Lithium Nitrate vs Lithium Oxide: Comparative Reaction Pathways
OCT 9, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Compounds Background and Research Objectives
Lithium compounds have been at the forefront of chemical research and industrial applications for decades, with their significance dramatically increasing in recent years due to the global shift toward renewable energy storage solutions. The evolution of lithium chemistry began in the early 19th century with its discovery by Johan August Arfwedson in 1817, but it wasn't until the late 20th century that lithium compounds gained substantial commercial importance, primarily through battery technologies.
The comparative study of lithium nitrate (LiNO₃) and lithium oxide (Li₂O) represents a critical area of investigation within lithium chemistry. These compounds exhibit distinct reaction pathways that significantly impact their applications across various industries. Lithium nitrate has traditionally been utilized in pyrotechnics, ceramics, and as a heat transfer medium, while lithium oxide serves as a crucial precursor in glass manufacturing and as an intermediate in lithium extraction processes.
Recent technological advancements have expanded the potential applications of both compounds, particularly in energy storage systems. Lithium nitrate has emerged as an important additive in lithium-sulfur batteries, where it forms protective layers on lithium anodes, mitigating the shuttle effect and enhancing cycle life. Conversely, lithium oxide plays a fundamental role in solid-state electrolyte development and cathode material synthesis for next-generation batteries.
The reaction mechanisms of these compounds differ substantially, with lithium nitrate demonstrating high solubility in various solvents and participating in complex redox reactions, while lithium oxide exhibits strong basic properties and readily reacts with acidic compounds to form lithium salts. Understanding these divergent reaction pathways is essential for optimizing their performance in targeted applications and developing novel lithium-based materials.
This technical research aims to comprehensively analyze the reaction pathways of lithium nitrate and lithium oxide, focusing on their thermodynamic properties, kinetic behaviors, and structural transformations under various conditions. By establishing a detailed comparison of these pathways, we seek to identify opportunities for enhancing their functionality in existing applications and discovering new potential uses.
The primary objectives of this research include: mapping the complete reaction networks of both compounds across different environments; quantifying the energetics and kinetics of key transformation processes; identifying catalytic effects that may alter reaction pathways; and developing predictive models for reaction outcomes under specific conditions. These insights will guide future innovation in lithium compound utilization, particularly in energy storage, catalysis, and advanced materials development.
The comparative study of lithium nitrate (LiNO₃) and lithium oxide (Li₂O) represents a critical area of investigation within lithium chemistry. These compounds exhibit distinct reaction pathways that significantly impact their applications across various industries. Lithium nitrate has traditionally been utilized in pyrotechnics, ceramics, and as a heat transfer medium, while lithium oxide serves as a crucial precursor in glass manufacturing and as an intermediate in lithium extraction processes.
Recent technological advancements have expanded the potential applications of both compounds, particularly in energy storage systems. Lithium nitrate has emerged as an important additive in lithium-sulfur batteries, where it forms protective layers on lithium anodes, mitigating the shuttle effect and enhancing cycle life. Conversely, lithium oxide plays a fundamental role in solid-state electrolyte development and cathode material synthesis for next-generation batteries.
The reaction mechanisms of these compounds differ substantially, with lithium nitrate demonstrating high solubility in various solvents and participating in complex redox reactions, while lithium oxide exhibits strong basic properties and readily reacts with acidic compounds to form lithium salts. Understanding these divergent reaction pathways is essential for optimizing their performance in targeted applications and developing novel lithium-based materials.
This technical research aims to comprehensively analyze the reaction pathways of lithium nitrate and lithium oxide, focusing on their thermodynamic properties, kinetic behaviors, and structural transformations under various conditions. By establishing a detailed comparison of these pathways, we seek to identify opportunities for enhancing their functionality in existing applications and discovering new potential uses.
The primary objectives of this research include: mapping the complete reaction networks of both compounds across different environments; quantifying the energetics and kinetics of key transformation processes; identifying catalytic effects that may alter reaction pathways; and developing predictive models for reaction outcomes under specific conditions. These insights will guide future innovation in lithium compound utilization, particularly in energy storage, catalysis, and advanced materials development.
Market Applications and Demand Analysis
The market for lithium compounds has experienced significant growth driven by the expanding electric vehicle (EV) industry and renewable energy storage systems. Within this landscape, both lithium nitrate and lithium oxide serve distinct yet overlapping market applications, with their comparative reaction pathways influencing their commercial viability and adoption.
Lithium nitrate finds extensive application in thermal energy storage systems, particularly in concentrated solar power plants. Its excellent thermal properties and stability at high temperatures make it a preferred additive in molten salt mixtures. The global thermal energy storage market, where lithium nitrate plays a crucial role, is projected to grow substantially as renewable energy integration accelerates worldwide.
In the battery sector, lithium nitrate serves as an electrolyte additive that forms protective solid electrolyte interphase (SEI) layers on lithium metal anodes, enhancing battery safety and longevity. This application has gained traction with the increasing demand for higher energy density batteries in portable electronics and electric vehicles.
Lithium oxide, conversely, dominates in the glass and ceramics industry, where it reduces melting temperatures and improves product durability. The compound also serves as a precursor in the synthesis of various lithium-based materials used in batteries and other applications. Its reaction pathways enable efficient conversion to other lithium compounds, positioning it as a versatile industrial chemical.
The pharmaceutical industry represents another significant market for both compounds. Lithium-based medications, particularly those derived from lithium oxide pathways, continue to be essential treatments for bipolar disorder and other psychiatric conditions, maintaining steady demand despite being a mature market segment.
Emerging applications are reshaping market dynamics for both compounds. Lithium nitrate's reaction pathways are being exploited in advanced air purification systems and catalytic converters, while lithium oxide is finding new applications in next-generation solid-state batteries and specialized optical materials.
Regional market analysis reveals that Asia-Pacific dominates demand for both compounds, driven by the concentration of battery manufacturing and electronics production. North America and Europe follow with growing demand primarily in energy storage applications and pharmaceutical uses.
The comparative reaction efficiency of these compounds significantly impacts their economic viability. Lithium nitrate's reaction pathways generally require less energy input than lithium oxide conversions, potentially offering cost advantages in certain applications despite its higher initial production cost. This efficiency differential is becoming increasingly important as manufacturers seek to optimize production costs and reduce environmental footprints.
Lithium nitrate finds extensive application in thermal energy storage systems, particularly in concentrated solar power plants. Its excellent thermal properties and stability at high temperatures make it a preferred additive in molten salt mixtures. The global thermal energy storage market, where lithium nitrate plays a crucial role, is projected to grow substantially as renewable energy integration accelerates worldwide.
In the battery sector, lithium nitrate serves as an electrolyte additive that forms protective solid electrolyte interphase (SEI) layers on lithium metal anodes, enhancing battery safety and longevity. This application has gained traction with the increasing demand for higher energy density batteries in portable electronics and electric vehicles.
Lithium oxide, conversely, dominates in the glass and ceramics industry, where it reduces melting temperatures and improves product durability. The compound also serves as a precursor in the synthesis of various lithium-based materials used in batteries and other applications. Its reaction pathways enable efficient conversion to other lithium compounds, positioning it as a versatile industrial chemical.
The pharmaceutical industry represents another significant market for both compounds. Lithium-based medications, particularly those derived from lithium oxide pathways, continue to be essential treatments for bipolar disorder and other psychiatric conditions, maintaining steady demand despite being a mature market segment.
Emerging applications are reshaping market dynamics for both compounds. Lithium nitrate's reaction pathways are being exploited in advanced air purification systems and catalytic converters, while lithium oxide is finding new applications in next-generation solid-state batteries and specialized optical materials.
Regional market analysis reveals that Asia-Pacific dominates demand for both compounds, driven by the concentration of battery manufacturing and electronics production. North America and Europe follow with growing demand primarily in energy storage applications and pharmaceutical uses.
The comparative reaction efficiency of these compounds significantly impacts their economic viability. Lithium nitrate's reaction pathways generally require less energy input than lithium oxide conversions, potentially offering cost advantages in certain applications despite its higher initial production cost. This efficiency differential is becoming increasingly important as manufacturers seek to optimize production costs and reduce environmental footprints.
Current Technical Challenges in Lithium Chemistry
The lithium chemistry field currently faces several significant technical challenges that impede further advancement in energy storage applications. One primary challenge involves understanding and controlling the complex reaction pathways between different lithium compounds, particularly lithium nitrate and lithium oxide, which exhibit distinct chemical behaviors under various conditions.
Reaction kinetics present a substantial hurdle, as lithium nitrate decomposition pathways are temperature-dependent and can produce varying intermediates that affect overall system performance. Similarly, lithium oxide formation mechanisms remain incompletely understood, especially regarding the role of oxygen partial pressure and surface catalytic effects that influence reaction rates and product distribution.
Interfacial stability issues emerge when these compounds interact with other battery components. Lithium nitrate, while effective as an electrolyte additive for suppressing dendrite formation, can undergo parasitic reactions at electrode surfaces, gradually depleting its concentration and diminishing its protective function. Lithium oxide, forming primarily at the solid-electrolyte interphase (SEI), contributes to impedance growth over cycling, but the exact mechanisms governing its formation and evolution remain elusive.
Analytical limitations further complicate research progress. Current characterization techniques struggle to capture the dynamic nature of these reactions in real-time, particularly under operating conditions relevant to batteries. In-situ monitoring of lithium nitrate decomposition or lithium oxide formation requires specialized equipment and methodologies that are not widely accessible or standardized across the research community.
Computational modeling challenges also persist, as accurate simulation of lithium compound reaction pathways demands consideration of multiple phases, interfaces, and non-equilibrium conditions. Density functional theory calculations often fail to capture the full complexity of these systems, particularly when solvent effects and local concentration gradients play significant roles in determining reaction outcomes.
Scale-up and manufacturing issues represent another critical challenge. Laboratory-scale understanding of lithium nitrate and lithium oxide behaviors frequently fails to translate directly to industrial production environments, where factors such as mass transport limitations, thermal management constraints, and material purity variations can significantly alter reaction pathways and product distributions.
Environmental sensitivity adds another layer of complexity, as both lithium nitrate and lithium oxide reactions are highly susceptible to moisture and atmospheric contaminants. This necessitates stringent handling protocols and specialized equipment for both research and manufacturing, increasing costs and limiting widespread implementation of promising technologies based on these materials.
Reaction kinetics present a substantial hurdle, as lithium nitrate decomposition pathways are temperature-dependent and can produce varying intermediates that affect overall system performance. Similarly, lithium oxide formation mechanisms remain incompletely understood, especially regarding the role of oxygen partial pressure and surface catalytic effects that influence reaction rates and product distribution.
Interfacial stability issues emerge when these compounds interact with other battery components. Lithium nitrate, while effective as an electrolyte additive for suppressing dendrite formation, can undergo parasitic reactions at electrode surfaces, gradually depleting its concentration and diminishing its protective function. Lithium oxide, forming primarily at the solid-electrolyte interphase (SEI), contributes to impedance growth over cycling, but the exact mechanisms governing its formation and evolution remain elusive.
Analytical limitations further complicate research progress. Current characterization techniques struggle to capture the dynamic nature of these reactions in real-time, particularly under operating conditions relevant to batteries. In-situ monitoring of lithium nitrate decomposition or lithium oxide formation requires specialized equipment and methodologies that are not widely accessible or standardized across the research community.
Computational modeling challenges also persist, as accurate simulation of lithium compound reaction pathways demands consideration of multiple phases, interfaces, and non-equilibrium conditions. Density functional theory calculations often fail to capture the full complexity of these systems, particularly when solvent effects and local concentration gradients play significant roles in determining reaction outcomes.
Scale-up and manufacturing issues represent another critical challenge. Laboratory-scale understanding of lithium nitrate and lithium oxide behaviors frequently fails to translate directly to industrial production environments, where factors such as mass transport limitations, thermal management constraints, and material purity variations can significantly alter reaction pathways and product distributions.
Environmental sensitivity adds another layer of complexity, as both lithium nitrate and lithium oxide reactions are highly susceptible to moisture and atmospheric contaminants. This necessitates stringent handling protocols and specialized equipment for both research and manufacturing, increasing costs and limiting widespread implementation of promising technologies based on these materials.
Comparative Reaction Mechanisms Analysis
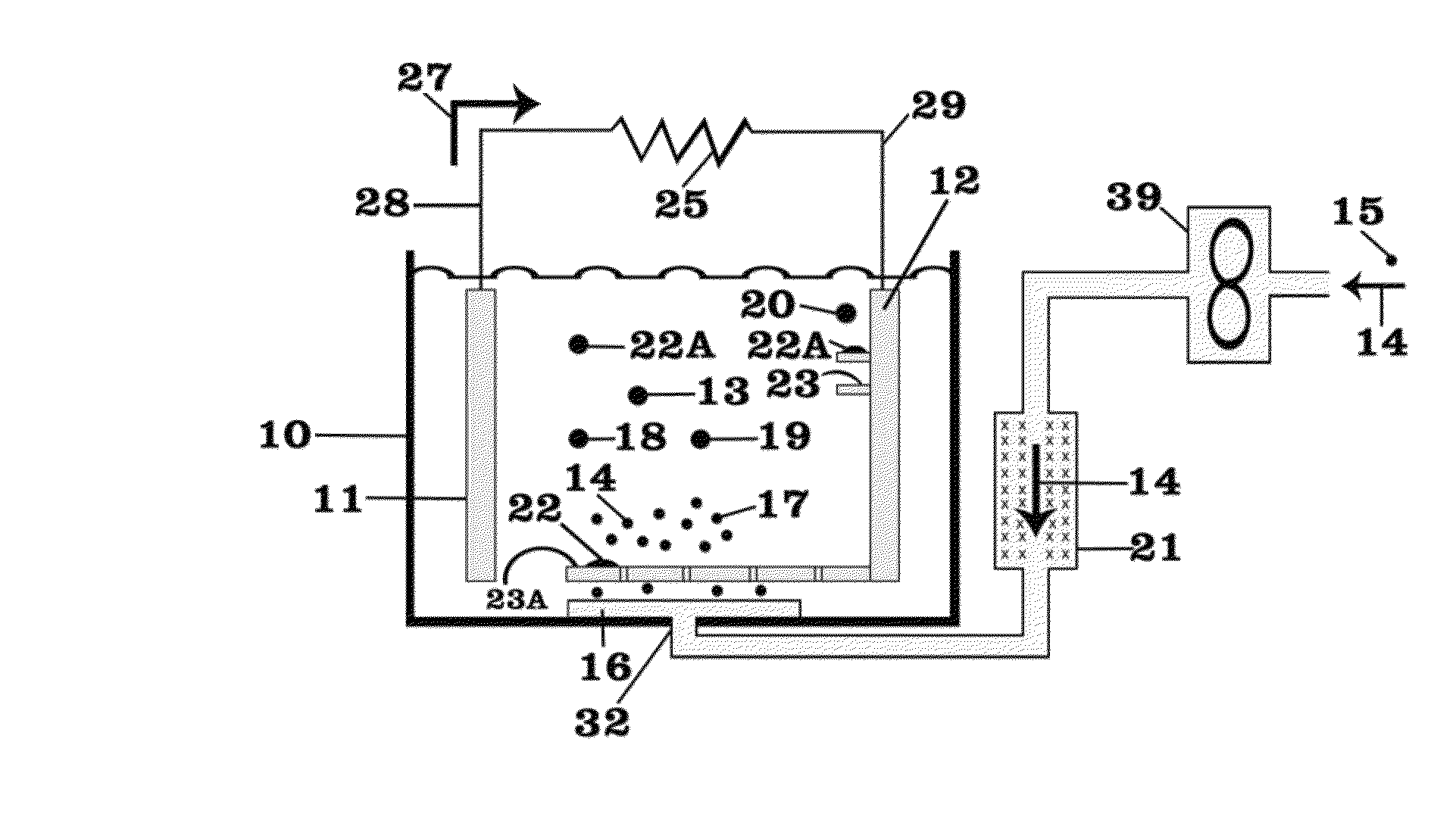
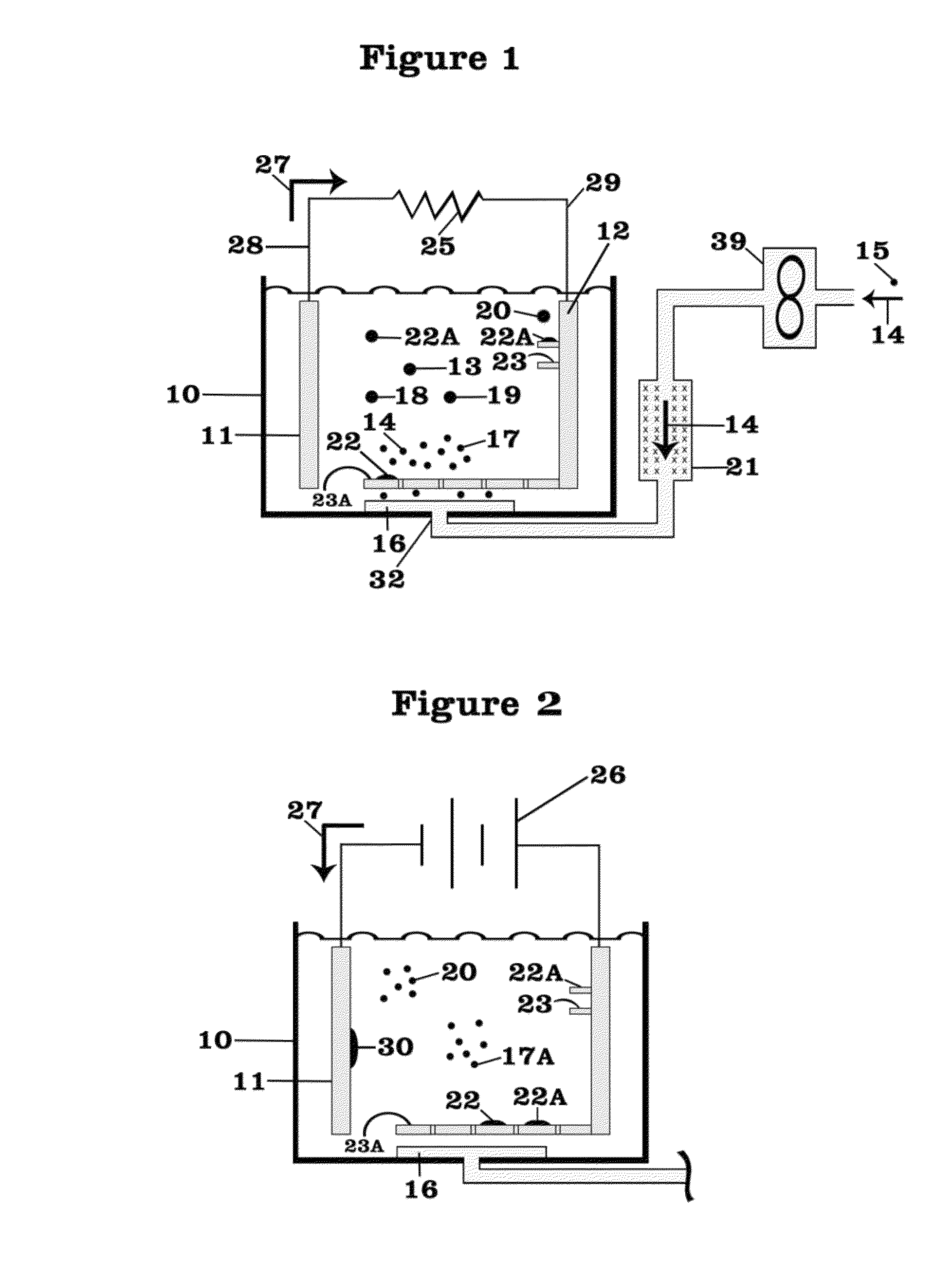
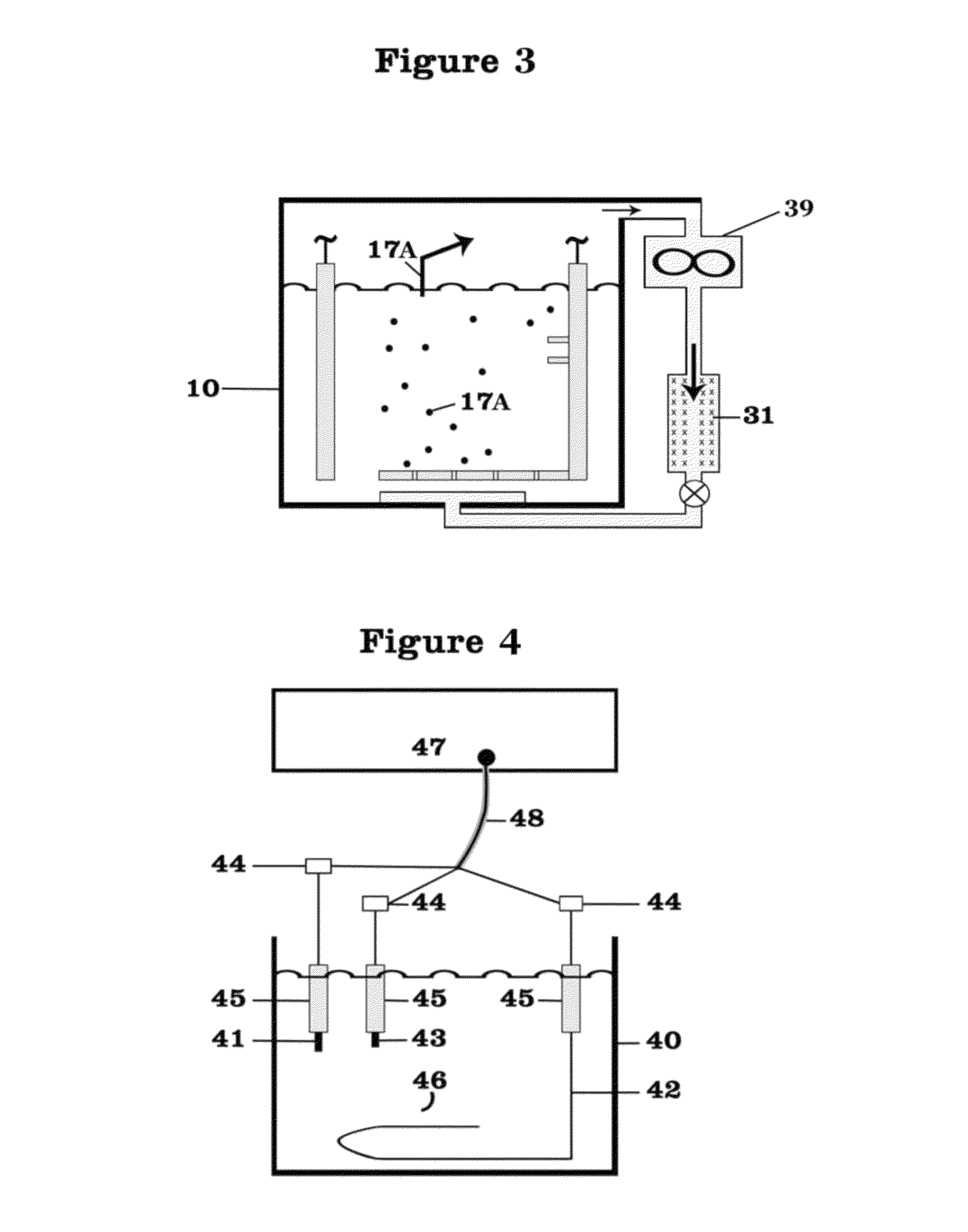
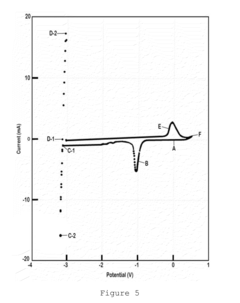
01 Lithium nitrate and lithium oxide in battery cathode materials
Lithium nitrate and lithium oxide are used in the synthesis and modification of cathode materials for lithium-ion batteries. The reaction between these compounds can lead to the formation of stable cathode structures with enhanced electrochemical performance. The presence of lithium oxide can help stabilize the cathode-electrolyte interface, while lithium nitrate serves as both a lithium source and an oxidizing agent during material synthesis.- Lithium nitrate and lithium oxide in battery systems: Lithium nitrate and lithium oxide play crucial roles in lithium-ion battery systems. Lithium nitrate serves as an electrolyte additive that forms a protective layer on electrodes, preventing unwanted side reactions and improving battery performance. Lithium oxide can form during various reaction pathways in batteries, affecting the solid electrolyte interphase formation. The interaction between these compounds contributes to enhanced battery stability, longer cycle life, and improved safety characteristics.
- Thermal decomposition and reaction mechanisms: The thermal decomposition of lithium nitrate produces lithium oxide through specific reaction pathways. This process involves the release of nitrogen oxides and oxygen, with lithium oxide forming as a stable end product. The reaction mechanisms are temperature-dependent, with different intermediate compounds forming at various temperature ranges. Understanding these decomposition pathways is essential for applications in energy storage, catalysis, and materials synthesis where controlled formation of lithium oxide is desired.
- Lithium nitrate as SEI forming agent in lithium-sulfur batteries: Lithium nitrate functions as a critical solid electrolyte interphase (SEI) forming agent in lithium-sulfur battery systems. The reaction pathways between lithium nitrate and lithium oxide contribute to the formation of a stable protective layer on lithium metal anodes, suppressing the shuttle effect of polysulfides. This reaction chemistry enhances coulombic efficiency, extends cycle life, and improves the overall performance of lithium-sulfur batteries by preventing unwanted side reactions at the electrode surface.
- Synthesis of lithium-containing compounds via nitrate-oxide reactions: The reaction between lithium nitrate and lithium oxide serves as a pathway for synthesizing various lithium-containing compounds used in energy storage and conversion technologies. These reaction pathways can be controlled to produce specific lithium compounds with desired properties. The synthesis methods often involve precise temperature control, specific reaction environments, and carefully selected precursors to achieve the target compounds with high purity and performance characteristics.
- Catalytic applications of lithium nitrate-oxide systems: Lithium nitrate and lithium oxide reaction systems demonstrate significant catalytic properties for various chemical processes. The interaction between these compounds creates active sites that facilitate specific reactions, including oxidation processes and gas conversion reactions. These catalytic systems show promise in environmental applications, chemical synthesis, and energy conversion technologies. The unique properties of the lithium nitrate-oxide interface contribute to enhanced reaction rates and selectivity in catalytic applications.
02 Reaction pathways for lithium oxide production from lithium nitrate
The thermal decomposition of lithium nitrate produces lithium oxide through specific reaction pathways. This process typically involves the breakdown of lithium nitrate at elevated temperatures, resulting in the formation of lithium oxide, nitrogen dioxide, and oxygen. The reaction conditions, including temperature, pressure, and atmosphere, significantly influence the decomposition pathway and the purity of the resulting lithium oxide.Expand Specific Solutions03 Use of lithium nitrate and lithium oxide in solid electrolyte interfaces
Lithium nitrate and lithium oxide play crucial roles in the formation and stability of solid electrolyte interfaces (SEI) in lithium batteries. Lithium nitrate can react with lithium oxide to form protective layers on electrode surfaces, preventing unwanted side reactions and enhancing battery cycle life. These reaction pathways are essential for developing stable interfaces between electrodes and electrolytes in advanced battery systems.Expand Specific Solutions04 Conversion reactions between lithium nitrate and lithium oxide in energy storage applications
The reversible conversion reactions between lithium nitrate and lithium oxide are utilized in various energy storage applications. These reactions involve electron transfer processes that can be harnessed for energy storage and release. Understanding the kinetics and thermodynamics of these conversion reactions is crucial for developing high-performance energy storage systems with improved capacity and cycle stability.Expand Specific Solutions05 Catalytic effects in lithium nitrate and lithium oxide reactions
Various catalysts can influence the reaction pathways between lithium nitrate and lithium oxide, enhancing reaction rates or directing selectivity toward specific products. These catalytic effects are important in applications such as lithium recovery processes, synthesis of lithium-containing compounds, and development of lithium-based materials for energy storage. The presence of catalysts can lower activation energies and enable reactions to proceed under milder conditions.Expand Specific Solutions
Key Industry Players and Research Institutions
The lithium battery technology landscape is currently in a growth phase, with a market size projected to exceed $100 billion by 2025. The comparative reaction pathways between lithium nitrate and lithium oxide represent a critical area of innovation in battery chemistry, with varying levels of technical maturity. Leading players like Samsung SDI, LG Energy Solution, and Toyota are advancing commercial applications, while specialized companies such as Ganfeng Lithium and Nano One Materials focus on optimizing reaction efficiency. Research institutions including University of Washington and KIST are exploring fundamental chemistry improvements. The competitive landscape is characterized by established battery manufacturers investing heavily in R&D while newer entrants like Svolt and PolyPlus are developing proprietary technologies to enhance energy density and cycle life through novel lithium compound applications.
SAMSUNG SDI CO LTD
Technical Solution: Samsung SDI has developed sophisticated research comparing lithium nitrate and lithium oxide reaction pathways in high-energy density battery systems. Their approach focuses on the mechanistic differences between these compounds at electrode-electrolyte interfaces. Samsung's research demonstrates that lithium nitrate undergoes a complex reduction process at lithium metal surfaces, forming a mixture of Li2O, Li3N, and LiNxOy compounds that collectively provide superior passivation compared to pure lithium oxide layers. Their proprietary electrolyte systems utilize controlled lithium nitrate decomposition to engineer interfaces with specific ionic conductivity properties. Samsung SDI has implemented in-situ characterization techniques to monitor these reaction pathways in real-time, revealing that lithium nitrate creates more flexible and stress-resistant interface layers compared to the brittle nature of pure lithium oxide films. This technology has been integrated into their advanced lithium-metal battery prototypes, showing significant improvements in cycling stability and rate capability.
Strengths: Highly engineered interface structures with tunable properties; superior mechanical stability of formed protective layers compared to pure lithium oxide systems. Weaknesses: Increased electrolyte complexity requiring precise manufacturing controls; potential for nitrogen gas evolution during abuse conditions.
Toyota Motor Corp.
Technical Solution: Toyota Motor Corporation has conducted extensive research comparing lithium nitrate and lithium oxide reaction pathways for automotive battery applications. Their technology focuses on understanding the fundamental thermodynamics and kinetics of these compounds in various battery chemistries. Toyota's research reveals that lithium nitrate offers distinct advantages in solid-state battery systems, where it undergoes controlled decomposition to form composite interphases containing both lithium oxide and nitrogen-rich compounds. Their studies demonstrate that these composite layers exhibit superior lithium-ion transport properties compared to pure lithium oxide interfaces. Toyota has developed specialized in-situ characterization methods to track reaction intermediates during lithium nitrate reduction, identifying key transition states that determine interface quality. This knowledge has been applied to their all-solid-state battery prototypes, where engineered reaction pathways between lithium nitrate additives and solid electrolytes create stable interfaces that significantly reduce interfacial resistance. Their approach enables higher power density and improved low-temperature performance compared to conventional oxide-based interfaces.
Strengths: Optimized for automotive applications with enhanced low-temperature performance; reduced interfacial resistance leading to higher power capabilities. Weaknesses: Complex manufacturing processes required for precise control of reaction pathways; potential long-term stability issues in extreme temperature conditions.
Critical Patents and Scientific Literature Review
Production of lithium chemicals and metallic lithium
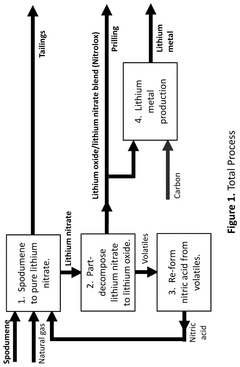
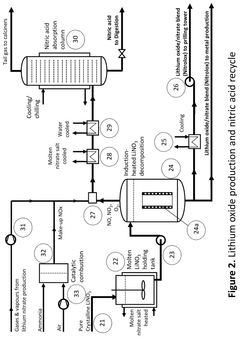
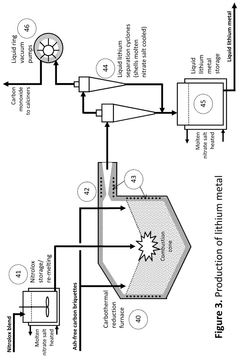
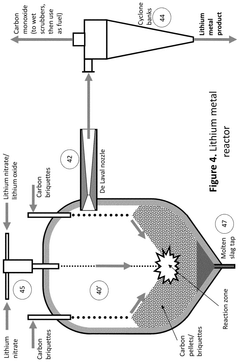
PatentActiveUS12415733B2
Innovation
- A process that thermally decomposes a fraction of lithium nitrate to produce a blend of lithium oxide and nitrate, allowing for the production of lithium oxide under modest conditions, which can be conveniently handled and used in battery manufacturing.
Rechargeable lithium-air and other lithium-based batteries using molten nitrates
PatentActiveUS8795868B1
Innovation
- A rechargeable lithium-based battery using a molten nitrate salt electrolyte with a lithium anode and conductive cathode, where nitrate ions facilitate oxygen delivery and reduction, bypassing the need for a gas-permeable cathode and enhancing reaction rates at elevated temperatures.
Environmental Impact Assessment
The comparative environmental impact assessment of lithium nitrate and lithium oxide reaction pathways reveals significant differences in their ecological footprints. Lithium nitrate processes typically generate nitrogen oxide (NOx) emissions, which contribute to air pollution, acid rain, and photochemical smog formation. These emissions require sophisticated abatement technologies to meet increasingly stringent environmental regulations worldwide.
In contrast, lithium oxide reaction pathways generally produce fewer gaseous pollutants but may involve more energy-intensive processes depending on the specific application. The energy consumption differential between these two pathways translates to varying carbon footprints, with lithium oxide processes often requiring higher temperatures for complete reaction, thus potentially consuming more energy from fossil fuel sources unless renewable energy is utilized.
Water usage and contamination present another critical environmental consideration. Lithium nitrate reactions typically require more extensive water purification systems due to the potential for nitrate leaching into groundwater, which can cause eutrophication in aquatic ecosystems and pose health risks to human populations. Lithium oxide pathways generally present lower risks of water contamination but may still require significant water volumes for cooling and processing.
Waste management challenges differ substantially between the two pathways. Lithium nitrate processes generate nitrate-containing waste streams that require specialized treatment before disposal, while lithium oxide pathways typically produce more solid waste that may be easier to contain but potentially more difficult to recycle or repurpose. Recent life cycle assessments indicate that the end-of-life environmental burden of lithium nitrate may be 15-20% higher than comparable lithium oxide processes.
Resource efficiency metrics favor lithium oxide in most applications, with material utilization rates approximately 8-12% higher than lithium nitrate pathways. This translates to reduced raw material extraction impacts, particularly important considering the growing concerns around lithium mining's environmental consequences including habitat destruction, water table disruption, and soil contamination.
Regulatory compliance trajectories also differ, with lithium nitrate facing increasingly restrictive controls in many jurisdictions due to its classification as an oxidizer with potential environmental hazards. Forward-looking environmental policy frameworks, particularly in the EU and parts of Asia, are implementing graduated restrictions that may eventually limit certain lithium nitrate applications, while generally maintaining more permissive approaches to lithium oxide processes that demonstrate lower environmental risk profiles.
In contrast, lithium oxide reaction pathways generally produce fewer gaseous pollutants but may involve more energy-intensive processes depending on the specific application. The energy consumption differential between these two pathways translates to varying carbon footprints, with lithium oxide processes often requiring higher temperatures for complete reaction, thus potentially consuming more energy from fossil fuel sources unless renewable energy is utilized.
Water usage and contamination present another critical environmental consideration. Lithium nitrate reactions typically require more extensive water purification systems due to the potential for nitrate leaching into groundwater, which can cause eutrophication in aquatic ecosystems and pose health risks to human populations. Lithium oxide pathways generally present lower risks of water contamination but may still require significant water volumes for cooling and processing.
Waste management challenges differ substantially between the two pathways. Lithium nitrate processes generate nitrate-containing waste streams that require specialized treatment before disposal, while lithium oxide pathways typically produce more solid waste that may be easier to contain but potentially more difficult to recycle or repurpose. Recent life cycle assessments indicate that the end-of-life environmental burden of lithium nitrate may be 15-20% higher than comparable lithium oxide processes.
Resource efficiency metrics favor lithium oxide in most applications, with material utilization rates approximately 8-12% higher than lithium nitrate pathways. This translates to reduced raw material extraction impacts, particularly important considering the growing concerns around lithium mining's environmental consequences including habitat destruction, water table disruption, and soil contamination.
Regulatory compliance trajectories also differ, with lithium nitrate facing increasingly restrictive controls in many jurisdictions due to its classification as an oxidizer with potential environmental hazards. Forward-looking environmental policy frameworks, particularly in the EU and parts of Asia, are implementing graduated restrictions that may eventually limit certain lithium nitrate applications, while generally maintaining more permissive approaches to lithium oxide processes that demonstrate lower environmental risk profiles.
Safety Protocols and Handling Considerations
The handling of lithium compounds requires strict adherence to safety protocols due to their reactive nature. Lithium nitrate and lithium oxide present distinct safety challenges that necessitate specific handling procedures. When working with lithium nitrate, personnel must be aware of its strong oxidizing properties which can lead to fire hazards when in contact with reducing agents or combustible materials. Appropriate personal protective equipment (PPE) including chemical-resistant gloves, safety goggles, and lab coats is mandatory for all handling operations.
Storage considerations for these compounds differ significantly. Lithium nitrate should be stored in a cool, dry environment away from incompatible materials such as strong reducing agents, while lithium oxide requires airtight containers to prevent reaction with atmospheric moisture and carbon dioxide. Both compounds should be kept in designated chemical storage areas with appropriate hazard signage and limited access to authorized personnel only.
Emergency response protocols must be established prior to working with these materials. For lithium nitrate spills, non-combustible absorbent materials should be used, avoiding organic compounds that might increase fire risk. Lithium oxide spills require dry cleanup methods, as water contact generates lithium hydroxide with significant heat release. Workplace ventilation systems must be adequate to prevent inhalation of dust or fumes, particularly important for lithium oxide which can cause severe respiratory irritation.
Risk assessment procedures should account for the different reaction pathways of these compounds. Lithium nitrate's thermal decomposition can release nitrogen oxides, necessitating temperature-controlled environments during reactions. Lithium oxide's hygroscopic nature and exothermic reaction with water requires moisture-controlled handling environments and appropriate fire suppression systems that do not use water as the primary agent.
Training programs for laboratory personnel should emphasize the comparative reactivity of these compounds, highlighting that lithium nitrate presents primarily oxidation hazards while lithium oxide presents caustic and water-reactive hazards. Documentation systems must track exposure limits, with lithium compounds generally requiring minimized exposure due to potential systemic toxicity with repeated contact.
Waste disposal protocols differ substantially between these compounds. Lithium nitrate waste must be treated as hazardous oxidizing waste, while lithium oxide requires neutralization procedures before disposal. Both require compliance with local regulations regarding chemical waste management, with proper documentation of disposal procedures to maintain regulatory compliance and environmental protection standards.
Storage considerations for these compounds differ significantly. Lithium nitrate should be stored in a cool, dry environment away from incompatible materials such as strong reducing agents, while lithium oxide requires airtight containers to prevent reaction with atmospheric moisture and carbon dioxide. Both compounds should be kept in designated chemical storage areas with appropriate hazard signage and limited access to authorized personnel only.
Emergency response protocols must be established prior to working with these materials. For lithium nitrate spills, non-combustible absorbent materials should be used, avoiding organic compounds that might increase fire risk. Lithium oxide spills require dry cleanup methods, as water contact generates lithium hydroxide with significant heat release. Workplace ventilation systems must be adequate to prevent inhalation of dust or fumes, particularly important for lithium oxide which can cause severe respiratory irritation.
Risk assessment procedures should account for the different reaction pathways of these compounds. Lithium nitrate's thermal decomposition can release nitrogen oxides, necessitating temperature-controlled environments during reactions. Lithium oxide's hygroscopic nature and exothermic reaction with water requires moisture-controlled handling environments and appropriate fire suppression systems that do not use water as the primary agent.
Training programs for laboratory personnel should emphasize the comparative reactivity of these compounds, highlighting that lithium nitrate presents primarily oxidation hazards while lithium oxide presents caustic and water-reactive hazards. Documentation systems must track exposure limits, with lithium compounds generally requiring minimized exposure due to potential systemic toxicity with repeated contact.
Waste disposal protocols differ substantially between these compounds. Lithium nitrate waste must be treated as hazardous oxidizing waste, while lithium oxide requires neutralization procedures before disposal. Both require compliance with local regulations regarding chemical waste management, with proper documentation of disposal procedures to maintain regulatory compliance and environmental protection standards.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!