Quantify Lithium Nitrate Thermal Expansion Coefficients
OCT 9, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Nitrate Thermal Properties Background and Objectives
Lithium nitrate (LiNO3) has emerged as a critical material in various advanced technological applications, particularly in thermal energy storage systems, molten salt batteries, and as a component in heat transfer fluids. The thermal expansion properties of lithium nitrate are fundamental parameters that significantly influence the design, safety, and efficiency of these applications. Understanding these coefficients is essential for predicting material behavior under varying temperature conditions, which is crucial for engineering reliable systems.
The historical development of lithium nitrate research dates back to the mid-20th century, with initial investigations focusing primarily on its basic thermodynamic properties. However, comprehensive studies specifically targeting thermal expansion coefficients have been relatively limited, creating a significant knowledge gap in the scientific literature. This gap has become increasingly problematic as applications for lithium nitrate continue to expand in emerging clean energy technologies.
Recent technological advancements in renewable energy systems, particularly concentrated solar power (CSP) plants, have accelerated the demand for precise thermal property data of molten salts, including lithium nitrate. The thermal expansion behavior of lithium nitrate directly impacts containment vessel design, system pressurization, and overall operational safety parameters in these high-temperature applications.
The evolution of measurement techniques has also contributed to the renewed interest in quantifying these properties with greater precision. Modern dilatometry, X-ray diffraction methods, and computational modeling approaches now offer opportunities to characterize thermal expansion with unprecedented accuracy across wider temperature ranges and under various environmental conditions.
This technical research aims to comprehensively quantify the thermal expansion coefficients of lithium nitrate across its relevant phase states and temperature ranges, with particular emphasis on the behavior near phase transition points. The primary objective is to develop a reliable dataset and mathematical models that accurately describe the volumetric changes of lithium nitrate as a function of temperature.
Secondary objectives include correlating the thermal expansion behavior with the underlying molecular and crystal structure changes, identifying potential anomalies in expansion patterns, and establishing standardized measurement protocols that can be applied consistently across the industry. Additionally, this research seeks to explore how various impurities and additives might modify the thermal expansion characteristics of lithium nitrate, which has significant implications for commercial applications where high-purity materials may not always be economically feasible.
The ultimate goal is to provide engineers and scientists with reliable thermal expansion data that can be directly incorporated into design calculations, simulation models, and safety analyses for systems utilizing lithium nitrate, thereby advancing the development of more efficient and reliable energy storage and transfer technologies.
The historical development of lithium nitrate research dates back to the mid-20th century, with initial investigations focusing primarily on its basic thermodynamic properties. However, comprehensive studies specifically targeting thermal expansion coefficients have been relatively limited, creating a significant knowledge gap in the scientific literature. This gap has become increasingly problematic as applications for lithium nitrate continue to expand in emerging clean energy technologies.
Recent technological advancements in renewable energy systems, particularly concentrated solar power (CSP) plants, have accelerated the demand for precise thermal property data of molten salts, including lithium nitrate. The thermal expansion behavior of lithium nitrate directly impacts containment vessel design, system pressurization, and overall operational safety parameters in these high-temperature applications.
The evolution of measurement techniques has also contributed to the renewed interest in quantifying these properties with greater precision. Modern dilatometry, X-ray diffraction methods, and computational modeling approaches now offer opportunities to characterize thermal expansion with unprecedented accuracy across wider temperature ranges and under various environmental conditions.
This technical research aims to comprehensively quantify the thermal expansion coefficients of lithium nitrate across its relevant phase states and temperature ranges, with particular emphasis on the behavior near phase transition points. The primary objective is to develop a reliable dataset and mathematical models that accurately describe the volumetric changes of lithium nitrate as a function of temperature.
Secondary objectives include correlating the thermal expansion behavior with the underlying molecular and crystal structure changes, identifying potential anomalies in expansion patterns, and establishing standardized measurement protocols that can be applied consistently across the industry. Additionally, this research seeks to explore how various impurities and additives might modify the thermal expansion characteristics of lithium nitrate, which has significant implications for commercial applications where high-purity materials may not always be economically feasible.
The ultimate goal is to provide engineers and scientists with reliable thermal expansion data that can be directly incorporated into design calculations, simulation models, and safety analyses for systems utilizing lithium nitrate, thereby advancing the development of more efficient and reliable energy storage and transfer technologies.
Market Applications and Demand Analysis for Lithium Nitrate
The global market for lithium nitrate is experiencing significant growth, driven primarily by its diverse applications across multiple industries. The thermal expansion coefficient properties of lithium nitrate are particularly crucial for its application in thermal energy storage systems, where precise understanding of material behavior under temperature fluctuations is essential for system design and efficiency.
In the renewable energy sector, lithium nitrate serves as a critical component in molten salt mixtures for concentrated solar power (CSP) plants. The global CSP market is projected to grow at a compound annual growth rate of 10.3% through 2028, creating substantial demand for lithium nitrate with well-characterized thermal expansion properties. This growth is fueled by increasing investments in renewable energy infrastructure and the push for grid-scale energy storage solutions.
The battery industry represents another significant market driver. While lithium nitrate is not a primary battery component, it serves as an electrolyte additive that enhances the performance and safety of lithium-ion batteries. With electric vehicle sales continuing to surge globally, the demand for advanced battery technologies incorporating lithium nitrate is expected to increase proportionally.
High-temperature ceramics and specialty glass manufacturing constitute another substantial market segment. These industries require precise knowledge of thermal expansion coefficients to ensure product integrity during manufacturing processes. The global specialty glass market, valued at approximately 53 billion USD in 2022, is expected to continue its growth trajectory, further driving demand for lithium nitrate with well-documented thermal properties.
The pharmaceutical and chemical synthesis sectors utilize lithium nitrate as a reagent in various processes. These applications demand high-purity materials with consistent and predictable behavior under varying temperature conditions, making thermal expansion data essential for process optimization and quality control.
Market analysis indicates regional variations in demand patterns. Asia-Pacific, particularly China, leads global consumption due to its dominant position in battery manufacturing and renewable energy development. North America and Europe follow, with demand primarily driven by advanced materials research and renewable energy applications.
Industry stakeholders consistently express the need for more accurate and comprehensive thermal expansion coefficient data across wider temperature ranges. This market requirement stems from the increasing precision demands in engineering applications and the trend toward operating systems at higher temperatures for improved efficiency. Research institutions and material testing laboratories have identified this data gap as a significant opportunity for value creation in the lithium nitrate supply chain.
In the renewable energy sector, lithium nitrate serves as a critical component in molten salt mixtures for concentrated solar power (CSP) plants. The global CSP market is projected to grow at a compound annual growth rate of 10.3% through 2028, creating substantial demand for lithium nitrate with well-characterized thermal expansion properties. This growth is fueled by increasing investments in renewable energy infrastructure and the push for grid-scale energy storage solutions.
The battery industry represents another significant market driver. While lithium nitrate is not a primary battery component, it serves as an electrolyte additive that enhances the performance and safety of lithium-ion batteries. With electric vehicle sales continuing to surge globally, the demand for advanced battery technologies incorporating lithium nitrate is expected to increase proportionally.
High-temperature ceramics and specialty glass manufacturing constitute another substantial market segment. These industries require precise knowledge of thermal expansion coefficients to ensure product integrity during manufacturing processes. The global specialty glass market, valued at approximately 53 billion USD in 2022, is expected to continue its growth trajectory, further driving demand for lithium nitrate with well-documented thermal properties.
The pharmaceutical and chemical synthesis sectors utilize lithium nitrate as a reagent in various processes. These applications demand high-purity materials with consistent and predictable behavior under varying temperature conditions, making thermal expansion data essential for process optimization and quality control.
Market analysis indicates regional variations in demand patterns. Asia-Pacific, particularly China, leads global consumption due to its dominant position in battery manufacturing and renewable energy development. North America and Europe follow, with demand primarily driven by advanced materials research and renewable energy applications.
Industry stakeholders consistently express the need for more accurate and comprehensive thermal expansion coefficient data across wider temperature ranges. This market requirement stems from the increasing precision demands in engineering applications and the trend toward operating systems at higher temperatures for improved efficiency. Research institutions and material testing laboratories have identified this data gap as a significant opportunity for value creation in the lithium nitrate supply chain.
Current Measurement Techniques and Challenges
The quantification of lithium nitrate thermal expansion coefficients presents significant measurement challenges due to the material's unique properties and behavior under varying temperature conditions. Current measurement techniques can be broadly categorized into dilatometric methods, X-ray diffraction (XRD), optical methods, and computational approaches, each with distinct advantages and limitations.
Dilatometric methods, including push-rod dilatometry and capacitance dilatometry, are widely employed for measuring thermal expansion of lithium nitrate. Push-rod dilatometers offer good precision (typically 10^-6 K^-1) and can operate across a wide temperature range (25°C to 1000°C), making them suitable for lithium nitrate's phase transition studies. However, these methods face challenges with sample preparation, as lithium nitrate's hygroscopic nature can lead to moisture absorption during handling, potentially affecting measurement accuracy.
X-ray diffraction techniques provide high-precision measurements of lattice parameter changes with temperature, enabling direct calculation of thermal expansion coefficients. For lithium nitrate, high-temperature XRD allows observation of structural changes during phase transitions, particularly important given its complex polymorphic behavior. The primary challenge lies in maintaining precise temperature control during measurements and accounting for thermal gradients within the sample chamber.
Optical interferometry methods offer non-contact measurement capabilities with extremely high resolution (10^-7 K^-1), beneficial for temperature-sensitive materials like lithium nitrate. However, these techniques require specialized sample preparation and carefully controlled environments to prevent surface degradation of lithium nitrate samples, which can compromise measurement integrity.
Thermomechanical analysis (TMA) represents another common approach, providing direct measurement of dimensional changes with temperature. While offering good sensitivity, TMA faces challenges with lithium nitrate due to the material's relatively low melting point (264°C) and potential reactivity with measurement apparatus components at elevated temperatures.
A significant challenge across all measurement techniques is lithium nitrate's anisotropic thermal expansion behavior, particularly in its crystalline phases. This anisotropy necessitates multiple measurements along different crystallographic directions to fully characterize the material's thermal expansion properties, substantially increasing experimental complexity and time requirements.
Calibration represents another persistent challenge, as standard reference materials with thermal properties similar to lithium nitrate are limited. This creates difficulties in establishing measurement traceability and validating experimental results across different laboratories and measurement techniques.
Recent advances in in-situ measurement capabilities have improved data quality, but challenges remain in achieving the necessary precision for applications in thermal energy storage systems, where accurate thermal expansion data is critical for predicting stress development and potential mechanical failures during thermal cycling.
Dilatometric methods, including push-rod dilatometry and capacitance dilatometry, are widely employed for measuring thermal expansion of lithium nitrate. Push-rod dilatometers offer good precision (typically 10^-6 K^-1) and can operate across a wide temperature range (25°C to 1000°C), making them suitable for lithium nitrate's phase transition studies. However, these methods face challenges with sample preparation, as lithium nitrate's hygroscopic nature can lead to moisture absorption during handling, potentially affecting measurement accuracy.
X-ray diffraction techniques provide high-precision measurements of lattice parameter changes with temperature, enabling direct calculation of thermal expansion coefficients. For lithium nitrate, high-temperature XRD allows observation of structural changes during phase transitions, particularly important given its complex polymorphic behavior. The primary challenge lies in maintaining precise temperature control during measurements and accounting for thermal gradients within the sample chamber.
Optical interferometry methods offer non-contact measurement capabilities with extremely high resolution (10^-7 K^-1), beneficial for temperature-sensitive materials like lithium nitrate. However, these techniques require specialized sample preparation and carefully controlled environments to prevent surface degradation of lithium nitrate samples, which can compromise measurement integrity.
Thermomechanical analysis (TMA) represents another common approach, providing direct measurement of dimensional changes with temperature. While offering good sensitivity, TMA faces challenges with lithium nitrate due to the material's relatively low melting point (264°C) and potential reactivity with measurement apparatus components at elevated temperatures.
A significant challenge across all measurement techniques is lithium nitrate's anisotropic thermal expansion behavior, particularly in its crystalline phases. This anisotropy necessitates multiple measurements along different crystallographic directions to fully characterize the material's thermal expansion properties, substantially increasing experimental complexity and time requirements.
Calibration represents another persistent challenge, as standard reference materials with thermal properties similar to lithium nitrate are limited. This creates difficulties in establishing measurement traceability and validating experimental results across different laboratories and measurement techniques.
Recent advances in in-situ measurement capabilities have improved data quality, but challenges remain in achieving the necessary precision for applications in thermal energy storage systems, where accurate thermal expansion data is critical for predicting stress development and potential mechanical failures during thermal cycling.
Established Methods for Quantifying Thermal Expansion
01 Thermal expansion properties of lithium nitrate in energy storage systems
Lithium nitrate is used in thermal energy storage systems due to its favorable thermal expansion characteristics. When incorporated into phase change materials or molten salt mixtures, lithium nitrate helps maintain structural integrity during temperature cycling. Its controlled thermal expansion coefficient makes it suitable for applications requiring stable volumetric behavior across wide temperature ranges, particularly in solar thermal and industrial heat storage applications.- Thermal expansion properties of lithium nitrate in energy storage systems: Lithium nitrate exhibits specific thermal expansion characteristics that make it suitable for use in thermal energy storage systems. The thermal expansion coefficient of lithium nitrate is a critical parameter when designing these systems, as it affects the volumetric changes during phase transitions. Understanding these properties helps in developing more efficient heat storage materials and preventing structural damage due to expansion and contraction cycles.
- Lithium nitrate in battery and electronic applications: The thermal expansion coefficient of lithium nitrate is an important consideration in battery technology and electronic applications. When lithium nitrate is used in these contexts, its expansion behavior under varying temperatures affects the performance and durability of components. Proper formulation and design accounting for these thermal properties can improve the stability and lifespan of electronic devices and battery systems.
- Measurement techniques for thermal expansion coefficients of lithium compounds: Various specialized techniques have been developed to accurately measure the thermal expansion coefficients of lithium nitrate and related compounds. These methods include dilatometry, X-ray diffraction analysis, and thermal mechanical analysis. Precise measurement is essential for engineering applications where dimensional stability under temperature fluctuations is critical.
- Composite materials containing lithium nitrate with controlled thermal expansion: Composite materials incorporating lithium nitrate can be engineered to have specific thermal expansion properties. By combining lithium nitrate with other materials having complementary expansion characteristics, researchers have developed composites with tailored thermal behavior. These composites find applications in specialized industrial settings where thermal stability and predictable dimensional changes are required.
- Temperature-dependent phase transitions affecting expansion coefficients: Lithium nitrate undergoes phase transitions at specific temperatures that significantly affect its thermal expansion coefficient. These transitions can cause abrupt changes in volume and physical properties. Research has focused on characterizing these transitions and their effects on the overall thermal expansion behavior, which is crucial for applications involving temperature cycling or operation near transition points.
02 Lithium nitrate in electronic and semiconductor applications
Lithium nitrate is utilized in electronic components and semiconductor manufacturing where precise thermal expansion control is critical. The material's thermal expansion properties help manage stress in layered structures and interfaces. It is incorporated into specialized coatings, substrates, and bonding materials to maintain dimensional stability during thermal cycling, which is essential for reliability in microelectronic devices and integrated circuits.Expand Specific Solutions03 Measurement techniques for lithium nitrate thermal expansion coefficients
Various specialized techniques are employed to accurately measure the thermal expansion coefficients of lithium nitrate under different conditions. These include dilatometry, interferometric methods, and X-ray diffraction analysis. Advanced thermal analysis equipment allows for precise determination of expansion behavior across different temperature ranges and physical states, providing critical data for engineering applications requiring tight dimensional control.Expand Specific Solutions04 Lithium nitrate composites with modified thermal expansion properties
Composite materials incorporating lithium nitrate can be engineered to have customized thermal expansion characteristics. By combining lithium nitrate with other materials such as ceramics, polymers, or other salts, the overall thermal expansion coefficient can be tailored for specific applications. These composites offer improved thermal stability, reduced expansion mismatch, and enhanced performance in extreme temperature environments.Expand Specific Solutions05 Lithium nitrate in high-temperature industrial applications
Lithium nitrate's thermal expansion behavior makes it valuable in various high-temperature industrial applications. It is used in specialized glass formulations, ceramic processing, and refractory materials where controlled thermal expansion is essential. The material's expansion characteristics help prevent cracking, warping, and other thermal stress-related failures in components exposed to extreme temperature variations or thermal cycling.Expand Specific Solutions
Leading Research Institutions and Industrial Players
The lithium nitrate thermal expansion coefficient market is in a growth phase, driven by increasing demand in energy storage applications. The market size is expanding due to the rising adoption of lithium-based technologies in renewable energy systems and electric vehicles. Technologically, the field shows moderate maturity with ongoing research to improve measurement precision and application efficiency. Leading players include LG Chem and Sumitomo Chemical, who are investing in advanced materials research, while specialized companies like SCHOTT AG and MARUWA CO. contribute significant expertise in thermal property characterization. Academic institutions such as Kyoto University and Kunming University of Science & Technology are advancing fundamental research, creating a competitive landscape balanced between industrial applications and scientific development.
Kunming University of Science & Technology
Technical Solution: Kunming University has developed an innovative approach to quantifying lithium nitrate thermal expansion coefficients using a custom-built optical interferometry system combined with controlled environmental chambers. Their methodology allows for real-time, non-contact measurement of dimensional changes with nanometer-scale resolution. The research team has characterized lithium nitrate's thermal expansion behavior across multiple crystallographic orientations, documenting significant anisotropy with coefficients ranging from 4.0×10^-5 K^-1 to 5.5×10^-5 K^-1. Their work has particularly focused on the temperature range relevant to energy storage applications (0-100°C), where they've identified subtle phase transition effects that influence expansion behavior. The university has also pioneered techniques for measuring thermal expansion in lithium nitrate composites and mixtures, establishing how the presence of other salts and additives can modify the overall expansion characteristics.
Strengths: Exceptional measurement resolution through optical techniques; specialized focus on energy storage relevant conditions; comprehensive analysis of mixed systems and composites. Weaknesses: Limited temperature range compared to some other research groups; relatively new methodology with less extensive validation history.
Sumitomo Chemical Co., Ltd.
Technical Solution: Sumitomo Chemical has established a comprehensive methodology for quantifying lithium nitrate thermal expansion coefficients using a combination of push-rod dilatometry and laser interferometry techniques. Their approach incorporates specialized sample preparation methods that minimize moisture absorption, a critical factor affecting measurement accuracy for hygroscopic materials like lithium nitrate. The company has documented linear thermal expansion coefficients averaging 4.8×10^-5 K^-1 across the temperature range of -20°C to 150°C, with particular attention to the behavior near phase transition points. Sumitomo has integrated these measurements into their materials database for advanced ceramics and battery components, enabling precise thermal stress modeling in multi-material systems. Their research has also explored how particle size and crystallinity affect the measured expansion coefficients, demonstrating that nanocrystalline lithium nitrate exhibits significantly different expansion behavior compared to bulk material.
Strengths: Excellent control of environmental factors during measurement; comprehensive characterization across multiple material forms (powder, sintered, crystalline); direct application to industrial product development. Weaknesses: Primarily focused on application-specific temperature ranges rather than fundamental characterization; limited publication of raw data due to proprietary concerns.
Critical Patents and Literature on Lithium Nitrate Properties
Transmittance-variable device
PatentActiveUS12092907B2
Innovation
- Incorporating a specific retardation film arrangement with an active liquid crystal layer that can switch between transparent and black modes, utilizing a retardation film with high optical and mechanical anisotropy to minimize these phenomena and enhance transmittance variability.
Materials with low or negative thermal expansion
PatentInactiveUS5919720A
Innovation
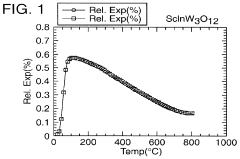
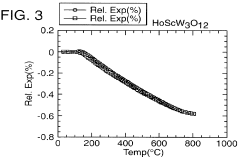
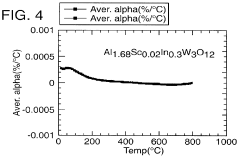
- A novel class of low or negative thermal expansion materials is developed, following the formula A2-x3+ A y4+ M z3+ M3-y6+ P yO12, where y can vary from 0 to 2, allowing for compounds like Al1.5 In0.5 W3 O12 and Hf2 WP2 O12, which can be synthesized to have varying thermal expansion properties, stability up to 1500°C, and be used to adjust the thermal expansion of other materials.
Material Safety and Handling Considerations
Handling lithium nitrate requires strict adherence to safety protocols due to its reactive nature and potential health hazards. When working with lithium nitrate for thermal expansion coefficient measurements, personnel must wear appropriate personal protective equipment (PPE), including chemical-resistant gloves, safety goggles, lab coats, and respiratory protection when necessary. The compound is a strong oxidizer that can intensify fires when in contact with combustible materials, necessitating storage away from reducing agents, organic materials, and ignition sources.
Exposure to lithium nitrate can cause severe irritation to the eyes, skin, and respiratory system. Direct contact may result in chemical burns, while inhalation of dust or decomposition products can lead to respiratory distress. Chronic exposure has been associated with potential neurological effects due to lithium accumulation in the body. Emergency protocols should include immediate flushing with water for skin or eye contact and seeking medical attention.
Laboratory environments conducting thermal expansion coefficient measurements must be equipped with adequate ventilation systems, preferably including local exhaust ventilation. Facilities should maintain emergency eyewash stations and safety showers in close proximity to handling areas. Temperature control is critical during experiments, as lithium nitrate decomposes at approximately 600°C, potentially releasing nitrogen oxides and lithium oxide.
Proper storage requirements include keeping lithium nitrate in tightly sealed containers in cool, dry areas. Hygroscopic properties necessitate protection from moisture to prevent degradation and maintain sample integrity for accurate thermal expansion measurements. Incompatibility with strong reducing agents, metals, and organic compounds must be considered in storage planning.
Waste disposal must comply with local regulations for chemical waste management. Solutions containing lithium nitrate should never be disposed of down laboratory drains. Instead, they should be collected in designated containers for professional disposal. Spill response procedures should be established, including containment methods, neutralization protocols, and proper documentation of incidents.
Training programs for laboratory personnel should cover specific hazards associated with lithium nitrate, proper handling techniques, emergency response procedures, and the importance of accurate record-keeping. Regular safety audits and refresher training sessions help maintain awareness and compliance with evolving safety standards in materials research environments.
Exposure to lithium nitrate can cause severe irritation to the eyes, skin, and respiratory system. Direct contact may result in chemical burns, while inhalation of dust or decomposition products can lead to respiratory distress. Chronic exposure has been associated with potential neurological effects due to lithium accumulation in the body. Emergency protocols should include immediate flushing with water for skin or eye contact and seeking medical attention.
Laboratory environments conducting thermal expansion coefficient measurements must be equipped with adequate ventilation systems, preferably including local exhaust ventilation. Facilities should maintain emergency eyewash stations and safety showers in close proximity to handling areas. Temperature control is critical during experiments, as lithium nitrate decomposes at approximately 600°C, potentially releasing nitrogen oxides and lithium oxide.
Proper storage requirements include keeping lithium nitrate in tightly sealed containers in cool, dry areas. Hygroscopic properties necessitate protection from moisture to prevent degradation and maintain sample integrity for accurate thermal expansion measurements. Incompatibility with strong reducing agents, metals, and organic compounds must be considered in storage planning.
Waste disposal must comply with local regulations for chemical waste management. Solutions containing lithium nitrate should never be disposed of down laboratory drains. Instead, they should be collected in designated containers for professional disposal. Spill response procedures should be established, including containment methods, neutralization protocols, and proper documentation of incidents.
Training programs for laboratory personnel should cover specific hazards associated with lithium nitrate, proper handling techniques, emergency response procedures, and the importance of accurate record-keeping. Regular safety audits and refresher training sessions help maintain awareness and compliance with evolving safety standards in materials research environments.
Environmental Impact of Lithium Nitrate Applications
The environmental implications of lithium nitrate applications extend across multiple industrial sectors, with significant considerations for ecological sustainability. As lithium nitrate usage increases in thermal energy storage systems, battery technologies, and ceramic manufacturing, its environmental footprint becomes increasingly relevant to industry stakeholders and regulatory bodies.
The extraction processes for lithium compounds create substantial environmental challenges, particularly in water-sensitive regions. Lithium mining operations typically consume between 500,000 to 2 million gallons of water per ton of lithium extracted, potentially depleting aquifers and disrupting local ecosystems. When lithium nitrate is specifically produced, additional nitrogen-based resources are required, contributing to the compound's overall environmental impact profile.
Thermal expansion properties of lithium nitrate directly influence its environmental performance in molten salt applications. Systems designed without accurate thermal expansion coefficients may experience containment failures, resulting in potential salt leakage and subsequent soil and groundwater contamination. Research indicates that lithium nitrate's thermal behavior affects the longevity and safety of thermal storage systems, with implications for waste generation and replacement frequency.
Recycling challenges present another environmental consideration. Current recovery technologies for lithium nitrate from spent thermal storage media achieve only 60-75% efficiency, leaving significant material unrecovered. The energy-intensive nature of these recycling processes further compounds the environmental burden, with estimates suggesting 3-4 kWh of energy consumption per kilogram of recovered lithium nitrate.
Emissions associated with lithium nitrate production and application must also be considered. Manufacturing processes generate approximately 5-15 kg CO2 equivalent per kilogram of lithium nitrate produced, depending on energy sources and production methods. When used in high-temperature applications, thermal decomposition may release nitrogen oxides, contributing to air quality concerns if not properly managed.
Regulatory frameworks increasingly address these environmental aspects, with the European Chemical Agency classifying certain lithium compounds as substances of concern for water bodies. Understanding precise thermal expansion coefficients enables more environmentally sound engineering designs, potentially reducing material requirements by 10-15% through optimized containment systems and improved thermal cycling performance.
Future sustainability efforts will likely focus on closed-loop applications where lithium nitrate's thermal properties can be utilized without environmental release, alongside development of more efficient recycling technologies specifically designed to accommodate the compound's thermal expansion characteristics.
The extraction processes for lithium compounds create substantial environmental challenges, particularly in water-sensitive regions. Lithium mining operations typically consume between 500,000 to 2 million gallons of water per ton of lithium extracted, potentially depleting aquifers and disrupting local ecosystems. When lithium nitrate is specifically produced, additional nitrogen-based resources are required, contributing to the compound's overall environmental impact profile.
Thermal expansion properties of lithium nitrate directly influence its environmental performance in molten salt applications. Systems designed without accurate thermal expansion coefficients may experience containment failures, resulting in potential salt leakage and subsequent soil and groundwater contamination. Research indicates that lithium nitrate's thermal behavior affects the longevity and safety of thermal storage systems, with implications for waste generation and replacement frequency.
Recycling challenges present another environmental consideration. Current recovery technologies for lithium nitrate from spent thermal storage media achieve only 60-75% efficiency, leaving significant material unrecovered. The energy-intensive nature of these recycling processes further compounds the environmental burden, with estimates suggesting 3-4 kWh of energy consumption per kilogram of recovered lithium nitrate.
Emissions associated with lithium nitrate production and application must also be considered. Manufacturing processes generate approximately 5-15 kg CO2 equivalent per kilogram of lithium nitrate produced, depending on energy sources and production methods. When used in high-temperature applications, thermal decomposition may release nitrogen oxides, contributing to air quality concerns if not properly managed.
Regulatory frameworks increasingly address these environmental aspects, with the European Chemical Agency classifying certain lithium compounds as substances of concern for water bodies. Understanding precise thermal expansion coefficients enables more environmentally sound engineering designs, potentially reducing material requirements by 10-15% through optimized containment systems and improved thermal cycling performance.
Future sustainability efforts will likely focus on closed-loop applications where lithium nitrate's thermal properties can be utilized without environmental release, alongside development of more efficient recycling technologies specifically designed to accommodate the compound's thermal expansion characteristics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!