Benchmark Lithium Nitrate Deliquescence Point Under Controlled Humidity
OCT 9, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Nitrate Deliquescence Background and Research Objectives
Lithium nitrate (LiNO3) has emerged as a critical compound in various industrial applications, particularly in energy storage systems, thermal energy storage, and as a component in electrolytes for lithium-ion batteries. The deliquescence behavior of lithium nitrate represents a fundamental property that significantly impacts its storage, handling, and application efficiency. Deliquescence refers to the process by which a substance absorbs moisture from the atmosphere until it dissolves in the absorbed water, forming a solution. This phenomenon is particularly relevant for hygroscopic salts like lithium nitrate.
The study of lithium nitrate's deliquescence point under controlled humidity conditions has gained increasing attention over the past decade, driven by the expanding applications of lithium compounds in renewable energy technologies. Historical research on hygroscopic properties of inorganic salts dates back to the early 20th century, but specific focus on lithium salts has intensified with the growth of the lithium battery industry since the 1990s.
Recent technological advancements in humidity control systems and analytical instrumentation have enabled more precise measurements of deliquescence points, creating opportunities for standardized benchmarking. The establishment of reliable benchmark data for lithium nitrate deliquescence is crucial for optimizing manufacturing processes, storage protocols, and application parameters across multiple industries.
The primary objective of this research is to establish definitive benchmark values for lithium nitrate deliquescence points across a spectrum of controlled humidity conditions, ranging from 20% to 90% relative humidity (RH) at various temperature points between 10°C and 50°C. This comprehensive mapping will provide essential reference data for industries utilizing lithium nitrate in their processes or products.
Secondary objectives include investigating the impact of particle size distribution on deliquescence behavior, examining the influence of common impurities on the deliquescence point, and developing predictive models that can accurately forecast deliquescence behavior under varying environmental conditions. These models would serve as valuable tools for quality control in manufacturing and storage facilities.
The technological evolution in this field is moving toward real-time monitoring systems that can detect early stages of deliquescence in industrial settings, preventing potential issues in production lines and storage facilities. Understanding the precise deliquescence point under various conditions represents a foundational step in this evolution, enabling the development of more sophisticated control systems and predictive maintenance protocols.
This research aligns with broader industry trends toward more precise material characterization and the growing importance of lithium compounds in sustainable energy solutions, making it a strategically significant area for technological advancement and standardization efforts.
The study of lithium nitrate's deliquescence point under controlled humidity conditions has gained increasing attention over the past decade, driven by the expanding applications of lithium compounds in renewable energy technologies. Historical research on hygroscopic properties of inorganic salts dates back to the early 20th century, but specific focus on lithium salts has intensified with the growth of the lithium battery industry since the 1990s.
Recent technological advancements in humidity control systems and analytical instrumentation have enabled more precise measurements of deliquescence points, creating opportunities for standardized benchmarking. The establishment of reliable benchmark data for lithium nitrate deliquescence is crucial for optimizing manufacturing processes, storage protocols, and application parameters across multiple industries.
The primary objective of this research is to establish definitive benchmark values for lithium nitrate deliquescence points across a spectrum of controlled humidity conditions, ranging from 20% to 90% relative humidity (RH) at various temperature points between 10°C and 50°C. This comprehensive mapping will provide essential reference data for industries utilizing lithium nitrate in their processes or products.
Secondary objectives include investigating the impact of particle size distribution on deliquescence behavior, examining the influence of common impurities on the deliquescence point, and developing predictive models that can accurately forecast deliquescence behavior under varying environmental conditions. These models would serve as valuable tools for quality control in manufacturing and storage facilities.
The technological evolution in this field is moving toward real-time monitoring systems that can detect early stages of deliquescence in industrial settings, preventing potential issues in production lines and storage facilities. Understanding the precise deliquescence point under various conditions represents a foundational step in this evolution, enabling the development of more sophisticated control systems and predictive maintenance protocols.
This research aligns with broader industry trends toward more precise material characterization and the growing importance of lithium compounds in sustainable energy solutions, making it a strategically significant area for technological advancement and standardization efforts.
Market Applications and Demand Analysis for Lithium Nitrate
The global lithium nitrate market has witnessed significant growth in recent years, driven primarily by its diverse applications across multiple industries. The compound's unique properties, particularly its deliquescence point characteristics under controlled humidity conditions, make it valuable for various commercial and industrial uses. Current market estimates value the global lithium nitrate market at approximately 320 million USD, with projections indicating a compound annual growth rate of 5.7% through 2028.
Energy storage represents the largest application segment for lithium nitrate, accounting for roughly 40% of total market demand. Within this sector, thermal energy storage systems for concentrated solar power (CSP) plants constitute the primary application. Lithium nitrate's ability to maintain stability at specific deliquescence points under controlled humidity environments makes it an ideal component in molten salt mixtures used as heat transfer fluids and storage media in these systems.
The pharmaceutical and chemical industries collectively represent the second-largest market segment, utilizing approximately 25% of global lithium nitrate production. Here, precise knowledge of deliquescence behavior is critical for formulation stability, storage conditions, and manufacturing processes. Pharmaceutical applications particularly benefit from understanding humidity thresholds that trigger phase changes in lithium nitrate-containing formulations.
Agriculture and food preservation applications account for approximately 15% of market demand. In these sectors, lithium nitrate serves as a specialized fertilizer component and preservation agent, where its hygroscopic properties must be carefully managed through controlled humidity environments to maintain product integrity and effectiveness.
Emerging applications in humidity control systems and advanced materials are driving new market growth, currently representing about 10% of demand but expanding rapidly at 8.3% annually. These applications directly leverage lithium nitrate's deliquescence characteristics for humidity regulation in sensitive environments and specialized material development.
Regional analysis reveals Asia-Pacific as the dominant market, consuming approximately 45% of global production, followed by North America (25%) and Europe (20%). China, Japan, and South Korea lead consumption in Asia-Pacific, while the United States dominates the North American market. Growth projections indicate particularly strong expansion in emerging economies where energy storage applications are gaining traction.
Market challenges include price volatility due to fluctuating lithium raw material costs and environmental concerns regarding lithium extraction. However, increasing research into lithium nitrate's deliquescence behavior under various humidity conditions is expected to expand application possibilities and drive further market growth, particularly in precision-controlled environments requiring specific humidity thresholds.
Energy storage represents the largest application segment for lithium nitrate, accounting for roughly 40% of total market demand. Within this sector, thermal energy storage systems for concentrated solar power (CSP) plants constitute the primary application. Lithium nitrate's ability to maintain stability at specific deliquescence points under controlled humidity environments makes it an ideal component in molten salt mixtures used as heat transfer fluids and storage media in these systems.
The pharmaceutical and chemical industries collectively represent the second-largest market segment, utilizing approximately 25% of global lithium nitrate production. Here, precise knowledge of deliquescence behavior is critical for formulation stability, storage conditions, and manufacturing processes. Pharmaceutical applications particularly benefit from understanding humidity thresholds that trigger phase changes in lithium nitrate-containing formulations.
Agriculture and food preservation applications account for approximately 15% of market demand. In these sectors, lithium nitrate serves as a specialized fertilizer component and preservation agent, where its hygroscopic properties must be carefully managed through controlled humidity environments to maintain product integrity and effectiveness.
Emerging applications in humidity control systems and advanced materials are driving new market growth, currently representing about 10% of demand but expanding rapidly at 8.3% annually. These applications directly leverage lithium nitrate's deliquescence characteristics for humidity regulation in sensitive environments and specialized material development.
Regional analysis reveals Asia-Pacific as the dominant market, consuming approximately 45% of global production, followed by North America (25%) and Europe (20%). China, Japan, and South Korea lead consumption in Asia-Pacific, while the United States dominates the North American market. Growth projections indicate particularly strong expansion in emerging economies where energy storage applications are gaining traction.
Market challenges include price volatility due to fluctuating lithium raw material costs and environmental concerns regarding lithium extraction. However, increasing research into lithium nitrate's deliquescence behavior under various humidity conditions is expected to expand application possibilities and drive further market growth, particularly in precision-controlled environments requiring specific humidity thresholds.
Current Challenges in Measuring Deliquescence Points
Measuring the deliquescence point of lithium nitrate presents significant challenges due to the complex nature of the deliquescence process and the sensitivity of measurements to environmental conditions. One primary challenge is maintaining precise humidity control throughout the experimental process. Even minor fluctuations in relative humidity can significantly impact results, requiring sophisticated environmental chambers with humidity control systems accurate to within ±0.1% RH.
Temperature stability poses another critical challenge, as deliquescence points are temperature-dependent. Research indicates that a 1°C temperature variation can shift the deliquescence point of lithium nitrate by approximately 0.2-0.5% RH. This necessitates temperature control systems capable of maintaining stability within ±0.2°C throughout extended measurement periods.
Sample preparation introduces additional variables affecting measurement accuracy. Particle size distribution, surface morphology, and the presence of impurities can all alter deliquescence behavior. Current methodologies struggle to standardize sample preparation protocols, leading to inconsistencies between research groups and limiting result reproducibility.
The detection of the precise moment of deliquescence onset represents a fundamental methodological challenge. Various techniques including gravimetric analysis, electrical conductivity measurements, and optical methods each present unique limitations. Gravimetric methods offer high sensitivity but suffer from slow response times. Electrical conductivity approaches provide real-time monitoring but may introduce measurement artifacts through electrode contact. Optical techniques enable non-contact measurement but require complex calibration and may be affected by sample opacity.
Hysteresis effects between deliquescence and efflorescence further complicate measurements. Lithium nitrate, like many hygroscopic materials, exhibits different critical relative humidity values during moisture absorption versus desorption cycles. This hysteresis can span several percentage points of relative humidity, making it difficult to establish a definitive deliquescence point without specifying the measurement direction and history.
Instrument calibration presents ongoing challenges, particularly for long-duration experiments. Drift in humidity sensors and measurement systems necessitates frequent recalibration against certified standards. However, suitable calibration standards for the specific humidity ranges relevant to lithium nitrate deliquescence are limited.
Data interpretation introduces additional complexity, as researchers must distinguish between surface adsorption phenomena and true deliquescence. The transition is often gradual rather than instantaneous, creating ambiguity in determining the precise deliquescence point. Current analytical frameworks lack standardized methods for processing raw measurement data and establishing consistent deliquescence criteria.
Temperature stability poses another critical challenge, as deliquescence points are temperature-dependent. Research indicates that a 1°C temperature variation can shift the deliquescence point of lithium nitrate by approximately 0.2-0.5% RH. This necessitates temperature control systems capable of maintaining stability within ±0.2°C throughout extended measurement periods.
Sample preparation introduces additional variables affecting measurement accuracy. Particle size distribution, surface morphology, and the presence of impurities can all alter deliquescence behavior. Current methodologies struggle to standardize sample preparation protocols, leading to inconsistencies between research groups and limiting result reproducibility.
The detection of the precise moment of deliquescence onset represents a fundamental methodological challenge. Various techniques including gravimetric analysis, electrical conductivity measurements, and optical methods each present unique limitations. Gravimetric methods offer high sensitivity but suffer from slow response times. Electrical conductivity approaches provide real-time monitoring but may introduce measurement artifacts through electrode contact. Optical techniques enable non-contact measurement but require complex calibration and may be affected by sample opacity.
Hysteresis effects between deliquescence and efflorescence further complicate measurements. Lithium nitrate, like many hygroscopic materials, exhibits different critical relative humidity values during moisture absorption versus desorption cycles. This hysteresis can span several percentage points of relative humidity, making it difficult to establish a definitive deliquescence point without specifying the measurement direction and history.
Instrument calibration presents ongoing challenges, particularly for long-duration experiments. Drift in humidity sensors and measurement systems necessitates frequent recalibration against certified standards. However, suitable calibration standards for the specific humidity ranges relevant to lithium nitrate deliquescence are limited.
Data interpretation introduces additional complexity, as researchers must distinguish between surface adsorption phenomena and true deliquescence. The transition is often gradual rather than instantaneous, creating ambiguity in determining the precise deliquescence point. Current analytical frameworks lack standardized methods for processing raw measurement data and establishing consistent deliquescence criteria.
Benchmark Methodologies for Controlled Humidity Testing
01 Deliquescence properties of lithium nitrate in energy storage applications
Lithium nitrate exhibits specific deliquescence characteristics that make it valuable in thermal energy storage systems. The compound's ability to absorb moisture from the atmosphere at certain humidity levels affects its performance in heat storage applications. Understanding the deliquescence point is crucial for designing stable energy storage materials that maintain their properties over time, particularly in phase change materials and thermal batteries where lithium nitrate serves as an additive or primary component.- Deliquescence properties of lithium nitrate in energy storage applications: Lithium nitrate exhibits specific deliquescence behavior that makes it valuable in thermal energy storage systems. The compound's ability to absorb moisture from the atmosphere at certain humidity levels affects its performance in heat storage applications. This deliquescence property can be controlled or utilized in phase change materials and thermal energy storage solutions, where the absorption or release of water molecules contributes to the overall energy storage mechanism.
- Prevention of lithium nitrate deliquescence in battery systems: In lithium-ion battery applications, the deliquescence of lithium nitrate can be problematic and requires specific prevention measures. Various formulations and protective coatings are employed to prevent moisture absorption by lithium nitrate when used as an electrolyte additive or component in battery systems. These approaches help maintain the stability and performance of batteries by controlling the hygroscopic nature of lithium nitrate under varying environmental conditions.
- Deliquescence control in lithium nitrate-containing heat transfer fluids: Heat transfer fluids containing lithium nitrate require careful management of deliquescence properties. The deliquescence point of lithium nitrate affects the stability and performance of these fluids in thermal management systems. Various additives and formulation techniques are employed to modify or control the moisture absorption characteristics of lithium nitrate in heat transfer applications, ensuring optimal performance across a range of temperature and humidity conditions.
- Utilization of lithium nitrate deliquescence in humidity control systems: The deliquescence properties of lithium nitrate can be advantageously used in humidity control and moisture management systems. By leveraging its specific deliquescence point, lithium nitrate can function as a humidity regulator in enclosed environments. These systems utilize the compound's ability to absorb moisture at specific relative humidity levels, making it effective for applications requiring precise atmospheric moisture control.
- Modification of lithium nitrate deliquescence point through compound formulations: The deliquescence point of lithium nitrate can be modified through specific formulations with other compounds. By creating mixtures or composite materials with lithium nitrate, the threshold at which it begins to absorb moisture from the atmosphere can be adjusted. These formulations allow for customization of the deliquescence behavior to suit specific applications, including energy storage, desiccants, and chemical processing, where controlled moisture absorption is critical.
02 Prevention of lithium nitrate deliquescence in battery systems
Various methods are employed to prevent or mitigate the deliquescence of lithium nitrate in battery applications. These include encapsulation techniques, protective coatings, and the use of hydrophobic additives that shield lithium nitrate from ambient moisture. Such approaches are particularly important in lithium-sulfur batteries where lithium nitrate functions as an electrolyte additive. Controlling the deliquescence behavior ensures longer battery life and improved performance by maintaining the integrity of the lithium nitrate component.Expand Specific Solutions03 Measurement and characterization of lithium nitrate deliquescence point
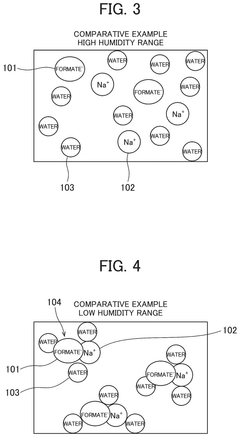
Specific analytical methods are used to determine the deliquescence point of lithium nitrate under various conditions. These include controlled humidity chambers, thermogravimetric analysis, and dynamic vapor sorption techniques. Research indicates that lithium nitrate begins to absorb moisture at relative humidity levels around 52-55% at standard temperature, though this can vary with temperature and the presence of other compounds. Accurate measurement of this property is essential for applications where moisture sensitivity impacts performance.Expand Specific Solutions04 Modification of lithium nitrate deliquescence behavior through formulation
The deliquescence point of lithium nitrate can be modified through formulation with other compounds. Mixing with certain salts, polymers, or ceramic materials can raise or lower the critical relative humidity at which deliquescence occurs. These formulations are designed to create materials with specific moisture absorption properties for applications such as desiccants, humidity control systems, or stabilized energy storage materials. The modified deliquescence behavior enables lithium nitrate to be used in environments where its natural hygroscopic properties would otherwise be problematic.Expand Specific Solutions05 Industrial applications leveraging lithium nitrate deliquescence properties
The deliquescence properties of lithium nitrate are utilized in various industrial applications. These include humidity control systems, heat transfer fluids, concrete additives, and specialized chemical processes. In some applications, the compound's ability to absorb moisture is beneficial, such as in certain desiccant formulations or humidity-triggered systems. In others, such as molten salt heat transfer systems, measures must be taken to prevent deliquescence that could compromise system performance. Understanding the deliquescence point allows engineers to design systems that either exploit or mitigate this property.Expand Specific Solutions
Leading Research Institutions and Industrial Players
The lithium nitrate deliquescence point benchmark market is in a growth phase, driven by increasing demand for lithium-ion battery technologies. The market size is expanding rapidly due to electric vehicle adoption and energy storage applications. Technologically, the field shows moderate maturity with ongoing refinement of measurement protocols under controlled humidity conditions. Key players include battery manufacturers like SAMSUNG SDI, CATL, and LG Energy Solution who are advancing research to improve battery stability and performance. Academic institutions such as Central South University and California Institute of Technology collaborate with industry leaders to develop standardized testing methodologies. Research organizations like the Institute of Process Engineering (Chinese Academy of Sciences) are contributing fundamental knowledge to address deliquescence challenges in lithium battery materials.
SAMSUNG SDI CO LTD
Technical Solution: Samsung SDI has developed advanced testing protocols for lithium nitrate deliquescence point measurement using controlled environmental chambers with precision humidity regulation systems. Their approach employs specialized humidity sensors with ±0.5% accuracy and temperature control within ±0.2°C to establish reliable benchmarks for lithium nitrate behavior under varying atmospheric conditions. The company utilizes gravimetric analysis combined with in-situ optical monitoring to detect the exact point of moisture absorption and phase transition. This methodology allows for real-time observation of deliquescence phenomena while maintaining strict environmental parameters, essential for battery materials research where lithium nitrate serves as an electrolyte additive to form protective cathode interfaces.
Strengths: Exceptional precision in humidity control systems; integration with battery manufacturing expertise allows direct application of findings to product development. Weaknesses: Proprietary nature of some methodologies limits academic collaboration; primarily focused on applications rather than fundamental science of deliquescence mechanisms.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering at the Chinese Academy of Sciences has developed a sophisticated experimental platform for precise measurement of lithium nitrate deliquescence points under meticulously controlled humidity conditions. Their methodology employs a custom-built environmental chamber with humidity control accurate to ±0.2% RH across a temperature range of 5-80°C. The system integrates high-precision analytical balances with 0.01 mg resolution to detect the onset of moisture absorption, while simultaneously employing in-situ Raman spectroscopy to monitor molecular-level changes during the deliquescence process. This multi-modal approach allows researchers to correlate macroscopic mass changes with microscopic structural transformations. The Institute has established standardized protocols for sample preparation, including controlled particle sizing and surface area normalization, ensuring reproducible results across different batches of lithium nitrate. Their research has revealed significant correlations between temperature, relative humidity, and deliquescence kinetics, providing fundamental data essential for lithium battery material handling and storage specifications.
Strengths: Exceptional precision in environmental control; comprehensive multi-parameter analysis approach; strong foundation in fundamental physical chemistry principles. Weaknesses: Laboratory-scale methodology may not fully translate to industrial environments; research primarily focused on pure materials rather than complex mixtures found in commercial applications.
Critical Patents and Literature on Lithium Salt Deliquescence
Humidity-controlling material
PatentPendingUS20250041794A1
Innovation
- A humidity-controlling material comprising a water-absorbing body and a humidity-controlling component that includes a first salt with a deliquescence point between 30% RH and 80% RH and a second salt with different anions and cations, allowing for effective moisture absorption and release across a wide humidity range.
Controlled humidity freeze drying process
PatentInactiveUS3964174A
Innovation
- Maintaining a controlled relative humidity during freeze drying using hydrating salts that transition from one hydrated state to another, ensuring a consistent moisture level throughout the food product, allowing for compression without damaging the tissue structure.
Environmental Factors Affecting Lithium Nitrate Stability
The stability of lithium nitrate is significantly influenced by various environmental factors, with humidity and temperature being the most critical parameters. Lithium nitrate (LiNO₃) exhibits strong hygroscopic properties, readily absorbing moisture from the surrounding atmosphere. This characteristic makes its deliquescence point—the relative humidity at which the salt begins to absorb enough water to form a solution—a crucial parameter for storage, handling, and application considerations.
Temperature plays a fundamental role in modulating the deliquescence behavior of lithium nitrate. Research indicates that as temperature increases, the deliquescence relative humidity (DRH) typically decreases, creating a more challenging storage environment. At standard room temperature (25°C), lithium nitrate begins to absorb moisture at approximately 52-55% relative humidity, but this threshold can drop significantly at elevated temperatures common in industrial settings or energy storage applications.
Atmospheric pressure variations, though less impactful than humidity and temperature, can still affect the stability of lithium nitrate compounds. Higher altitudes with lower atmospheric pressure may slightly alter the deliquescence behavior, requiring adjustments in humidity control protocols for facilities located at different elevations.
Air quality factors, particularly the presence of acidic or basic gaseous compounds, can accelerate degradation processes in lithium nitrate. Exposure to carbon dioxide can lead to the formation of lithium carbonate over time, while sulfur dioxide and nitrogen oxides may catalyze undesired side reactions, compromising the salt's purity and functional properties.
Light exposure, especially UV radiation, has been documented to induce photochemical reactions in nitrate compounds. While lithium nitrate is generally considered photostable compared to other nitrate salts, prolonged exposure to intense light sources may contribute to gradual decomposition, particularly in solutions or when combined with photosensitive additives.
Cycling between different humidity conditions presents a particularly challenging scenario for lithium nitrate stability. Repeated deliquescence and recrystallization cycles can alter the crystal structure and particle size distribution, potentially affecting the material's performance in applications such as thermal energy storage systems or battery technologies.
Understanding these environmental factors is essential for establishing appropriate handling protocols and storage conditions that maintain lithium nitrate in its optimal state for intended applications, whether in energy storage, heat transfer fluids, or other industrial processes.
Temperature plays a fundamental role in modulating the deliquescence behavior of lithium nitrate. Research indicates that as temperature increases, the deliquescence relative humidity (DRH) typically decreases, creating a more challenging storage environment. At standard room temperature (25°C), lithium nitrate begins to absorb moisture at approximately 52-55% relative humidity, but this threshold can drop significantly at elevated temperatures common in industrial settings or energy storage applications.
Atmospheric pressure variations, though less impactful than humidity and temperature, can still affect the stability of lithium nitrate compounds. Higher altitudes with lower atmospheric pressure may slightly alter the deliquescence behavior, requiring adjustments in humidity control protocols for facilities located at different elevations.
Air quality factors, particularly the presence of acidic or basic gaseous compounds, can accelerate degradation processes in lithium nitrate. Exposure to carbon dioxide can lead to the formation of lithium carbonate over time, while sulfur dioxide and nitrogen oxides may catalyze undesired side reactions, compromising the salt's purity and functional properties.
Light exposure, especially UV radiation, has been documented to induce photochemical reactions in nitrate compounds. While lithium nitrate is generally considered photostable compared to other nitrate salts, prolonged exposure to intense light sources may contribute to gradual decomposition, particularly in solutions or when combined with photosensitive additives.
Cycling between different humidity conditions presents a particularly challenging scenario for lithium nitrate stability. Repeated deliquescence and recrystallization cycles can alter the crystal structure and particle size distribution, potentially affecting the material's performance in applications such as thermal energy storage systems or battery technologies.
Understanding these environmental factors is essential for establishing appropriate handling protocols and storage conditions that maintain lithium nitrate in its optimal state for intended applications, whether in energy storage, heat transfer fluids, or other industrial processes.
Standardization and Quality Control Protocols
To ensure consistent and reliable results in benchmarking lithium nitrate deliquescence points under controlled humidity conditions, comprehensive standardization and quality control protocols must be established. These protocols should encompass all aspects of the experimental process, from sample preparation to data analysis.
Sample preparation requires strict adherence to purity standards, with lithium nitrate samples meeting a minimum purity of 99.5%. Samples should be characterized using X-ray diffraction and differential scanning calorimetry to confirm crystalline structure and phase purity before testing. Standardized sample sizes (typically 1-5g) and surface area exposure must be maintained across all experiments to ensure reproducibility.
Environmental control represents a critical aspect of the protocol. Testing chambers must maintain humidity levels within ±1% of target values and temperature stability within ±0.5°C. Calibration of humidity sensors should be performed using certified salt solutions as reference standards at least bi-weekly, with verification checks before each experimental run. Temperature gradients within the chamber should be mapped and documented to ensure uniform conditions across all sample positions.
Measurement procedures must follow a time-sequenced approach, with standardized equilibration periods established for each humidity level. Visual observation protocols should be supplemented with gravimetric analysis, with weight measurements taken at predetermined intervals using analytical balances with precision of at least 0.1mg. The deliquescence point determination should follow the established criterion of the first observable liquid formation, confirmed by a sudden increase in sample mass.
Data validation requires statistical analysis of replicate measurements, with a minimum of five replicates per condition. Acceptance criteria should include maximum standard deviation thresholds (typically ±1.5% RH) and outlier identification using Grubbs' test. Control samples of known deliquescence behavior (such as sodium chloride) should be included in each experimental batch to verify system performance.
Documentation standards must include comprehensive recording of all experimental parameters, raw data, calibration records, and environmental conditions. Each experiment should receive a unique identifier linking all associated data, enabling complete traceability and reproducibility. Regular proficiency testing among different laboratories using identical protocols should be implemented to establish interlaboratory reproducibility and identify potential systematic errors in methodology.
Sample preparation requires strict adherence to purity standards, with lithium nitrate samples meeting a minimum purity of 99.5%. Samples should be characterized using X-ray diffraction and differential scanning calorimetry to confirm crystalline structure and phase purity before testing. Standardized sample sizes (typically 1-5g) and surface area exposure must be maintained across all experiments to ensure reproducibility.
Environmental control represents a critical aspect of the protocol. Testing chambers must maintain humidity levels within ±1% of target values and temperature stability within ±0.5°C. Calibration of humidity sensors should be performed using certified salt solutions as reference standards at least bi-weekly, with verification checks before each experimental run. Temperature gradients within the chamber should be mapped and documented to ensure uniform conditions across all sample positions.
Measurement procedures must follow a time-sequenced approach, with standardized equilibration periods established for each humidity level. Visual observation protocols should be supplemented with gravimetric analysis, with weight measurements taken at predetermined intervals using analytical balances with precision of at least 0.1mg. The deliquescence point determination should follow the established criterion of the first observable liquid formation, confirmed by a sudden increase in sample mass.
Data validation requires statistical analysis of replicate measurements, with a minimum of five replicates per condition. Acceptance criteria should include maximum standard deviation thresholds (typically ±1.5% RH) and outlier identification using Grubbs' test. Control samples of known deliquescence behavior (such as sodium chloride) should be included in each experimental batch to verify system performance.
Documentation standards must include comprehensive recording of all experimental parameters, raw data, calibration records, and environmental conditions. Each experiment should receive a unique identifier linking all associated data, enabling complete traceability and reproducibility. Regular proficiency testing among different laboratories using identical protocols should be implemented to establish interlaboratory reproducibility and identify potential systematic errors in methodology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!