How to Detect Lithium Nitrate Phase Change via DTA Techniques
OCT 9, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Nitrate DTA Detection Background and Objectives
Lithium nitrate (LiNO3) has emerged as a critical material in various advanced technological applications, particularly in energy storage systems, thermal energy storage, and as a component in molten salt mixtures for concentrated solar power plants. The phase change behavior of lithium nitrate is of paramount importance in these applications, as it directly impacts the efficiency, safety, and longevity of the systems in which it is employed. Differential Thermal Analysis (DTA) represents one of the most effective analytical techniques for characterizing phase transitions in materials like lithium nitrate.
The evolution of DTA techniques for detecting phase changes in inorganic salts dates back to the early 20th century, with significant advancements occurring in the 1960s and 1970s when thermal analysis became more sophisticated and widely accessible. Over the past two decades, the integration of computerized data acquisition systems and advanced signal processing algorithms has dramatically enhanced the precision and reliability of DTA measurements for lithium nitrate and similar compounds.
Current technological trends in this field are moving toward higher sensitivity detection methods, miniaturization of equipment, and the development of in-situ monitoring capabilities that allow for real-time phase change detection in operational systems. These advancements are particularly relevant for lithium nitrate applications in next-generation energy storage technologies, where precise thermal management is essential.
The primary objective of this technical research is to comprehensively evaluate the state-of-the-art DTA methodologies specifically optimized for detecting phase transitions in lithium nitrate. This includes examining various experimental parameters such as heating/cooling rates, sample preparation techniques, and signal processing methods that influence the accuracy and reproducibility of measurements.
Additionally, this research aims to identify the limitations of current DTA techniques when applied to lithium nitrate, particularly in challenging conditions such as under varying pressure environments, in the presence of impurities, or when integrated into complex material systems. Understanding these constraints is crucial for developing improved detection methodologies.
Furthermore, this investigation seeks to establish standardized protocols for lithium nitrate phase change detection via DTA, which could serve as reference procedures for both research and industrial applications. Such standardization would facilitate more consistent and comparable results across different laboratories and manufacturing facilities, ultimately accelerating technological development in related fields.
The long-term technical goal is to contribute to the development of more efficient energy storage and thermal management systems by providing precise characterization tools for lithium nitrate phase behavior, thereby supporting innovation in renewable energy technologies and sustainable industrial processes.
The evolution of DTA techniques for detecting phase changes in inorganic salts dates back to the early 20th century, with significant advancements occurring in the 1960s and 1970s when thermal analysis became more sophisticated and widely accessible. Over the past two decades, the integration of computerized data acquisition systems and advanced signal processing algorithms has dramatically enhanced the precision and reliability of DTA measurements for lithium nitrate and similar compounds.
Current technological trends in this field are moving toward higher sensitivity detection methods, miniaturization of equipment, and the development of in-situ monitoring capabilities that allow for real-time phase change detection in operational systems. These advancements are particularly relevant for lithium nitrate applications in next-generation energy storage technologies, where precise thermal management is essential.
The primary objective of this technical research is to comprehensively evaluate the state-of-the-art DTA methodologies specifically optimized for detecting phase transitions in lithium nitrate. This includes examining various experimental parameters such as heating/cooling rates, sample preparation techniques, and signal processing methods that influence the accuracy and reproducibility of measurements.
Additionally, this research aims to identify the limitations of current DTA techniques when applied to lithium nitrate, particularly in challenging conditions such as under varying pressure environments, in the presence of impurities, or when integrated into complex material systems. Understanding these constraints is crucial for developing improved detection methodologies.
Furthermore, this investigation seeks to establish standardized protocols for lithium nitrate phase change detection via DTA, which could serve as reference procedures for both research and industrial applications. Such standardization would facilitate more consistent and comparable results across different laboratories and manufacturing facilities, ultimately accelerating technological development in related fields.
The long-term technical goal is to contribute to the development of more efficient energy storage and thermal management systems by providing precise characterization tools for lithium nitrate phase behavior, thereby supporting innovation in renewable energy technologies and sustainable industrial processes.
Market Applications for Lithium Nitrate Phase Change Analysis
The market for lithium nitrate phase change analysis via DTA techniques spans multiple high-value industries, with thermal energy storage representing the most significant application sector. Lithium nitrate's exceptional thermal properties—including high energy density, favorable melting point (255°C), and excellent thermal stability—make it an ideal candidate for concentrated solar power (CSP) systems. The global CSP market, projected to reach $24.5 billion by 2025 with a CAGR of 15.3%, relies heavily on precise phase change materials for efficient energy storage and transfer.
Battery manufacturing represents another crucial market segment, where lithium nitrate serves as an electrolyte additive in lithium-ion and solid-state batteries. Accurate phase change analysis enables manufacturers to optimize battery formulations, enhancing performance and safety characteristics. This application is particularly valuable as the electric vehicle battery market continues its exponential growth trajectory, expected to surpass $95 billion by 2028.
The aerospace and defense sectors utilize lithium nitrate phase change analysis for developing advanced thermal management systems in aircraft, satellites, and military equipment. These applications require materials with precisely characterized thermal properties to maintain optimal operating temperatures in extreme environments. The aerospace thermal management market alone is valued at approximately $8.7 billion with steady growth projections.
In the pharmaceutical industry, DTA-based phase change analysis of lithium compounds supports drug formulation processes, ensuring stability and efficacy across temperature ranges. This analytical capability is particularly valuable for temperature-sensitive medications and biologics, representing a specialized but high-margin application segment.
Electronics manufacturing benefits from lithium nitrate phase change analysis in developing thermal interface materials and cooling solutions for high-performance computing systems. As data centers and AI infrastructure expand globally, demand for advanced thermal management solutions continues to grow, creating sustained market opportunities.
The construction industry has emerging applications in phase change materials for building envelopes and HVAC systems, where lithium nitrate compounds can provide passive temperature regulation. This market segment, though currently smaller, shows promising growth potential as green building standards become more stringent worldwide.
Research institutions and analytical laboratories represent a consistent market for DTA instrumentation and methodologies specific to lithium compound analysis, driven by ongoing materials science research and development activities across multiple disciplines.
Battery manufacturing represents another crucial market segment, where lithium nitrate serves as an electrolyte additive in lithium-ion and solid-state batteries. Accurate phase change analysis enables manufacturers to optimize battery formulations, enhancing performance and safety characteristics. This application is particularly valuable as the electric vehicle battery market continues its exponential growth trajectory, expected to surpass $95 billion by 2028.
The aerospace and defense sectors utilize lithium nitrate phase change analysis for developing advanced thermal management systems in aircraft, satellites, and military equipment. These applications require materials with precisely characterized thermal properties to maintain optimal operating temperatures in extreme environments. The aerospace thermal management market alone is valued at approximately $8.7 billion with steady growth projections.
In the pharmaceutical industry, DTA-based phase change analysis of lithium compounds supports drug formulation processes, ensuring stability and efficacy across temperature ranges. This analytical capability is particularly valuable for temperature-sensitive medications and biologics, representing a specialized but high-margin application segment.
Electronics manufacturing benefits from lithium nitrate phase change analysis in developing thermal interface materials and cooling solutions for high-performance computing systems. As data centers and AI infrastructure expand globally, demand for advanced thermal management solutions continues to grow, creating sustained market opportunities.
The construction industry has emerging applications in phase change materials for building envelopes and HVAC systems, where lithium nitrate compounds can provide passive temperature regulation. This market segment, though currently smaller, shows promising growth potential as green building standards become more stringent worldwide.
Research institutions and analytical laboratories represent a consistent market for DTA instrumentation and methodologies specific to lithium compound analysis, driven by ongoing materials science research and development activities across multiple disciplines.
Current DTA Techniques and Limitations for Lithium Compounds
Differential Thermal Analysis (DTA) is a thermoanalytical technique that has been widely applied to study phase transitions in various materials, including lithium compounds. For lithium nitrate specifically, current DTA techniques involve measuring the temperature difference between a sample and an inert reference material as both are subjected to identical temperature regimes. The technique relies on detecting the heat absorbed or released during phase transitions, which manifest as peaks or valleys in the DTA curve.
Standard DTA equipment typically consists of a furnace, temperature sensors, sample holders, and data acquisition systems. For lithium nitrate analysis, platinum or alumina crucibles are commonly used due to their chemical inertness and thermal stability. The temperature range for lithium nitrate phase change detection typically spans from ambient temperature to approximately 350°C, covering its melting point of around 264°C.
Despite its widespread use, conventional DTA techniques face several limitations when applied to lithium compounds. One significant challenge is the high reactivity of lithium compounds with moisture and air, necessitating specialized sample preparation and handling procedures. Even trace amounts of contamination can significantly alter the thermal behavior and phase transition characteristics of lithium nitrate.
Another limitation is the thermal lag inherent in DTA measurements, which can lead to discrepancies between the actual and measured transition temperatures. This issue becomes particularly problematic when studying fast phase transitions or when precise temperature determination is critical for lithium nitrate applications in thermal energy storage systems.
Resolution constraints also affect the ability to detect subtle phase changes in lithium compounds. Minor transitions or those with low enthalpy changes may be obscured by baseline drift or instrumental noise. This is particularly challenging when analyzing complex lithium-based systems where multiple phase transitions may occur in close temperature proximity.
Sample size inconsistencies represent another limitation, as they can lead to variability in results. Smaller samples may provide better resolution but suffer from reduced signal strength, while larger samples may introduce thermal gradients within the sample itself, complicating the interpretation of phase change data for lithium nitrate.
Calibration issues further complicate DTA analysis of lithium compounds. The choice of calibration standards and procedures significantly impacts measurement accuracy. Many laboratories use different calibration protocols, leading to inconsistencies in reported transition temperatures across research publications on lithium nitrate phase behavior.
Advanced DTA techniques have emerged to address some of these limitations, including modulated DTA and high-pressure DTA systems. However, these specialized approaches often require expensive equipment and extensive operator expertise, limiting their accessibility for routine analysis of lithium compounds in many research and industrial settings.
Standard DTA equipment typically consists of a furnace, temperature sensors, sample holders, and data acquisition systems. For lithium nitrate analysis, platinum or alumina crucibles are commonly used due to their chemical inertness and thermal stability. The temperature range for lithium nitrate phase change detection typically spans from ambient temperature to approximately 350°C, covering its melting point of around 264°C.
Despite its widespread use, conventional DTA techniques face several limitations when applied to lithium compounds. One significant challenge is the high reactivity of lithium compounds with moisture and air, necessitating specialized sample preparation and handling procedures. Even trace amounts of contamination can significantly alter the thermal behavior and phase transition characteristics of lithium nitrate.
Another limitation is the thermal lag inherent in DTA measurements, which can lead to discrepancies between the actual and measured transition temperatures. This issue becomes particularly problematic when studying fast phase transitions or when precise temperature determination is critical for lithium nitrate applications in thermal energy storage systems.
Resolution constraints also affect the ability to detect subtle phase changes in lithium compounds. Minor transitions or those with low enthalpy changes may be obscured by baseline drift or instrumental noise. This is particularly challenging when analyzing complex lithium-based systems where multiple phase transitions may occur in close temperature proximity.
Sample size inconsistencies represent another limitation, as they can lead to variability in results. Smaller samples may provide better resolution but suffer from reduced signal strength, while larger samples may introduce thermal gradients within the sample itself, complicating the interpretation of phase change data for lithium nitrate.
Calibration issues further complicate DTA analysis of lithium compounds. The choice of calibration standards and procedures significantly impacts measurement accuracy. Many laboratories use different calibration protocols, leading to inconsistencies in reported transition temperatures across research publications on lithium nitrate phase behavior.
Advanced DTA techniques have emerged to address some of these limitations, including modulated DTA and high-pressure DTA systems. However, these specialized approaches often require expensive equipment and extensive operator expertise, limiting their accessibility for routine analysis of lithium compounds in many research and industrial settings.
Established DTA Protocols for Lithium Nitrate Characterization
01 Lithium nitrate as phase change material for thermal energy storage
Lithium nitrate is utilized as a phase change material (PCM) for thermal energy storage applications due to its high latent heat of fusion and suitable phase change temperature. When incorporated into thermal energy storage systems, lithium nitrate can absorb, store, and release large amounts of energy during its phase transition, making it effective for applications requiring efficient heat management and energy conservation.- Lithium nitrate as phase change material for thermal energy storage: Lithium nitrate is utilized as a phase change material (PCM) for thermal energy storage applications due to its favorable thermophysical properties. It undergoes phase transitions at specific temperatures, allowing it to absorb, store, and release large amounts of thermal energy. This makes it particularly valuable for solar thermal systems, building temperature regulation, and other applications requiring efficient heat management. The high energy density and relatively stable phase change characteristics of lithium nitrate make it an effective component in thermal storage solutions.
- Lithium nitrate in salt mixtures and eutectic compositions: Lithium nitrate is frequently incorporated into salt mixtures and eutectic compositions to modify and optimize phase change properties. By combining lithium nitrate with other salts like sodium nitrate, potassium nitrate, or calcium nitrate, the melting point and thermal storage capacity can be tailored for specific applications. These eutectic mixtures often exhibit lower melting points than the individual components while maintaining high energy storage density. The synergistic effects in these compositions enhance thermal conductivity and cycling stability, making them suitable for concentrated solar power plants and industrial heat storage systems.
- Phase change behavior and crystallization characteristics of lithium nitrate: The phase change behavior and crystallization characteristics of lithium nitrate are critical for its performance in thermal applications. During phase transitions, lithium nitrate exhibits specific crystallization patterns, nucleation rates, and growth kinetics that affect its thermal energy storage efficiency. Research focuses on understanding these mechanisms to control supercooling, improve cycling stability, and enhance heat transfer rates. Various techniques are employed to study the phase change dynamics, including differential scanning calorimetry, X-ray diffraction, and thermal analysis methods. Understanding these fundamental properties enables the development of more efficient thermal storage systems.
- Lithium nitrate in battery and electrochemical applications: Beyond thermal storage, lithium nitrate plays a significant role in battery and electrochemical applications where phase change properties are relevant. It is used as an additive in lithium-sulfur batteries to passivate lithium metal surfaces and suppress the shuttle effect. The phase behavior of lithium nitrate in electrolyte solutions affects the formation of solid-electrolyte interphase layers and influences battery performance. Additionally, lithium nitrate's phase transitions can be utilized in thermal management systems for batteries, helping to regulate operating temperatures and improve safety and efficiency.
- Encapsulation and stabilization techniques for lithium nitrate phase change materials: Various encapsulation and stabilization techniques are employed to enhance the performance and longevity of lithium nitrate as a phase change material. These methods include microencapsulation, nanoencapsulation, and incorporation into supporting matrices like polymers, ceramics, or metal foams. Such techniques help prevent leakage during the molten phase, reduce reactivity with container materials, improve thermal conductivity, and maintain cycling stability. Additionally, various additives and surface modifications are used to control nucleation, reduce supercooling, and enhance the overall thermal performance of lithium nitrate-based phase change materials.
02 Lithium nitrate in salt mixtures and eutectic compositions
Lithium nitrate is often combined with other salts to form eutectic mixtures with optimized phase change properties. These mixtures can achieve lower melting points, broader operating temperature ranges, and enhanced thermal stability compared to pure lithium nitrate. Common components in these mixtures include sodium nitrate, potassium nitrate, and calcium nitrate, which together create phase change materials with tailored thermal characteristics for specific applications.Expand Specific Solutions03 Lithium nitrate in battery and electrochemical applications
Lithium nitrate plays a significant role in battery technology, particularly in lithium-sulfur and lithium-ion batteries. During phase transitions, it can form protective layers on electrodes, improve electrolyte stability, and enhance the overall electrochemical performance. The phase behavior of lithium nitrate in these systems affects battery cycle life, capacity retention, and safety characteristics.Expand Specific Solutions04 Encapsulation and stabilization of lithium nitrate for phase change applications
Various encapsulation techniques are employed to stabilize lithium nitrate during phase change cycles and prevent leakage or degradation. These methods include microencapsulation, incorporation into supporting matrices, and composite formation with materials like graphene, carbon nanotubes, or polymers. Such stabilization approaches enhance the cycling stability, thermal conductivity, and practical applicability of lithium nitrate-based phase change materials.Expand Specific Solutions05 Phase change characteristics and modification of lithium nitrate
Research focuses on understanding and modifying the phase change characteristics of lithium nitrate, including its melting point, supercooling behavior, and crystallization kinetics. Various additives and processing techniques are employed to enhance phase change properties, improve thermal conductivity, and reduce supercooling. These modifications optimize the performance of lithium nitrate in thermal energy storage and other applications requiring controlled phase transitions.Expand Specific Solutions
Leading Manufacturers and Research Institutions in DTA Technology
The lithium nitrate phase change detection via DTA techniques market is currently in a growth phase, characterized by increasing research activities across academic and industrial sectors. The market size is expanding due to rising demand for energy storage solutions, particularly in lithium-ion battery applications. Technologically, this field shows moderate maturity with established players like NOVONIX Battery Technology Solutions, LG Energy Solution, and Agilent Technologies leading commercial applications, while academic institutions such as Arizona State University, The Ohio State University, and Tianjin University drive fundamental research. Companies like Murata Manufacturing and AVL List are advancing specialized instrumentation, while emerging players like Shanghai Mek Sheng Energy Technology are developing innovative diagnostic systems, creating a competitive landscape balanced between established analytical equipment providers and specialized energy storage technology developers.
AGILENT TECHNOLOGIES INC
Technical Solution: Agilent Technologies has developed advanced Differential Thermal Analysis (DTA) systems specifically optimized for lithium nitrate phase change detection. Their approach combines high-sensitivity thermal sensors with proprietary signal processing algorithms to accurately identify the endothermic and exothermic reactions associated with lithium nitrate phase transitions. The technology employs platinum-rhodium thermocouples with temperature accuracy of ±0.1°C and heat flow sensitivity down to 0.2μW. Their systems feature automated baseline correction and peak integration capabilities that can distinguish between overlapping thermal events common in complex lithium salt mixtures. Agilent's DTA instruments incorporate specialized sample holders designed to contain lithium compounds safely during heating cycles up to 600°C, with controlled atmosphere options to prevent sample oxidation or contamination during analysis.
Strengths: Superior signal-to-noise ratio allowing detection of subtle phase transitions; comprehensive software suite for data analysis; excellent reproducibility with standard deviation <1% for transition temperatures. Weaknesses: Higher cost compared to simpler thermal analysis systems; requires specialized training for optimal operation; sample preparation protocols can be time-consuming.
Beijing Institute of Technology
Technical Solution: Beijing Institute of Technology has pioneered an innovative DTA methodology specifically tailored for lithium nitrate phase change detection in energy storage applications. Their approach combines traditional DTA techniques with specialized sample preparation protocols that maintain the chemical integrity of lithium nitrate during thermal cycling. The system employs custom-designed crucibles with corrosion-resistant linings that prevent sample contamination while ensuring optimal thermal contact. BIT's technology features a dual-differential measurement system that simultaneously compares the sample against both an inert reference and a calibration standard, enabling precise determination of both the temperature and enthalpy of phase transitions. Their analytical software incorporates machine learning algorithms trained on extensive lithium salt thermal databases to automatically identify characteristic phase change signatures and distinguish them from other thermal events. The system achieves temperature resolution of ±0.05°C and can detect phase transitions with enthalpies as low as 0.5 J/g.
Strengths: Exceptional reproducibility with standard deviation <0.8% for transition temperatures; specialized algorithms for lithium compound analysis; excellent baseline stability during long-duration experiments. Weaknesses: Limited commercial availability outside research partnerships; requires specialized training for operation; higher initial setup complexity compared to commercial systems.
Critical Parameters Affecting Lithium Nitrate Phase Change Detection
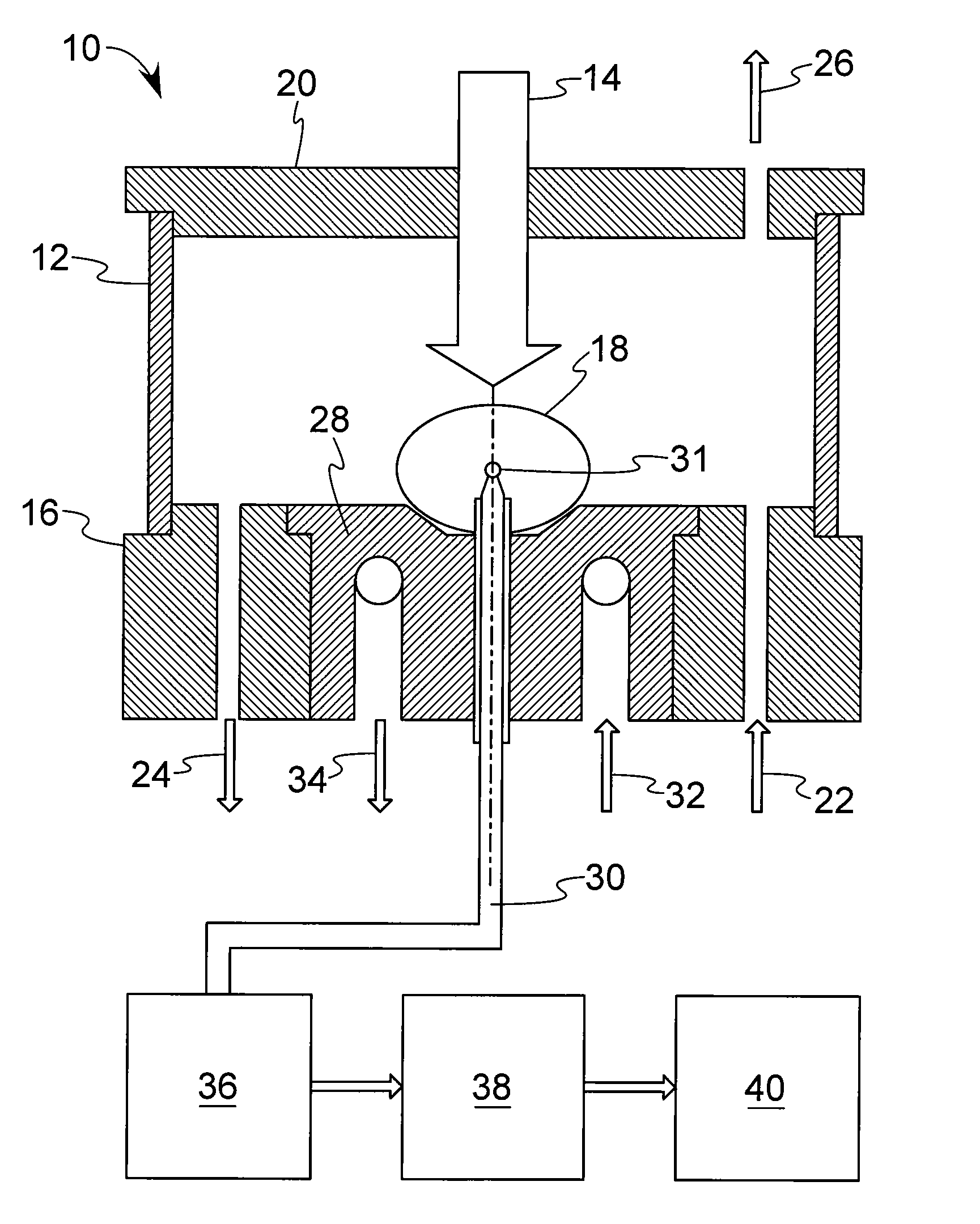
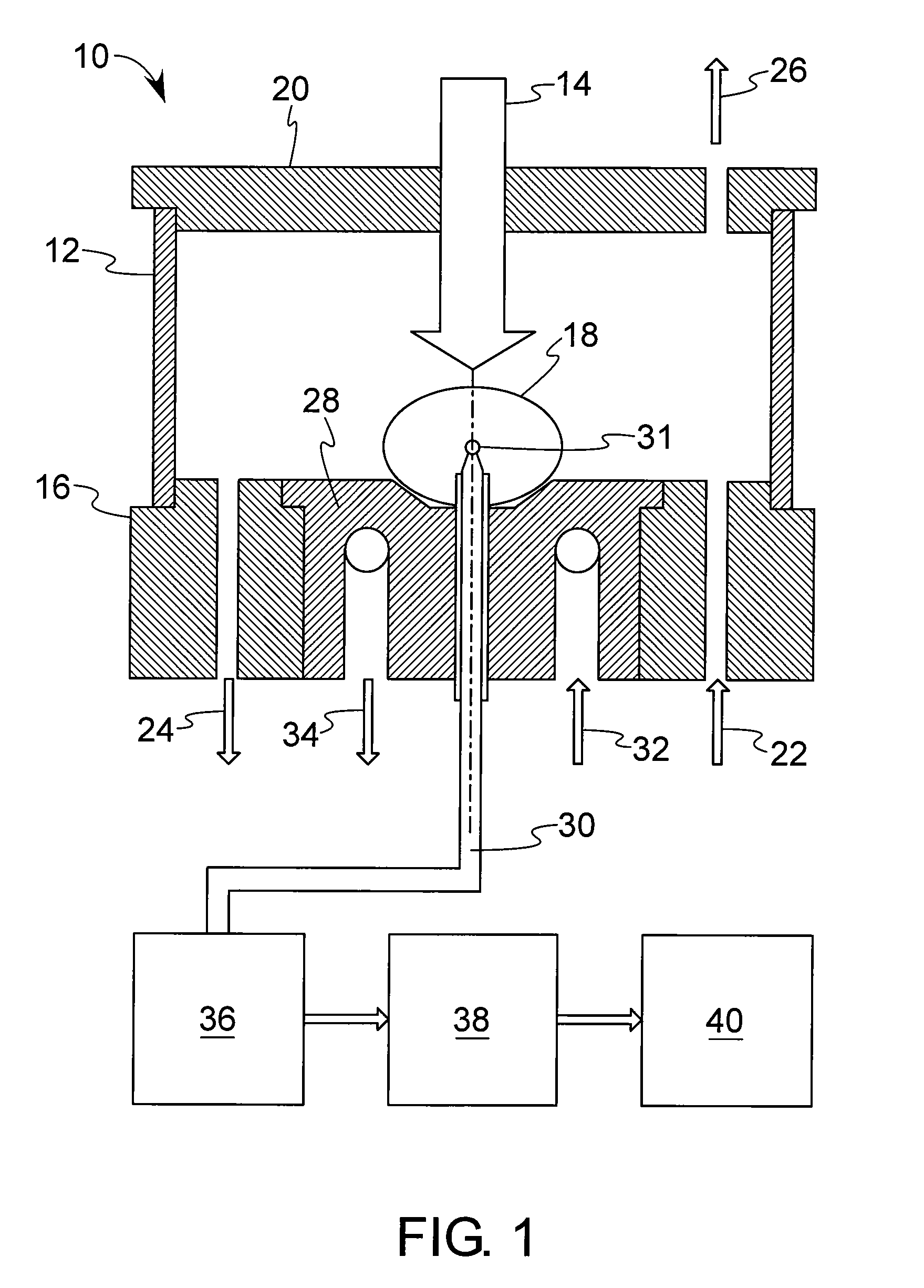
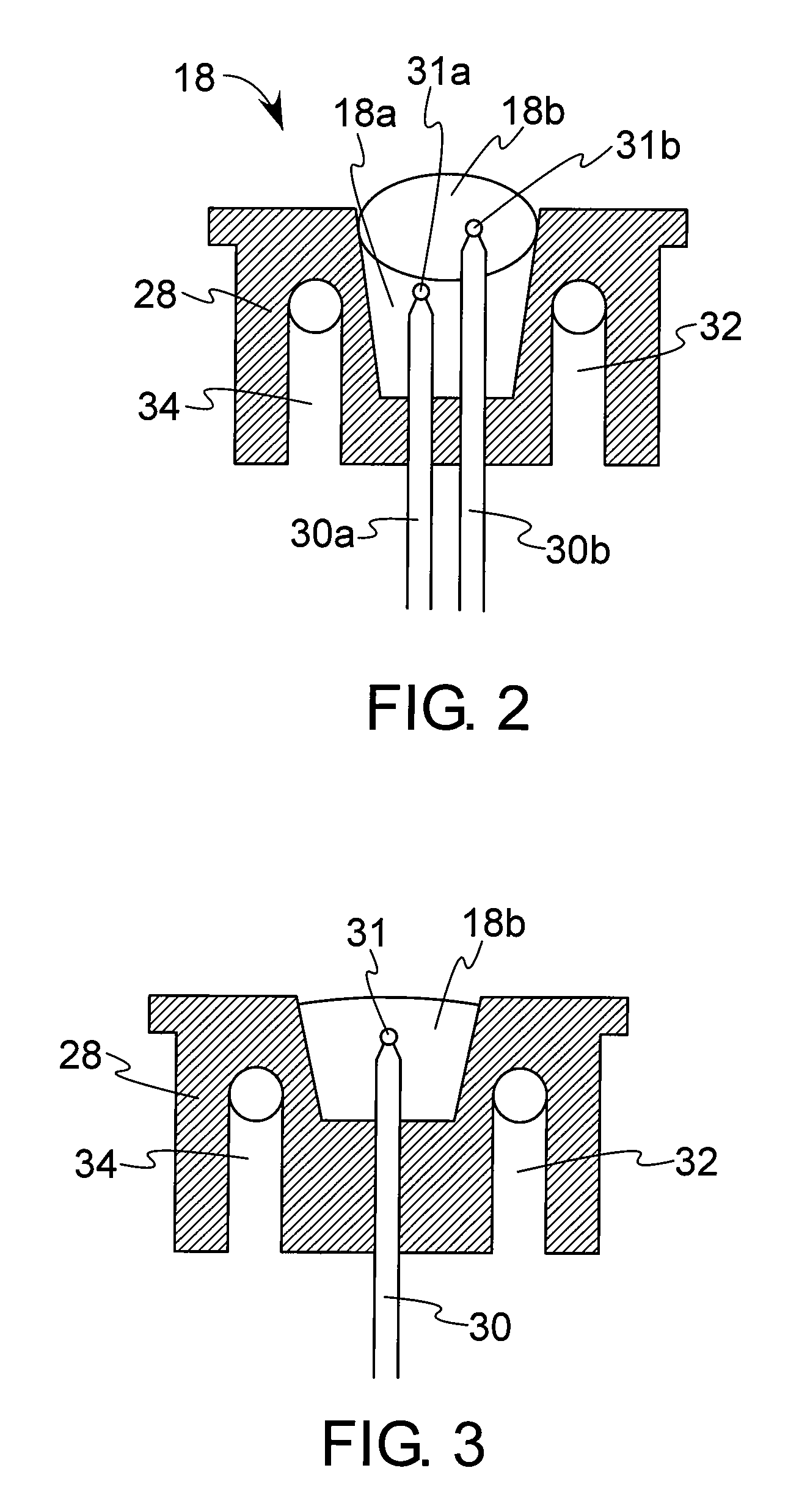
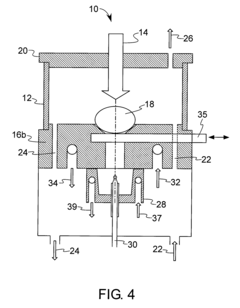
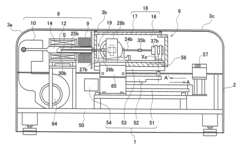
Method and device for investigation of phase transformations in metals and alloys
PatentActiveUS20090119057A1
Innovation
- The Single Sensor Differential Thermal Analysis (SSDTA) method calculates reference temperatures using numerical modeling, eliminating the need for a reference sensor and allowing for precise determination of phase transformation temperatures and structural changes under both simulated and actual processing conditions, including high heating and cooling rates.
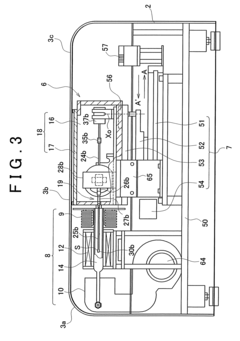
Thermal analysis apparatus
PatentInactiveUS7922386B2
Innovation
- A thermal analysis apparatus design where the sample temperature control unit remains stationary, and a sample moving unit, such as a sliding balance beam, allows for sample exchange at a distant position outside the temperature control unit, reducing the need for large-scale mechanisms and simplifying the structure for sample replacement.
Safety Considerations in Lithium Compound Analysis
The handling of lithium compounds, particularly lithium nitrate, during Differential Thermal Analysis (DTA) requires stringent safety protocols due to their reactive nature. When conducting phase change detection experiments, researchers must be aware that lithium nitrate can release toxic nitrogen oxide gases when heated above decomposition temperatures (approximately 600°C). Laboratory ventilation systems must be properly maintained and calibrated to handle these potential emissions, with fume hoods operating at optimal face velocities of 80-120 ft/min.
Personal protective equipment is essential when working with lithium compounds. This includes chemical-resistant gloves (nitrile or neoprene), safety goggles with side shields, lab coats, and in some cases, respiratory protection if ventilation is inadequate. Researchers should be trained in proper handling techniques to minimize exposure risks through inhalation, ingestion, or skin contact.
Fire safety represents another critical consideration as lithium nitrate is an oxidizer that can intensify fires when in contact with combustible materials. DTA laboratories must be equipped with appropriate fire extinguishers (Class D for lithium metal fires, if present) and fire suppression systems. Water-based firefighting methods should be avoided with certain lithium compounds due to potential violent reactions.
Chemical storage protocols must be strictly followed, keeping lithium compounds separated from incompatible materials such as reducing agents, organic compounds, and acids. Temperature-controlled storage is recommended to prevent degradation and maintain sample integrity for accurate DTA results.
Emergency response procedures specific to lithium compound incidents should be established and regularly practiced. This includes spill containment protocols, evacuation procedures, and decontamination methods. Safety data sheets for all lithium compounds must be readily accessible in the laboratory.
Waste management presents unique challenges, as lithium compounds require specialized disposal methods. DTA sample residues containing lithium nitrate should be collected separately and processed according to local hazardous waste regulations, never disposed of in regular trash or sink drains.
Instrument-specific safety considerations for DTA equipment include regular maintenance checks of gas lines, temperature controllers, and pressure relief systems. Calibration should be performed with safer reference materials before working with lithium compounds to ensure proper instrument function and minimize unexpected reactions during analysis.
Personal protective equipment is essential when working with lithium compounds. This includes chemical-resistant gloves (nitrile or neoprene), safety goggles with side shields, lab coats, and in some cases, respiratory protection if ventilation is inadequate. Researchers should be trained in proper handling techniques to minimize exposure risks through inhalation, ingestion, or skin contact.
Fire safety represents another critical consideration as lithium nitrate is an oxidizer that can intensify fires when in contact with combustible materials. DTA laboratories must be equipped with appropriate fire extinguishers (Class D for lithium metal fires, if present) and fire suppression systems. Water-based firefighting methods should be avoided with certain lithium compounds due to potential violent reactions.
Chemical storage protocols must be strictly followed, keeping lithium compounds separated from incompatible materials such as reducing agents, organic compounds, and acids. Temperature-controlled storage is recommended to prevent degradation and maintain sample integrity for accurate DTA results.
Emergency response procedures specific to lithium compound incidents should be established and regularly practiced. This includes spill containment protocols, evacuation procedures, and decontamination methods. Safety data sheets for all lithium compounds must be readily accessible in the laboratory.
Waste management presents unique challenges, as lithium compounds require specialized disposal methods. DTA sample residues containing lithium nitrate should be collected separately and processed according to local hazardous waste regulations, never disposed of in regular trash or sink drains.
Instrument-specific safety considerations for DTA equipment include regular maintenance checks of gas lines, temperature controllers, and pressure relief systems. Calibration should be performed with safer reference materials before working with lithium compounds to ensure proper instrument function and minimize unexpected reactions during analysis.
Data Processing Algorithms for DTA Signal Interpretation
The interpretation of Differential Thermal Analysis (DTA) signals for lithium nitrate phase change detection requires sophisticated data processing algorithms to extract meaningful information from raw thermal data. These algorithms serve as the bridge between experimental measurements and scientific conclusions, enabling researchers to accurately identify phase transitions and characterize thermal properties.
Signal filtering techniques represent the first critical step in DTA data processing. Raw DTA signals often contain various noise components from instrumental fluctuations, environmental interference, and random thermal events. Advanced digital filters including Savitzky-Golay, wavelet transforms, and adaptive filtering methods can effectively separate the genuine thermal response from background noise while preserving the critical features of phase transition peaks.
Baseline correction algorithms address the non-linear baseline drift commonly observed in DTA measurements of lithium nitrate. Polynomial fitting, spline interpolation, and asymmetric least squares approaches enable the isolation of phase change signals from the underlying baseline trend. For lithium nitrate specifically, temperature-dependent baseline models that account for the material's changing heat capacity across different phases yield superior results compared to simpler linear correction methods.
Peak detection and characterization algorithms constitute the core analytical component for phase transition identification. These algorithms employ derivative analysis, pattern recognition techniques, and statistical methods to precisely locate transition temperatures, measure enthalpy changes, and characterize peak shapes. Machine learning approaches, particularly convolutional neural networks, have recently demonstrated exceptional capability in identifying subtle phase transitions that might be overlooked by conventional threshold-based detection methods.
Deconvolution algorithms address overlapping thermal events that frequently occur during lithium nitrate phase transitions. Mathematical models based on Gaussian, Lorentzian, or asymmetric peak functions can separate complex thermal signatures into individual components, allowing for the identification of closely spaced phase transitions or simultaneous thermal events.
Calibration and standardization algorithms ensure measurement accuracy across different DTA instruments and experimental conditions. These algorithms incorporate reference materials with well-characterized phase transitions to establish temperature and enthalpy calibration curves, compensating for systematic instrumental biases and enabling quantitative comparison between different experimental datasets.
Data visualization techniques transform processed DTA signals into interpretable graphical representations. Advanced plotting methods including 3D surface plots, heat maps, and interactive visualization tools allow researchers to examine phase transition behavior across multiple dimensions such as temperature, heating rate, and sample composition simultaneously.
Signal filtering techniques represent the first critical step in DTA data processing. Raw DTA signals often contain various noise components from instrumental fluctuations, environmental interference, and random thermal events. Advanced digital filters including Savitzky-Golay, wavelet transforms, and adaptive filtering methods can effectively separate the genuine thermal response from background noise while preserving the critical features of phase transition peaks.
Baseline correction algorithms address the non-linear baseline drift commonly observed in DTA measurements of lithium nitrate. Polynomial fitting, spline interpolation, and asymmetric least squares approaches enable the isolation of phase change signals from the underlying baseline trend. For lithium nitrate specifically, temperature-dependent baseline models that account for the material's changing heat capacity across different phases yield superior results compared to simpler linear correction methods.
Peak detection and characterization algorithms constitute the core analytical component for phase transition identification. These algorithms employ derivative analysis, pattern recognition techniques, and statistical methods to precisely locate transition temperatures, measure enthalpy changes, and characterize peak shapes. Machine learning approaches, particularly convolutional neural networks, have recently demonstrated exceptional capability in identifying subtle phase transitions that might be overlooked by conventional threshold-based detection methods.
Deconvolution algorithms address overlapping thermal events that frequently occur during lithium nitrate phase transitions. Mathematical models based on Gaussian, Lorentzian, or asymmetric peak functions can separate complex thermal signatures into individual components, allowing for the identification of closely spaced phase transitions or simultaneous thermal events.
Calibration and standardization algorithms ensure measurement accuracy across different DTA instruments and experimental conditions. These algorithms incorporate reference materials with well-characterized phase transitions to establish temperature and enthalpy calibration curves, compensating for systematic instrumental biases and enabling quantitative comparison between different experimental datasets.
Data visualization techniques transform processed DTA signals into interpretable graphical representations. Advanced plotting methods including 3D surface plots, heat maps, and interactive visualization tools allow researchers to examine phase transition behavior across multiple dimensions such as temperature, heating rate, and sample composition simultaneously.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!