How to Increase Lithium Nitrate Compatibility with Organic Additives
OCT 9, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Nitrate Compatibility Background and Objectives
Lithium nitrate (LiNO3) has emerged as a critical component in advanced energy storage systems, particularly in lithium-sulfur batteries and other next-generation energy storage technologies. The evolution of this technology can be traced back to the early 2000s when researchers began exploring additives to mitigate the "shuttle effect" in lithium-sulfur batteries, a phenomenon that significantly reduces battery efficiency and lifespan.
The technical progression of lithium nitrate applications has seen significant advancements over the past decade, transitioning from laboratory curiosity to commercial consideration. Initially identified as a passive film-forming agent on lithium anodes, its role has expanded to include polysulfide suppression and electrolyte stabilization functions, making it an indispensable component in high-performance energy storage systems.
Current research indicates that while lithium nitrate offers substantial benefits in battery performance, its compatibility with organic additives presents significant challenges. These challenges stem from chemical interactions that can lead to decomposition, precipitation, or neutralization of beneficial effects when lithium nitrate is combined with certain organic compounds commonly used in electrolyte formulations.
The primary technical objective of enhancing lithium nitrate compatibility with organic additives is to develop stable, high-performance electrolyte systems that maintain the beneficial properties of both components. This includes preserving the solid electrolyte interphase (SEI) formation capabilities of lithium nitrate while leveraging the conductivity, viscosity, and stability improvements offered by organic additives.
Industry trends suggest a growing focus on tailored electrolyte systems that can address specific application requirements, from high-energy density consumer electronics to long-duration grid storage solutions. The ability to successfully integrate lithium nitrate with organic additives represents a key enabling technology for these diverse applications.
Recent publications highlight several promising approaches, including molecular engineering of organic additives specifically designed to complement lithium nitrate's properties, development of novel solvent systems that mediate interactions between components, and exploration of sequential addition protocols that optimize the formation of protective interfaces.
The technical trajectory points toward increasingly sophisticated multi-component electrolyte systems where lithium nitrate serves as one element in a carefully orchestrated chemical environment. Understanding and controlling the molecular-level interactions between lithium nitrate and organic additives represents the frontier of this research area, with potential implications extending beyond battery technologies to other electrochemical systems.
The technical progression of lithium nitrate applications has seen significant advancements over the past decade, transitioning from laboratory curiosity to commercial consideration. Initially identified as a passive film-forming agent on lithium anodes, its role has expanded to include polysulfide suppression and electrolyte stabilization functions, making it an indispensable component in high-performance energy storage systems.
Current research indicates that while lithium nitrate offers substantial benefits in battery performance, its compatibility with organic additives presents significant challenges. These challenges stem from chemical interactions that can lead to decomposition, precipitation, or neutralization of beneficial effects when lithium nitrate is combined with certain organic compounds commonly used in electrolyte formulations.
The primary technical objective of enhancing lithium nitrate compatibility with organic additives is to develop stable, high-performance electrolyte systems that maintain the beneficial properties of both components. This includes preserving the solid electrolyte interphase (SEI) formation capabilities of lithium nitrate while leveraging the conductivity, viscosity, and stability improvements offered by organic additives.
Industry trends suggest a growing focus on tailored electrolyte systems that can address specific application requirements, from high-energy density consumer electronics to long-duration grid storage solutions. The ability to successfully integrate lithium nitrate with organic additives represents a key enabling technology for these diverse applications.
Recent publications highlight several promising approaches, including molecular engineering of organic additives specifically designed to complement lithium nitrate's properties, development of novel solvent systems that mediate interactions between components, and exploration of sequential addition protocols that optimize the formation of protective interfaces.
The technical trajectory points toward increasingly sophisticated multi-component electrolyte systems where lithium nitrate serves as one element in a carefully orchestrated chemical environment. Understanding and controlling the molecular-level interactions between lithium nitrate and organic additives represents the frontier of this research area, with potential implications extending beyond battery technologies to other electrochemical systems.
Market Analysis for Enhanced Electrolyte Solutions
The global market for enhanced electrolyte solutions has experienced significant growth in recent years, primarily driven by the expanding lithium-ion battery sector. As of 2023, the lithium-ion battery market reached approximately $59 billion, with projections indicating growth to $182 billion by 2030, representing a compound annual growth rate of 17.3%. Within this ecosystem, electrolyte solutions account for roughly 15-20% of the battery component market value, highlighting their critical importance.
The demand for improved lithium nitrate-based electrolytes stems from multiple market segments. Electric vehicles represent the largest and fastest-growing application sector, with automotive manufacturers increasingly seeking battery technologies that offer enhanced safety profiles and longer lifespans. Consumer electronics continues to be a stable market driver, while grid storage applications are emerging as a significant growth opportunity, particularly in regions with aggressive renewable energy adoption targets.
Market research indicates that electrolyte solutions capable of successfully incorporating lithium nitrate with organic additives command premium pricing, typically 30-40% higher than conventional electrolytes. This price differential reflects the performance advantages, including improved cycle life and enhanced safety characteristics that these advanced formulations provide.
Regional analysis shows Asia-Pacific dominating the market with approximately 65% share, led by China, Japan, and South Korea. North America and Europe follow with roughly 20% and 12% respectively, though both regions are investing heavily in domestic battery production capabilities that will likely alter this distribution by 2025.
Customer segmentation reveals distinct requirements across different applications. EV manufacturers prioritize electrolyte solutions that enable fast charging while maintaining thermal stability. Consumer electronics producers focus on energy density and cycle life, while grid storage applications emphasize long-term stability and cost-effectiveness.
Market barriers include supply chain constraints for high-purity lithium nitrate, with current global production capacity struggling to meet projected demand growth. Intellectual property landscapes present another challenge, as several key formulation approaches are protected by patents held by major battery manufacturers and chemical companies.
Competitive analysis identifies three tiers of market participants: established chemical conglomerates with extensive R&D capabilities, specialized battery material suppliers with proprietary formulations, and emerging startups focused on novel additive combinations. Recent market consolidation through strategic acquisitions suggests the industry recognizes the strategic importance of advanced electrolyte technology.
The demand for improved lithium nitrate-based electrolytes stems from multiple market segments. Electric vehicles represent the largest and fastest-growing application sector, with automotive manufacturers increasingly seeking battery technologies that offer enhanced safety profiles and longer lifespans. Consumer electronics continues to be a stable market driver, while grid storage applications are emerging as a significant growth opportunity, particularly in regions with aggressive renewable energy adoption targets.
Market research indicates that electrolyte solutions capable of successfully incorporating lithium nitrate with organic additives command premium pricing, typically 30-40% higher than conventional electrolytes. This price differential reflects the performance advantages, including improved cycle life and enhanced safety characteristics that these advanced formulations provide.
Regional analysis shows Asia-Pacific dominating the market with approximately 65% share, led by China, Japan, and South Korea. North America and Europe follow with roughly 20% and 12% respectively, though both regions are investing heavily in domestic battery production capabilities that will likely alter this distribution by 2025.
Customer segmentation reveals distinct requirements across different applications. EV manufacturers prioritize electrolyte solutions that enable fast charging while maintaining thermal stability. Consumer electronics producers focus on energy density and cycle life, while grid storage applications emphasize long-term stability and cost-effectiveness.
Market barriers include supply chain constraints for high-purity lithium nitrate, with current global production capacity struggling to meet projected demand growth. Intellectual property landscapes present another challenge, as several key formulation approaches are protected by patents held by major battery manufacturers and chemical companies.
Competitive analysis identifies three tiers of market participants: established chemical conglomerates with extensive R&D capabilities, specialized battery material suppliers with proprietary formulations, and emerging startups focused on novel additive combinations. Recent market consolidation through strategic acquisitions suggests the industry recognizes the strategic importance of advanced electrolyte technology.
Technical Challenges in LiNO3-Organic Additive Systems
The integration of lithium nitrate (LiNO3) with organic additives presents significant technical challenges that must be addressed to enhance lithium-sulfur (Li-S) battery performance. The primary obstacle lies in the chemical incompatibility between LiNO3, a strong oxidizing agent, and many organic additives. When combined, these components often undergo undesired redox reactions, leading to premature decomposition of the organic additives and formation of byproducts that can impair battery functionality.
Solubility mismatch represents another major challenge. LiNO3 exhibits high solubility in polar solvents, whereas many effective organic additives are designed for non-polar or moderately polar environments. This fundamental difference creates phase separation issues in the electrolyte, resulting in non-uniform distribution of additives and compromised protective film formation on electrode surfaces.
The stability of LiNO3-organic additive systems across wide temperature ranges poses additional difficulties. At elevated temperatures, accelerated reaction rates between LiNO3 and organic components can lead to rapid degradation of the electrolyte system. Conversely, at lower temperatures, differential precipitation rates may cause component separation and electrolyte inhomogeneity.
Electrochemical window compatibility presents a further challenge. The effective voltage range where LiNO3 forms beneficial SEI layers must align with the stability window of organic additives. Misalignment can result in preferential decomposition of certain components, reducing the synergistic effects sought from the combination.
Long-term cycling stability remains problematic as the continuous consumption of LiNO3 during battery operation can alter the ratio between LiNO3 and organic additives over time. This changing composition progressively modifies the protective layer characteristics, often leading to diminished effectiveness in polysulfide suppression.
Manufacturing challenges also exist in ensuring homogeneous mixing and stable formulations at industrial scales. The sensitivity of these systems to moisture and oxygen requires stringent production environments, while achieving consistent quality across large production volumes demands precise process control.
Analytical limitations further complicate development efforts. Current characterization techniques struggle to provide real-time, in-situ monitoring of the complex interactions between LiNO3 and organic additives within operational batteries. This knowledge gap hinders systematic optimization of these multi-component systems.
Solubility mismatch represents another major challenge. LiNO3 exhibits high solubility in polar solvents, whereas many effective organic additives are designed for non-polar or moderately polar environments. This fundamental difference creates phase separation issues in the electrolyte, resulting in non-uniform distribution of additives and compromised protective film formation on electrode surfaces.
The stability of LiNO3-organic additive systems across wide temperature ranges poses additional difficulties. At elevated temperatures, accelerated reaction rates between LiNO3 and organic components can lead to rapid degradation of the electrolyte system. Conversely, at lower temperatures, differential precipitation rates may cause component separation and electrolyte inhomogeneity.
Electrochemical window compatibility presents a further challenge. The effective voltage range where LiNO3 forms beneficial SEI layers must align with the stability window of organic additives. Misalignment can result in preferential decomposition of certain components, reducing the synergistic effects sought from the combination.
Long-term cycling stability remains problematic as the continuous consumption of LiNO3 during battery operation can alter the ratio between LiNO3 and organic additives over time. This changing composition progressively modifies the protective layer characteristics, often leading to diminished effectiveness in polysulfide suppression.
Manufacturing challenges also exist in ensuring homogeneous mixing and stable formulations at industrial scales. The sensitivity of these systems to moisture and oxygen requires stringent production environments, while achieving consistent quality across large production volumes demands precise process control.
Analytical limitations further complicate development efforts. Current characterization techniques struggle to provide real-time, in-situ monitoring of the complex interactions between LiNO3 and organic additives within operational batteries. This knowledge gap hinders systematic optimization of these multi-component systems.
Current Approaches to Improve LiNO3 Compatibility
01 Compatibility with battery electrolytes
Lithium nitrate serves as an important additive in battery electrolytes, particularly for lithium-sulfur batteries, where it helps form a stable solid electrolyte interphase (SEI) layer. Its compatibility with various solvents and other electrolyte components is crucial for battery performance and longevity. The addition of lithium nitrate to electrolyte formulations can suppress the shuttle effect of polysulfides and improve cycling stability.- Lithium nitrate in battery electrolytes: Lithium nitrate is widely used as an additive in battery electrolytes, particularly for lithium-sulfur batteries, where it helps form a stable solid electrolyte interphase (SEI) layer. This compound improves battery performance by preventing polysulfide shuttling, enhancing cycling stability, and increasing coulombic efficiency. The compatibility of lithium nitrate with other electrolyte components is crucial for maintaining these beneficial effects without compromising battery safety or longevity.
- Thermal energy storage applications: Lithium nitrate is utilized in thermal energy storage systems due to its favorable thermophysical properties. It can be combined with other nitrate salts to form eutectic mixtures with lower melting points and higher thermal stability. These mixtures serve as heat transfer fluids or thermal storage media in concentrated solar power plants and other thermal energy applications. The compatibility of lithium nitrate with containment materials and other salts in these systems is essential for preventing corrosion and ensuring long-term stability.
- Compatibility with polymer materials: The interaction between lithium nitrate and various polymer materials is significant in applications such as solid-state batteries and composite materials. Lithium nitrate can be incorporated into polymer matrices to enhance ionic conductivity or serve as a flame retardant. The compatibility between lithium nitrate and polymers depends on factors such as the polymer's chemical structure, crystallinity, and functional groups. Proper selection of compatible polymers ensures optimal performance and stability of the resulting materials.
- Corrosion inhibition properties: Lithium nitrate exhibits corrosion inhibition properties when used with various metals and alloys. It can form protective layers on metal surfaces, preventing oxidation and degradation. The compatibility of lithium nitrate with different metallic materials is important in applications such as heat exchangers, cooling systems, and structural components exposed to harsh environments. The effectiveness of lithium nitrate as a corrosion inhibitor depends on factors such as concentration, temperature, and the presence of other chemical species.
- Chemical stability with other salts and compounds: The chemical stability of lithium nitrate when mixed with other salts and compounds is crucial for many applications. Lithium nitrate can participate in various chemical reactions, including oxidation-reduction processes and complex formation. Understanding these interactions is essential for formulating stable mixtures for applications in catalysis, electrochemistry, and materials synthesis. Factors affecting the compatibility include temperature, pressure, concentration, and the presence of moisture or impurities.
02 Thermal energy storage applications
Lithium nitrate is used in thermal energy storage systems due to its favorable thermophysical properties. Its compatibility with containment materials and other phase change materials is essential for effective heat storage applications. When combined with other nitrate salts, it can form eutectic mixtures with lower melting points and higher thermal stability, making these compositions suitable for concentrated solar power plants and other thermal energy storage systems.Expand Specific Solutions03 Corrosion inhibition properties
Lithium nitrate exhibits compatibility with various metal surfaces where it functions as a corrosion inhibitor. Its interaction with metal substrates forms protective layers that prevent oxidation and degradation. This property makes lithium nitrate valuable in formulations designed to protect metal components in various environments, particularly in high-temperature applications where conventional inhibitors might decompose.Expand Specific Solutions04 Compatibility with polymer matrices
The incorporation of lithium nitrate into polymer matrices creates composite materials with enhanced properties. Its compatibility with various polymers determines the mechanical and electrochemical characteristics of the resulting composites. These polymer-lithium nitrate composites find applications in solid-state electrolytes, where the lithium salt provides ionic conductivity while the polymer matrix offers mechanical stability and processability.Expand Specific Solutions05 Compatibility in concrete and construction materials
Lithium nitrate shows compatibility with cement and concrete mixtures, where it functions as an admixture to control setting time and enhance certain properties. Its interaction with other concrete components affects the overall performance of the construction material. When used in appropriate concentrations, lithium nitrate can help mitigate alkali-silica reactions in concrete, extending the service life of structures and reducing maintenance requirements.Expand Specific Solutions
Leading Companies in Battery Electrolyte Development
The lithium nitrate compatibility with organic additives market is in a growth phase, driven by increasing demand for high-performance batteries in electric vehicles and energy storage systems. The global market size is expanding rapidly, with projections indicating substantial growth as battery technology advances. Technologically, this field is moderately mature but still evolving, with key players demonstrating varying levels of innovation. Companies like Samsung SDI, Panasonic Holdings, and Sila Nanotechnologies lead commercial applications, while research institutions such as National Institute for Materials Science and Central South University contribute fundamental advancements. Specialized battery material manufacturers including POSCO Future M, Svolt Energy, and Zhejiang Zhonglan are developing proprietary solutions to enhance electrolyte stability and performance, creating a competitive landscape balanced between established corporations and emerging technology providers.
SAMSUNG SDI CO LTD
Technical Solution: Samsung SDI has developed a novel approach to increase lithium nitrate (LiNO3) compatibility with organic additives in lithium-sulfur (Li-S) batteries. Their technology involves creating a protective interface layer using LiNO3 combined with specifically designed organic additives like fluorinated ethers and sulfone derivatives. This combination forms a stable solid electrolyte interphase (SEI) on the lithium metal anode while simultaneously addressing the polysulfide shuttle effect. Samsung's approach includes a gradient concentration distribution of LiNO3 across the electrolyte, with higher concentrations near the lithium anode and lower concentrations near the sulfur cathode, optimizing the protective effects while minimizing negative interactions with organic additives. They've also developed proprietary solvent systems that enhance LiNO3 solubility and stability when combined with functional organic additives, resulting in extended cycle life exceeding 500 cycles with capacity retention above 80%.
Strengths: Samsung's approach effectively addresses the dual challenges of lithium metal protection and polysulfide suppression while maintaining compatibility between LiNO3 and organic additives. Their gradient concentration strategy is particularly innovative. Weaknesses: The complex formulation may increase production costs, and the long-term stability of these electrolyte systems under extreme temperature conditions remains a concern.
Hydro-Québec
Technical Solution: Hydro-Québec has pioneered an innovative approach to enhance lithium nitrate compatibility with organic additives through their patented "co-solvation strategy." This technique involves using specifically designed solvating polymers that form coordinated structures with lithium nitrate, effectively encapsulating it while maintaining its beneficial properties. Their research has shown that incorporating crown ethers and polyethylene oxide derivatives creates a molecular environment where LiNO3 can coexist with functional organic additives without detrimental interactions. The company has developed a multi-layer electrolyte system where LiNO3 is concentrated in a gel-polymer layer adjacent to the lithium metal anode, while organic additives are primarily distributed in a liquid electrolyte phase near the cathode. This spatial separation prevents direct chemical interactions while allowing each component to perform its intended function. Testing has demonstrated that this approach enables stable cycling of lithium-metal batteries for over 1000 cycles with minimal capacity fade, representing a significant advancement in electrolyte design for next-generation energy storage systems.
Strengths: Hydro-Québec's co-solvation approach effectively addresses the fundamental chemical incompatibility issues between LiNO3 and many organic additives, enabling their simultaneous use in advanced battery systems. Their spatial separation strategy is particularly effective. Weaknesses: The complex polymer structures may increase electrolyte viscosity, potentially reducing ionic conductivity and rate capability, especially at lower temperatures.
Key Patents in Electrolyte Additive Synergy
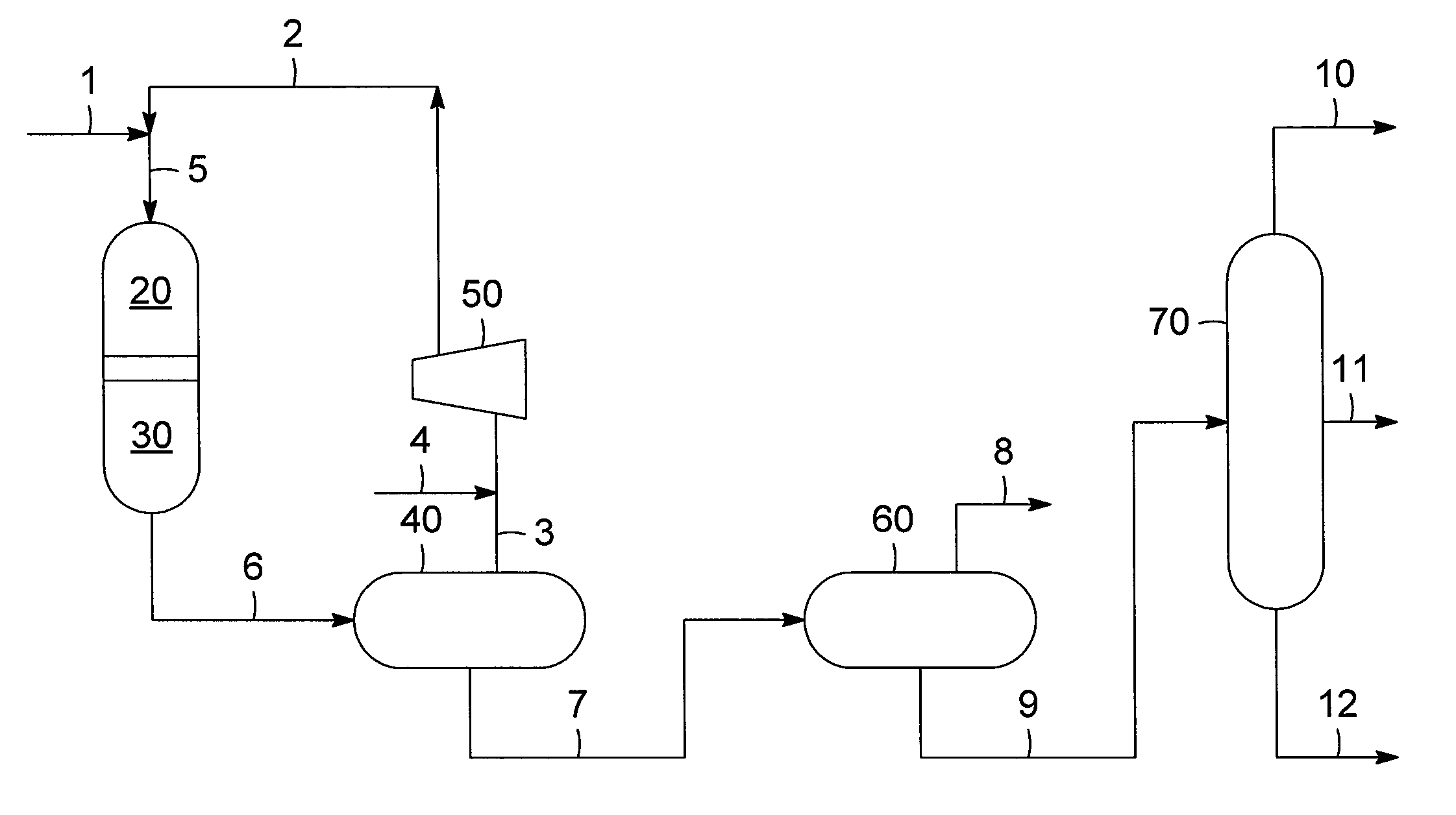
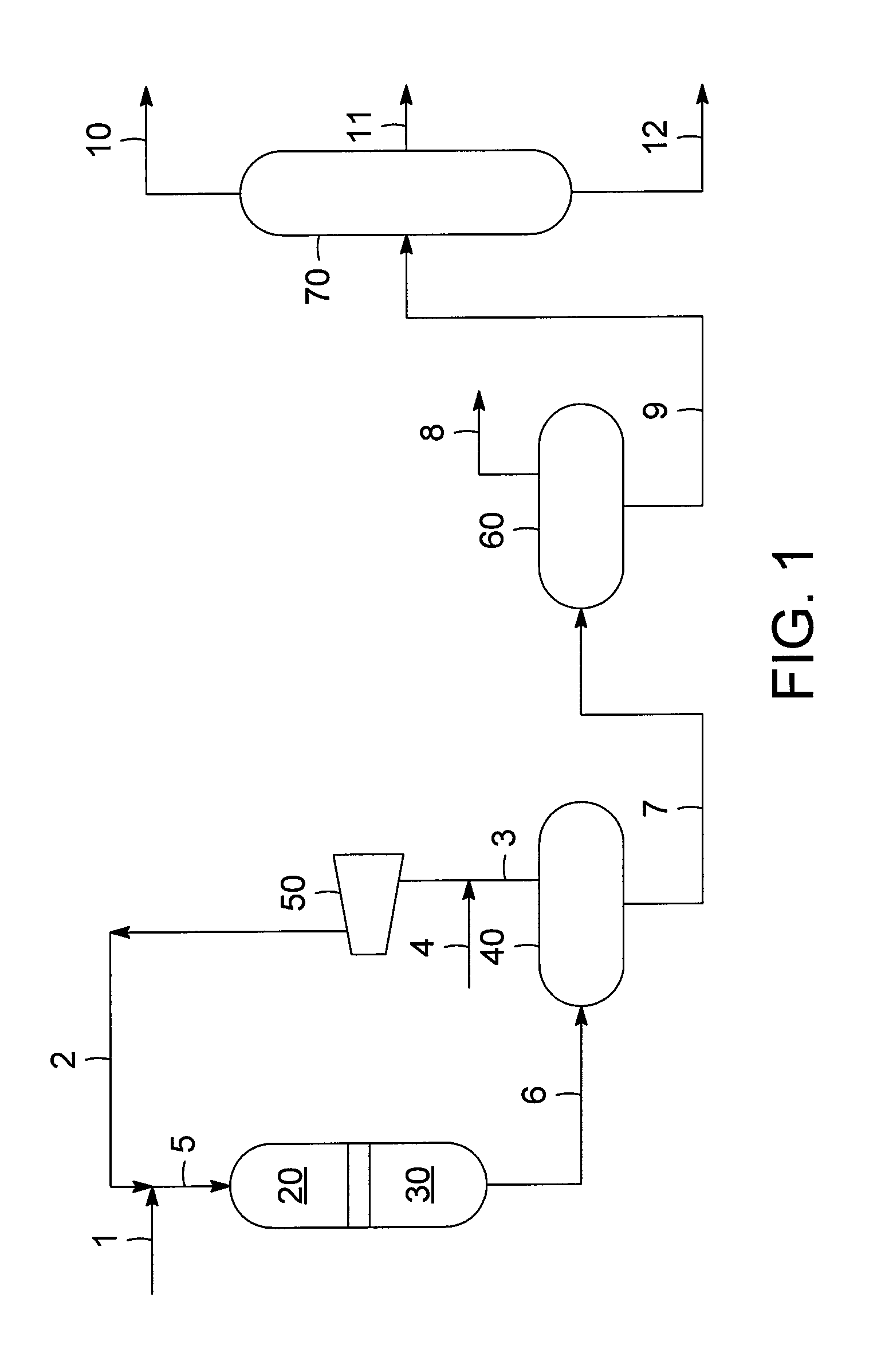
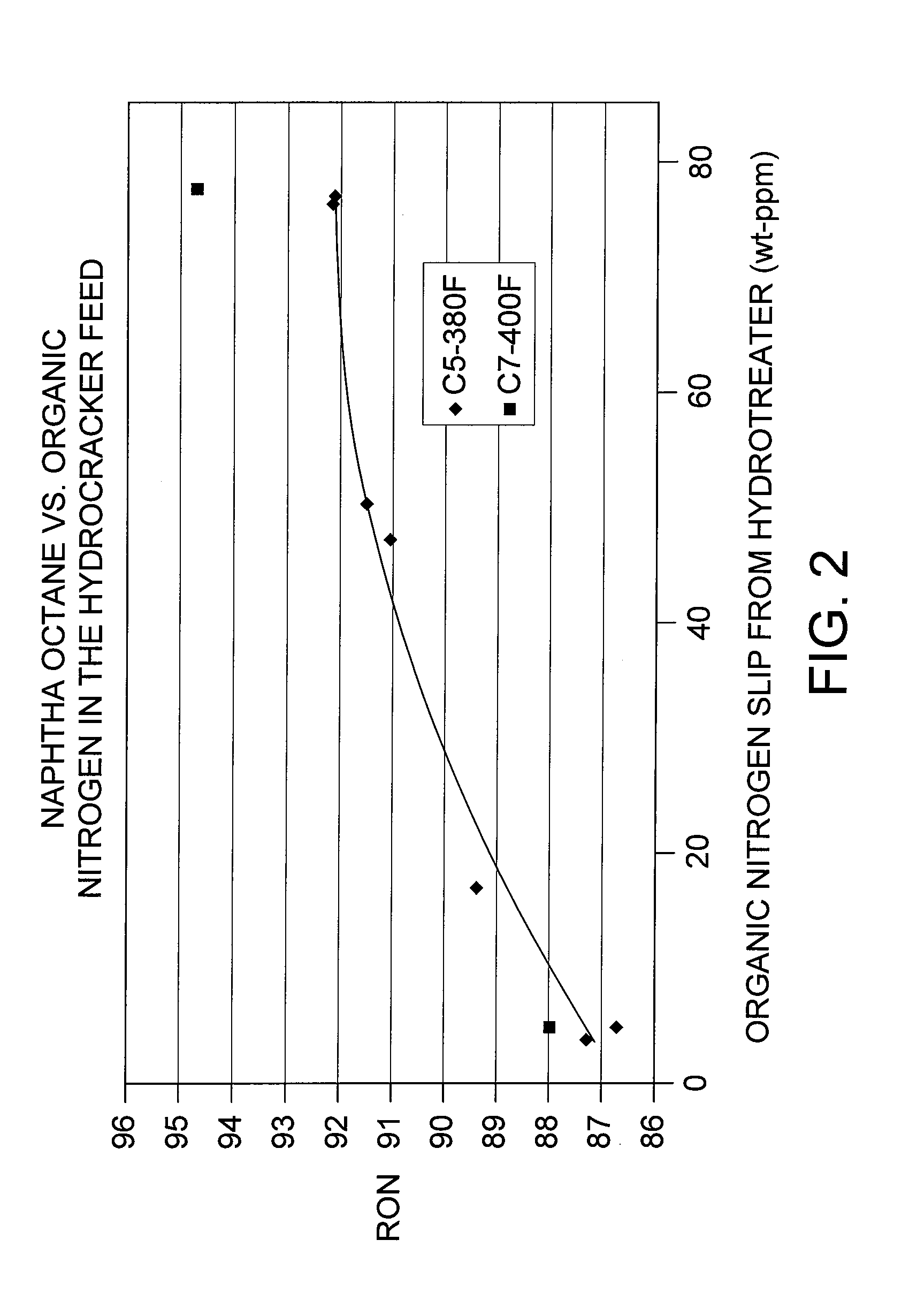
Combination of mild hydrotreating and hydrocracking for making low sulfur diesel and high octane naphtha
PatentActiveUS20100116712A1
Innovation
- A combination of mild hydrotreating and hydrocracking is employed, allowing organic nitrogen to 'slip' from the hydrotreating to the hydrocracking catalyst bed, which suppresses hydrogenation and retains aromatic compounds, thereby improving the quality of naphtha and diesel products.
Safety and Stability Considerations for Electrolyte Systems
The integration of lithium nitrate (LiNO3) with organic additives in electrolyte systems presents significant safety and stability challenges that must be carefully addressed. When combining these components, thermal stability becomes a primary concern as LiNO3 is a strong oxidizing agent that can react exothermically with organic materials under certain conditions. Laboratory testing has demonstrated that temperatures exceeding 80°C may trigger decomposition reactions, potentially leading to gas evolution and pressure build-up within battery cells.
Chemical compatibility assessments reveal that LiNO3 can undergo redox reactions with certain functional groups commonly found in organic additives, particularly those containing easily oxidizable moieties such as thiols, aldehydes, and some unsaturated compounds. These reactions not only compromise the intended function of the additives but may generate reactive intermediates that further degrade the electrolyte system.
Storage stability represents another critical consideration, as LiNO3-containing electrolytes have demonstrated accelerated aging when formulated with incompatible organic additives. Long-term testing indicates that such combinations can lead to gradual color changes, precipitate formation, and viscosity increases—all indicators of unwanted side reactions occurring within the electrolyte matrix.
From a safety perspective, the potential for gas generation deserves particular attention. When LiNO3 decomposes in the presence of certain organic compounds, nitrogen oxides (NOx) may be produced, creating both pressure and toxicity hazards. This risk necessitates robust cell design with appropriate pressure relief mechanisms and thorough safety validation protocols.
Handling procedures must be modified when working with LiNO3-enriched electrolytes. Standard protocols for conventional organic electrolytes may be insufficient due to the oxidizing nature of nitrate salts. Specialized training for manufacturing personnel and modified production environments with controlled humidity and temperature are essential to minimize safety risks during large-scale production.
Environmental factors significantly impact the stability of these complex electrolyte systems. Exposure to moisture can accelerate decomposition pathways, while UV light may trigger photochemical reactions in certain organic additives, subsequently affecting their interaction with LiNO3. Proper packaging and storage conditions must therefore be established to maintain electrolyte integrity throughout the product lifecycle.
Regulatory compliance adds another layer of complexity, as transportation and storage of oxidizing agents like LiNO3 are subject to strict regulations. When combined with organic solvents, these mixtures may fall under different hazard classifications, potentially requiring specialized containment systems and handling protocols to meet international safety standards.
Chemical compatibility assessments reveal that LiNO3 can undergo redox reactions with certain functional groups commonly found in organic additives, particularly those containing easily oxidizable moieties such as thiols, aldehydes, and some unsaturated compounds. These reactions not only compromise the intended function of the additives but may generate reactive intermediates that further degrade the electrolyte system.
Storage stability represents another critical consideration, as LiNO3-containing electrolytes have demonstrated accelerated aging when formulated with incompatible organic additives. Long-term testing indicates that such combinations can lead to gradual color changes, precipitate formation, and viscosity increases—all indicators of unwanted side reactions occurring within the electrolyte matrix.
From a safety perspective, the potential for gas generation deserves particular attention. When LiNO3 decomposes in the presence of certain organic compounds, nitrogen oxides (NOx) may be produced, creating both pressure and toxicity hazards. This risk necessitates robust cell design with appropriate pressure relief mechanisms and thorough safety validation protocols.
Handling procedures must be modified when working with LiNO3-enriched electrolytes. Standard protocols for conventional organic electrolytes may be insufficient due to the oxidizing nature of nitrate salts. Specialized training for manufacturing personnel and modified production environments with controlled humidity and temperature are essential to minimize safety risks during large-scale production.
Environmental factors significantly impact the stability of these complex electrolyte systems. Exposure to moisture can accelerate decomposition pathways, while UV light may trigger photochemical reactions in certain organic additives, subsequently affecting their interaction with LiNO3. Proper packaging and storage conditions must therefore be established to maintain electrolyte integrity throughout the product lifecycle.
Regulatory compliance adds another layer of complexity, as transportation and storage of oxidizing agents like LiNO3 are subject to strict regulations. When combined with organic solvents, these mixtures may fall under different hazard classifications, potentially requiring specialized containment systems and handling protocols to meet international safety standards.
Environmental Impact of Advanced Electrolyte Formulations
The environmental implications of advanced electrolyte formulations containing lithium nitrate and organic additives extend beyond performance considerations to encompass their full lifecycle impact. These formulations, while promising for battery technology advancement, introduce complex environmental challenges that require thorough assessment.
When lithium nitrate interacts with organic additives in electrolyte systems, the resulting compounds may exhibit varying degrees of biodegradability. Research indicates that certain organic additives can significantly reduce the environmental persistence of electrolyte components, while others may form more stable compounds that resist natural degradation processes. The compatibility enhancement techniques between lithium nitrate and organic additives must therefore consider end-of-life decomposition pathways.
Water contamination represents a critical environmental concern with these advanced formulations. Lithium compounds can mobilize in aquatic environments, potentially affecting freshwater ecosystems and drinking water sources. The addition of organic components may alter the solubility and transport characteristics of lithium compounds, sometimes creating more mobile species with greater potential for widespread contamination. Comprehensive leaching studies under various environmental conditions are essential to fully understand these risks.
Manufacturing processes for compatible lithium nitrate-organic additive formulations often require specialized solvents and processing aids that carry their own environmental footprints. Energy-intensive purification steps may be necessary to achieve the desired compatibility, contributing to increased carbon emissions. Alternative synthesis routes utilizing green chemistry principles show promise for reducing these impacts, with recent developments in solvent-free processing techniques demonstrating up to 40% reduction in process-related emissions.
Recycling considerations present both challenges and opportunities. The complex nature of these advanced electrolyte formulations can complicate traditional battery recycling processes, potentially reducing recovery rates for valuable materials. However, innovative separation technologies specifically designed for these formulations are emerging, with selective precipitation methods showing recovery efficiencies exceeding 85% for lithium compounds from mixed electrolyte systems.
Regulatory frameworks worldwide are increasingly addressing the environmental aspects of battery technologies, with particular attention to novel chemical formulations. The European Union's Battery Directive revisions and similar initiatives in Asia and North America are establishing more stringent requirements for lifecycle assessment and end-of-life management of advanced battery components, including electrolyte systems containing reactive additives like lithium nitrate.
When lithium nitrate interacts with organic additives in electrolyte systems, the resulting compounds may exhibit varying degrees of biodegradability. Research indicates that certain organic additives can significantly reduce the environmental persistence of electrolyte components, while others may form more stable compounds that resist natural degradation processes. The compatibility enhancement techniques between lithium nitrate and organic additives must therefore consider end-of-life decomposition pathways.
Water contamination represents a critical environmental concern with these advanced formulations. Lithium compounds can mobilize in aquatic environments, potentially affecting freshwater ecosystems and drinking water sources. The addition of organic components may alter the solubility and transport characteristics of lithium compounds, sometimes creating more mobile species with greater potential for widespread contamination. Comprehensive leaching studies under various environmental conditions are essential to fully understand these risks.
Manufacturing processes for compatible lithium nitrate-organic additive formulations often require specialized solvents and processing aids that carry their own environmental footprints. Energy-intensive purification steps may be necessary to achieve the desired compatibility, contributing to increased carbon emissions. Alternative synthesis routes utilizing green chemistry principles show promise for reducing these impacts, with recent developments in solvent-free processing techniques demonstrating up to 40% reduction in process-related emissions.
Recycling considerations present both challenges and opportunities. The complex nature of these advanced electrolyte formulations can complicate traditional battery recycling processes, potentially reducing recovery rates for valuable materials. However, innovative separation technologies specifically designed for these formulations are emerging, with selective precipitation methods showing recovery efficiencies exceeding 85% for lithium compounds from mixed electrolyte systems.
Regulatory frameworks worldwide are increasingly addressing the environmental aspects of battery technologies, with particular attention to novel chemical formulations. The European Union's Battery Directive revisions and similar initiatives in Asia and North America are establishing more stringent requirements for lifecycle assessment and end-of-life management of advanced battery components, including electrolyte systems containing reactive additives like lithium nitrate.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!