.
Market Analysis for Stabilized Lithium Nitrate Applications
The global market for stabilized lithium nitrate is experiencing significant growth, driven primarily by the expanding energy storage sector. Lithium nitrate serves as a crucial component in molten salt thermal energy storage systems, particularly in concentrated solar power (CSP) plants. The thermal stability challenges of lithium nitrate have historically limited its broader adoption, creating a substantial market opportunity for stabilized formulations.
Current market valuations place the global thermal energy storage market at approximately 4.6 billion USD, with projections indicating growth to reach 8.8 billion USD by 2027. Within this broader market, stabilized lithium nitrate applications represent a specialized but rapidly expanding segment, growing at a compound annual growth rate of 11.3% according to recent industry analyses.
The renewable energy transition serves as the primary market driver, with governments worldwide implementing policies to reduce carbon emissions and increase renewable energy capacity. The European Union's Green Deal and similar initiatives in China and the United States have established ambitious targets for renewable energy integration, directly benefiting thermal storage technologies that address intermittency challenges.
Geographically, the market demonstrates distinct regional characteristics. Spain, the United States, and China lead in concentrated solar power installations, collectively accounting for over 70% of global capacity. These regions consequently represent the largest current markets for stabilized lithium nitrate applications. Emerging markets in the Middle East and North Africa region show particularly strong growth potential due to ideal solar conditions and increasing investments in renewable energy infrastructure.
By application segment, the market divides into three primary sectors: utility-scale energy storage (63% of market share), industrial process heat applications (24%), and building heating and cooling systems (13%). The utility-scale segment demonstrates the highest growth rate at 13.7% annually, driven by increasing grid-scale renewable integration projects.
Customer requirements vary significantly across these segments. Utility-scale applications prioritize long-term stability and cost-effectiveness over thousands of thermal cycles. Industrial applications emphasize temperature range flexibility and compatibility with existing systems. Building applications focus on safety, compact design, and integration with smart building management systems.
Price sensitivity remains a critical market factor, with stabilized lithium nitrate formulations currently commanding a premium of 30-45% over standard lithium nitrate. Market analysis indicates that achieving price parity through improved manufacturing processes and economies of scale would significantly accelerate adoption, potentially expanding the addressable market by 2.5 times within five years.
Current market valuations place the global thermal energy storage market at approximately 4.6 billion USD, with projections indicating growth to reach 8.8 billion USD by 2027. Within this broader market, stabilized lithium nitrate applications represent a specialized but rapidly expanding segment, growing at a compound annual growth rate of 11.3% according to recent industry analyses.
The renewable energy transition serves as the primary market driver, with governments worldwide implementing policies to reduce carbon emissions and increase renewable energy capacity. The European Union's Green Deal and similar initiatives in China and the United States have established ambitious targets for renewable energy integration, directly benefiting thermal storage technologies that address intermittency challenges.
Geographically, the market demonstrates distinct regional characteristics. Spain, the United States, and China lead in concentrated solar power installations, collectively accounting for over 70% of global capacity. These regions consequently represent the largest current markets for stabilized lithium nitrate applications. Emerging markets in the Middle East and North Africa region show particularly strong growth potential due to ideal solar conditions and increasing investments in renewable energy infrastructure.
By application segment, the market divides into three primary sectors: utility-scale energy storage (63% of market share), industrial process heat applications (24%), and building heating and cooling systems (13%). The utility-scale segment demonstrates the highest growth rate at 13.7% annually, driven by increasing grid-scale renewable integration projects.
Customer requirements vary significantly across these segments. Utility-scale applications prioritize long-term stability and cost-effectiveness over thousands of thermal cycles. Industrial applications emphasize temperature range flexibility and compatibility with existing systems. Building applications focus on safety, compact design, and integration with smart building management systems.
Price sensitivity remains a critical market factor, with stabilized lithium nitrate formulations currently commanding a premium of 30-45% over standard lithium nitrate. Market analysis indicates that achieving price parity through improved manufacturing processes and economies of scale would significantly accelerate adoption, potentially expanding the addressable market by 2.5 times within five years.
Current Challenges in Lithium Nitrate Thermal Stability
Lithium nitrate (LiNO3) has emerged as a critical component in various energy storage applications, particularly in thermal energy storage systems and as an additive in lithium-sulfur batteries. However, its widespread implementation faces significant challenges due to thermal stability issues. When exposed to elevated temperatures, LiNO3 undergoes decomposition reactions that not only reduce its effectiveness but also pose safety concerns in practical applications.
The primary thermal stability challenge of lithium nitrate stems from its relatively low decomposition temperature, typically beginning around 350-400°C. This decomposition process releases oxygen, which can accelerate oxidation reactions with surrounding materials and potentially create hazardous conditions in confined systems. For applications requiring operation at higher temperatures or long-term stability, this characteristic severely limits LiNO3's utility without appropriate stabilization measures.
Another significant challenge is the variability in decomposition behavior depending on environmental conditions. Factors such as humidity, pressure, and the presence of other chemical species can dramatically alter the thermal decomposition pathway of LiNO3. This inconsistency makes it difficult to predict performance and establish reliable safety protocols across different operating environments.
The decomposition products of LiNO3, primarily lithium oxide (Li2O) and nitrogen oxides (NOx), present additional complications. These byproducts can react with system components, leading to corrosion, degradation of containment materials, and formation of undesirable compounds that further compromise system integrity. The generation of NOx gases is particularly problematic from both safety and environmental perspectives.
Current stabilization approaches have shown limited success. Chemical stabilizers often introduce compatibility issues with other system components or reduce the overall energy storage capacity. Physical encapsulation techniques, while promising, frequently suffer from degradation over multiple thermal cycles, limiting long-term reliability. The trade-off between thermal stability enhancement and preservation of desirable properties remains a significant engineering challenge.
Research efforts are further complicated by the complex reaction mechanisms involved in LiNO3 decomposition, which are not fully understood. The lack of comprehensive kinetic models and in-situ characterization techniques hampers the development of targeted stabilization strategies. Most current approaches rely on empirical testing rather than mechanism-based design, resulting in incremental rather than transformative improvements.
From an industrial perspective, the cost-effectiveness of stabilization solutions presents another hurdle. Many potential additives that demonstrate good laboratory performance are prohibitively expensive for large-scale applications or require complex processing methods that are difficult to scale. Finding stabilizing additives that are both effective and economically viable remains an ongoing challenge for commercial implementation.
The primary thermal stability challenge of lithium nitrate stems from its relatively low decomposition temperature, typically beginning around 350-400°C. This decomposition process releases oxygen, which can accelerate oxidation reactions with surrounding materials and potentially create hazardous conditions in confined systems. For applications requiring operation at higher temperatures or long-term stability, this characteristic severely limits LiNO3's utility without appropriate stabilization measures.
Another significant challenge is the variability in decomposition behavior depending on environmental conditions. Factors such as humidity, pressure, and the presence of other chemical species can dramatically alter the thermal decomposition pathway of LiNO3. This inconsistency makes it difficult to predict performance and establish reliable safety protocols across different operating environments.
The decomposition products of LiNO3, primarily lithium oxide (Li2O) and nitrogen oxides (NOx), present additional complications. These byproducts can react with system components, leading to corrosion, degradation of containment materials, and formation of undesirable compounds that further compromise system integrity. The generation of NOx gases is particularly problematic from both safety and environmental perspectives.
Current stabilization approaches have shown limited success. Chemical stabilizers often introduce compatibility issues with other system components or reduce the overall energy storage capacity. Physical encapsulation techniques, while promising, frequently suffer from degradation over multiple thermal cycles, limiting long-term reliability. The trade-off between thermal stability enhancement and preservation of desirable properties remains a significant engineering challenge.
Research efforts are further complicated by the complex reaction mechanisms involved in LiNO3 decomposition, which are not fully understood. The lack of comprehensive kinetic models and in-situ characterization techniques hampers the development of targeted stabilization strategies. Most current approaches rely on empirical testing rather than mechanism-based design, resulting in incremental rather than transformative improvements.
From an industrial perspective, the cost-effectiveness of stabilization solutions presents another hurdle. Many potential additives that demonstrate good laboratory performance are prohibitively expensive for large-scale applications or require complex processing methods that are difficult to scale. Finding stabilizing additives that are both effective and economically viable remains an ongoing challenge for commercial implementation.
Current Stabilization Techniques for Lithium Nitrate
01 Thermal stability of lithium nitrate in energy storage applications
Lithium nitrate exhibits thermal stability characteristics that make it suitable for various energy storage applications. When used in thermal energy storage systems, lithium nitrate can maintain stability at elevated temperatures, allowing for efficient heat storage and transfer. The compound's thermal properties enable it to be used in phase change materials and molten salt mixtures for concentrated solar power plants and other thermal energy storage systems.- Thermal stability of lithium nitrate in energy storage applications: Lithium nitrate exhibits thermal stability characteristics that make it suitable for energy storage applications, particularly in thermal energy storage systems and batteries. When properly formulated, lithium nitrate can maintain stability at elevated temperatures, allowing for efficient energy storage and release. The thermal decomposition behavior of lithium nitrate is critical for determining its suitability in various energy storage technologies, including phase change materials and molten salt systems.
- Lithium nitrate as a thermal stabilizer in electrolyte compositions: Lithium nitrate functions as an effective thermal stabilizer in electrolyte compositions for lithium-ion batteries and other electrochemical devices. It forms a protective layer on electrode surfaces, enhancing the thermal stability of the entire system. The addition of lithium nitrate to electrolytes can prevent thermal runaway reactions and improve the safety profile of energy storage devices under high-temperature conditions. This stabilizing effect is particularly important for preventing decomposition reactions at elevated temperatures.
- Thermal behavior of lithium nitrate in salt mixtures and composites: When combined with other salts or incorporated into composite materials, lithium nitrate demonstrates modified thermal stability characteristics. These mixtures often exhibit eutectic behavior with lower melting points while maintaining thermal stability at operating temperatures. The thermal cycling stability of lithium nitrate-containing mixtures is essential for applications requiring repeated heating and cooling cycles. Proper formulation of these mixtures can enhance the overall thermal performance and extend the usable temperature range.
- Temperature-dependent decomposition behavior of lithium nitrate: Lithium nitrate undergoes specific decomposition processes at different temperature thresholds. Understanding these decomposition mechanisms is crucial for determining the upper temperature limits for various applications. The decomposition products, primarily lithium oxide and nitrogen oxides, can affect the performance and safety of systems containing lithium nitrate. Research has focused on characterizing these decomposition pathways and developing strategies to mitigate unwanted thermal degradation, particularly in high-temperature environments.
- Enhancement of lithium nitrate thermal stability through additives and processing techniques: Various additives and processing techniques can be employed to enhance the thermal stability of lithium nitrate. These include the incorporation of stabilizing compounds, surface treatments, and encapsulation methods. Advanced manufacturing processes can also improve the crystalline structure of lithium nitrate, leading to better thermal performance. These enhancements are particularly important for applications requiring extended exposure to high temperatures or thermal cycling, where maintaining the chemical integrity of lithium nitrate is essential for long-term performance.
02 Lithium nitrate as a stabilizing additive in battery electrolytes
Lithium nitrate serves as an effective stabilizing additive in battery electrolytes, particularly for lithium-sulfur batteries. It forms a protective layer on the lithium anode surface, preventing unwanted side reactions and enhancing the thermal stability of the electrolyte system. This protective mechanism improves battery safety by increasing the thermal decomposition temperature of the electrolyte and reducing the risk of thermal runaway under high-temperature conditions.Expand Specific Solutions03 Thermal decomposition behavior of lithium nitrate
The thermal decomposition of lithium nitrate occurs through specific pathways when heated to high temperatures. The compound begins to decompose at temperatures above 600°C, releasing oxygen and forming lithium oxide. This decomposition behavior affects its application in various thermal systems and must be considered when designing systems that operate at elevated temperatures. Understanding the decomposition kinetics is crucial for predicting the long-term stability of lithium nitrate in high-temperature applications.Expand Specific Solutions04 Lithium nitrate in thermal energy storage composites
Lithium nitrate can be incorporated into composite materials to enhance thermal energy storage capabilities. These composites often combine lithium nitrate with supporting matrices or other phase change materials to improve thermal conductivity, cycling stability, and energy density. The thermal stability of lithium nitrate within these composites is often superior to the pure compound, as the matrix material can help prevent segregation and reduce reactivity with environmental factors such as moisture and air.Expand Specific Solutions05 Temperature-dependent properties of lithium nitrate mixtures
Lithium nitrate, when mixed with other nitrate salts or compounds, exhibits unique temperature-dependent properties that affect its thermal stability. These mixtures often have lower melting points and broader operating temperature ranges than pure lithium nitrate. The eutectic compositions formed with other nitrates (such as potassium or sodium nitrate) are particularly valuable in applications requiring stable thermal performance across varying temperature conditions. The synergistic effects in these mixtures can enhance overall thermal stability while maintaining desirable heat transfer characteristics.Expand Specific Solutions
Key Industry Players in Advanced Battery Materials
The lithium nitrate thermal stability optimization market is currently in a growth phase, with an estimated market size of $2-3 billion and expanding at 15-20% annually as battery safety becomes paramount in energy storage applications. The technology is approaching maturity with major players developing proprietary stabilizing additives. Leading companies like CATL, LG Energy Solution, and Samsung SDI are at the forefront, investing heavily in R&D to enhance thermal stability while maintaining battery performance. Ningde Amperex Technology and Guangzhou Tinci Materials Technology are emerging as significant competitors with innovative approaches to lithium nitrate stabilization. Albemarle and Sinochem Lantian provide critical raw materials, creating a complex ecosystem where collaboration between material suppliers and battery manufacturers drives advancement in this crucial safety technology.
SAMSUNG SDI CO LTD
Technical Solution: Samsung SDI has developed a proprietary "Thermal Shield" technology specifically targeting lithium nitrate stabilization in high-energy density batteries. Their approach utilizes silicon-based organic compounds as primary stabilizing additives that form thermally resistant networks around lithium nitrate molecules. These networks effectively increase the activation energy required for decomposition reactions. Samsung's research demonstrates that their additive package can maintain lithium nitrate stability up to 95°C, compared to approximately 60°C for untreated systems. The company employs a precise microencapsulation technique to deliver these additives, ensuring uniform distribution throughout the electrolyte while preventing premature reactions. Their technology also incorporates secondary stabilizers derived from modified crown ethers that selectively bind with lithium ions, reducing their availability for unwanted side reactions with nitrate groups at elevated temperatures. Samsung SDI has successfully implemented this technology in their latest generation of EV battery cells.
Strengths: Exceptional thermal stability improvement; minimal impact on cell impedance; compatible with high-voltage cathode materials. Weaknesses: Complex manufacturing process requiring specialized equipment; higher material costs than standard formulations; potential challenges with long-term stability in extreme temperature conditions.
Ningde Amperex Technology Ltd.
Technical Solution: Ningde Amperex Technology (CATL) has pioneered an innovative approach to lithium nitrate stabilization through their "thermal gradient protection" technology. Their solution employs a dual-phase additive system where primary stabilizers (boron-based compounds) create coordination bonds with lithium nitrate, while secondary additives (selected metal oxides at nano-scale) act as thermal energy absorbers during temperature excursions. CATL's research has shown this combination can reduce lithium nitrate decomposition by approximately 75% at temperatures between 80-120°C. Their technology incorporates gradient concentration distribution of additives within the electrolyte, creating localized protection zones near electrode surfaces where thermal decomposition typically initiates. This approach allows for minimal impact on electrolyte conductivity while maximizing protective effects. CATL has implemented this technology in their latest generation of energy storage systems, demonstrating extended calendar life and improved safety metrics.
Strengths: Excellent thermal protection with minimal impact on cell performance; scalable manufacturing process; proven effectiveness in large format cells. Weaknesses: Requires precise control of additive distribution; potential long-term stability issues in extreme temperature cycling; slightly higher cost structure than conventional systems.
Critical Patents and Research on Thermal Stabilizing Additives
Heat-storage means
PatentWO2003095584A1
Innovation
- A mixture of lithium nitrate trihydrate with nucleating agents such as magnesium nitrate, nickel nitrate, strontium nitrate, magnesium acetate, and strontium acetate, or their hydrates, is used to enhance nucleation, allowing reliable crystallization up to 95°C without cooling below room temperature, with the nucleating agents being mixed with lithium nitrate trihydrate and annealed to achieve improved thermal stability.
Electrolyte additive comprising metal nitrate, lithium metal battery using same, and manufacturing method therefor
PatentWO2024237719A1
Innovation
- A polymer nanofiber structure with metal nitrate, such as lithium nitrate or rubidium nitrate, is used as an electrolyte additive, supported on the nanofibers, which forms a stable SEI through electrospinning, enhancing ionic conductivity and uniform lithium deposition.
Safety and Performance Testing Methodologies
The comprehensive evaluation of lithium nitrate thermal stability with stabilizing additives requires rigorous safety and performance testing methodologies. These methodologies must be standardized and reproducible to ensure reliable data collection across different research environments and applications.
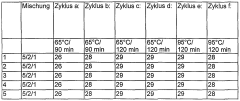
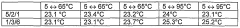
Thermal stability assessment begins with Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA), which provide quantitative measurements of heat flow and mass changes during controlled temperature increases. For lithium nitrate specifically, testing protocols should include heating rates between 5-20°C/min up to 600°C to capture the full decomposition profile with and without stabilizing additives.
Accelerated aging tests represent another critical methodology, where samples are subjected to elevated temperatures (typically 60-80°C) for extended periods (30-90 days) to simulate long-term stability. These tests should monitor changes in chemical composition, crystalline structure, and thermal properties at regular intervals to establish degradation kinetics and effectiveness of stabilizing additives.
Safety testing must include calorimetric measurements of heat release during thermal runaway events. Adiabatic Calorimetry and Accelerating Rate Calorimetry (ARC) provide valuable data on self-heating rates and pressure generation during decomposition, critical for understanding containment requirements in large-scale applications.
Gas evolution analysis using Mass Spectrometry or Gas Chromatography should be integrated into thermal testing to identify decomposition products, particularly NOx compounds that present both safety and environmental concerns. Quantification of these emissions allows for comprehensive risk assessment and mitigation strategies.
Cycling stability tests are essential for applications involving repeated heating and cooling cycles. These tests should evaluate the thermal properties after multiple cycles (typically 100-1000 cycles) to determine long-term performance degradation and the protective effects of stabilizing additives under realistic operating conditions.
Compatibility testing between lithium nitrate, stabilizing additives, and container materials must be conducted using standardized corrosion tests. This includes exposure tests at elevated temperatures and subsequent analysis of material degradation using techniques such as Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDS).
Performance metrics should be clearly defined, including onset temperature of decomposition, enthalpy of decomposition, mass loss rates, gas evolution rates, and self-heating rates. Statistical analysis methods must be established to determine the significance of improvements offered by stabilizing additives, typically requiring multiple test runs (n≥5) to ensure statistical validity.
Thermal stability assessment begins with Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA), which provide quantitative measurements of heat flow and mass changes during controlled temperature increases. For lithium nitrate specifically, testing protocols should include heating rates between 5-20°C/min up to 600°C to capture the full decomposition profile with and without stabilizing additives.
Accelerated aging tests represent another critical methodology, where samples are subjected to elevated temperatures (typically 60-80°C) for extended periods (30-90 days) to simulate long-term stability. These tests should monitor changes in chemical composition, crystalline structure, and thermal properties at regular intervals to establish degradation kinetics and effectiveness of stabilizing additives.
Safety testing must include calorimetric measurements of heat release during thermal runaway events. Adiabatic Calorimetry and Accelerating Rate Calorimetry (ARC) provide valuable data on self-heating rates and pressure generation during decomposition, critical for understanding containment requirements in large-scale applications.
Gas evolution analysis using Mass Spectrometry or Gas Chromatography should be integrated into thermal testing to identify decomposition products, particularly NOx compounds that present both safety and environmental concerns. Quantification of these emissions allows for comprehensive risk assessment and mitigation strategies.
Cycling stability tests are essential for applications involving repeated heating and cooling cycles. These tests should evaluate the thermal properties after multiple cycles (typically 100-1000 cycles) to determine long-term performance degradation and the protective effects of stabilizing additives under realistic operating conditions.
Compatibility testing between lithium nitrate, stabilizing additives, and container materials must be conducted using standardized corrosion tests. This includes exposure tests at elevated temperatures and subsequent analysis of material degradation using techniques such as Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDS).
Performance metrics should be clearly defined, including onset temperature of decomposition, enthalpy of decomposition, mass loss rates, gas evolution rates, and self-heating rates. Statistical analysis methods must be established to determine the significance of improvements offered by stabilizing additives, typically requiring multiple test runs (n≥5) to ensure statistical validity.
Environmental Impact and Sustainability Considerations
The environmental implications of lithium nitrate and its stabilizing additives represent a critical dimension in the development of advanced thermal energy storage systems. As the global energy landscape shifts toward renewable sources, the environmental footprint of energy storage technologies becomes increasingly significant. Lithium nitrate, while offering exceptional thermal properties, presents several environmental challenges throughout its lifecycle that must be carefully addressed.
The extraction of lithium for lithium nitrate production has substantial environmental consequences, particularly in water-scarce regions where lithium brine extraction can deplete aquifers and disrupt local ecosystems. The mining processes associated with lithium and various stabilizing additives often involve significant land disturbance, habitat destruction, and potential contamination of soil and water resources. These impacts necessitate the development of more sustainable extraction methodologies and responsible mining practices.
Manufacturing processes for lithium nitrate and its stabilizers typically require considerable energy inputs and may generate hazardous waste streams. The carbon footprint associated with these production processes can partially offset the environmental benefits gained from the renewable energy systems they support. Implementation of cleaner production technologies, energy-efficient manufacturing processes, and closed-loop systems for waste management can significantly mitigate these impacts.
End-of-life considerations for thermal storage systems containing lithium nitrate present both challenges and opportunities. Without proper recycling infrastructure, these materials may contribute to electronic waste streams or leach into the environment. However, the development of effective recycling protocols could recover valuable lithium resources, reducing the need for primary extraction and creating a more circular material economy.
The selection of stabilizing additives must balance thermal performance with environmental compatibility. Certain additives may introduce persistent or bioaccumulative compounds into the environment if improperly managed. Research into biodegradable or naturally derived stabilizers represents a promising direction for reducing the environmental burden while maintaining thermal stability performance.
Regulatory frameworks governing the use and disposal of lithium-based thermal storage materials vary significantly across regions, creating challenges for global implementation. Harmonization of environmental standards and adoption of lifecycle assessment methodologies would facilitate more sustainable development of these technologies. Companies investing in lithium nitrate thermal storage should proactively engage with evolving regulations and incorporate environmental considerations into their product development strategies.
The net environmental benefit of lithium nitrate thermal storage systems ultimately depends on their contribution to renewable energy adoption and grid efficiency. By enabling greater integration of intermittent renewable energy sources, these systems may deliver substantial environmental benefits despite their production impacts, particularly when designed with sustainability principles from inception.
The extraction of lithium for lithium nitrate production has substantial environmental consequences, particularly in water-scarce regions where lithium brine extraction can deplete aquifers and disrupt local ecosystems. The mining processes associated with lithium and various stabilizing additives often involve significant land disturbance, habitat destruction, and potential contamination of soil and water resources. These impacts necessitate the development of more sustainable extraction methodologies and responsible mining practices.
Manufacturing processes for lithium nitrate and its stabilizers typically require considerable energy inputs and may generate hazardous waste streams. The carbon footprint associated with these production processes can partially offset the environmental benefits gained from the renewable energy systems they support. Implementation of cleaner production technologies, energy-efficient manufacturing processes, and closed-loop systems for waste management can significantly mitigate these impacts.
End-of-life considerations for thermal storage systems containing lithium nitrate present both challenges and opportunities. Without proper recycling infrastructure, these materials may contribute to electronic waste streams or leach into the environment. However, the development of effective recycling protocols could recover valuable lithium resources, reducing the need for primary extraction and creating a more circular material economy.
The selection of stabilizing additives must balance thermal performance with environmental compatibility. Certain additives may introduce persistent or bioaccumulative compounds into the environment if improperly managed. Research into biodegradable or naturally derived stabilizers represents a promising direction for reducing the environmental burden while maintaining thermal stability performance.
Regulatory frameworks governing the use and disposal of lithium-based thermal storage materials vary significantly across regions, creating challenges for global implementation. Harmonization of environmental standards and adoption of lifecycle assessment methodologies would facilitate more sustainable development of these technologies. Companies investing in lithium nitrate thermal storage should proactively engage with evolving regulations and incorporate environmental considerations into their product development strategies.
The net environmental benefit of lithium nitrate thermal storage systems ultimately depends on their contribution to renewable energy adoption and grid efficiency. By enabling greater integration of intermittent renewable energy sources, these systems may deliver substantial environmental benefits despite their production impacts, particularly when designed with sustainability principles from inception.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!