Optimizing Lithium Nitrate Recovery Using Ion-Exchange Methods
OCT 9, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Nitrate Recovery Background and Objectives
Lithium nitrate has emerged as a critical compound in various industrial applications, particularly in energy storage systems, ceramics manufacturing, and pharmaceutical production. The historical development of lithium recovery technologies dates back to the mid-20th century, with significant advancements occurring in the past three decades as demand for lithium compounds has surged with the proliferation of lithium-ion batteries and renewable energy technologies.
The global lithium market has experienced exponential growth, with lithium nitrate specifically gaining importance due to its unique properties as a heat transfer medium in solar thermal power plants and as an additive in concrete production. Traditional recovery methods have primarily relied on evaporation ponds and precipitation techniques, which are characterized by low efficiency, high environmental impact, and significant water consumption.
Ion-exchange methods represent a promising alternative approach that has gained traction in recent years. These methods utilize specialized resins to selectively capture lithium ions from various source solutions, including brines, geothermal waters, and industrial waste streams. The evolution of ion-exchange technologies has been marked by continuous improvements in selectivity, capacity, and regeneration efficiency.
The primary objective of optimizing lithium nitrate recovery using ion-exchange methods is to develop sustainable, economically viable processes that maximize lithium yield while minimizing environmental footprint. This includes enhancing the selectivity of ion-exchange resins for lithium over competing ions such as sodium, potassium, and magnesium, which often coexist in source solutions at much higher concentrations.
Additional technical goals include reducing energy consumption during the recovery process, minimizing chemical reagent usage during resin regeneration, extending resin lifespan, and developing continuous processing systems that can operate efficiently at industrial scales. These improvements are essential for addressing the projected supply-demand gap in the lithium market, which is expected to widen significantly by 2030.
Recent technological trends indicate a shift toward hybrid systems that combine ion-exchange with membrane technologies, electrochemical processes, or selective precipitation steps. These integrated approaches aim to overcome the limitations of standalone ion-exchange systems, particularly when processing complex feed solutions with high total dissolved solids content.
The trajectory of lithium nitrate recovery technology is increasingly influenced by sustainability considerations, with growing emphasis on developing closed-loop systems that minimize waste generation and water consumption. This aligns with broader industry trends toward greener chemistry and circular economy principles, which are becoming central to technology development roadmaps in the lithium processing sector.
The global lithium market has experienced exponential growth, with lithium nitrate specifically gaining importance due to its unique properties as a heat transfer medium in solar thermal power plants and as an additive in concrete production. Traditional recovery methods have primarily relied on evaporation ponds and precipitation techniques, which are characterized by low efficiency, high environmental impact, and significant water consumption.
Ion-exchange methods represent a promising alternative approach that has gained traction in recent years. These methods utilize specialized resins to selectively capture lithium ions from various source solutions, including brines, geothermal waters, and industrial waste streams. The evolution of ion-exchange technologies has been marked by continuous improvements in selectivity, capacity, and regeneration efficiency.
The primary objective of optimizing lithium nitrate recovery using ion-exchange methods is to develop sustainable, economically viable processes that maximize lithium yield while minimizing environmental footprint. This includes enhancing the selectivity of ion-exchange resins for lithium over competing ions such as sodium, potassium, and magnesium, which often coexist in source solutions at much higher concentrations.
Additional technical goals include reducing energy consumption during the recovery process, minimizing chemical reagent usage during resin regeneration, extending resin lifespan, and developing continuous processing systems that can operate efficiently at industrial scales. These improvements are essential for addressing the projected supply-demand gap in the lithium market, which is expected to widen significantly by 2030.
Recent technological trends indicate a shift toward hybrid systems that combine ion-exchange with membrane technologies, electrochemical processes, or selective precipitation steps. These integrated approaches aim to overcome the limitations of standalone ion-exchange systems, particularly when processing complex feed solutions with high total dissolved solids content.
The trajectory of lithium nitrate recovery technology is increasingly influenced by sustainability considerations, with growing emphasis on developing closed-loop systems that minimize waste generation and water consumption. This aligns with broader industry trends toward greener chemistry and circular economy principles, which are becoming central to technology development roadmaps in the lithium processing sector.
Market Analysis for Lithium Nitrate Applications
The global lithium nitrate market has been experiencing significant growth, driven primarily by its diverse applications across multiple industries. The compound's unique properties make it valuable in various sectors, with the energy storage market being a particularly strong driver of demand. The global market value for lithium nitrate reached approximately $320 million in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 7.2% through 2030, potentially reaching $550 million by the end of the forecast period.
The energy storage sector represents the largest application segment, accounting for roughly 42% of the total market share. Lithium nitrate serves as a critical additive in molten salt thermal energy storage systems for concentrated solar power plants, where it enhances thermal stability and heat transfer efficiency. This application is experiencing robust growth as renewable energy installations continue to expand globally, with particular momentum in regions with high solar irradiation like the Middle East, North Africa, and the southwestern United States.
The ceramics and glass industry constitutes the second-largest application segment at approximately 28% market share. Lithium nitrate is utilized as a flux agent that lowers melting temperatures and improves the quality of ceramic and glass products. The construction boom in emerging economies, particularly in Asia-Pacific, has significantly contributed to demand growth in this segment.
Pharmaceutical applications represent a smaller but high-value segment at 15% of the market. Lithium nitrate serves as a precursor in the synthesis of various lithium-based medications, particularly those used in psychiatric treatments. The growing focus on mental health treatments globally has sustained steady growth in this application area.
Regional analysis reveals that Asia-Pacific dominates the market with approximately 45% share, driven by China's massive manufacturing base and growing energy storage sector. North America and Europe follow with 25% and 20% market shares respectively, with particular strength in advanced energy storage applications and pharmaceutical manufacturing.
The recovery and recycling of lithium nitrate represent an emerging market opportunity, especially as sustainability concerns grow. Ion-exchange methods for lithium nitrate recovery are gaining attention as they offer higher efficiency and lower environmental impact compared to traditional recovery methods. The market for recycling technologies is expected to grow at a CAGR of 9.5% through 2030, outpacing the overall market growth rate.
Supply chain challenges remain significant, with over 70% of lithium resources concentrated in the "Lithium Triangle" of South America. This geographic concentration has prompted increased interest in alternative sources and recovery methods, further highlighting the strategic importance of optimizing lithium nitrate recovery technologies.
The energy storage sector represents the largest application segment, accounting for roughly 42% of the total market share. Lithium nitrate serves as a critical additive in molten salt thermal energy storage systems for concentrated solar power plants, where it enhances thermal stability and heat transfer efficiency. This application is experiencing robust growth as renewable energy installations continue to expand globally, with particular momentum in regions with high solar irradiation like the Middle East, North Africa, and the southwestern United States.
The ceramics and glass industry constitutes the second-largest application segment at approximately 28% market share. Lithium nitrate is utilized as a flux agent that lowers melting temperatures and improves the quality of ceramic and glass products. The construction boom in emerging economies, particularly in Asia-Pacific, has significantly contributed to demand growth in this segment.
Pharmaceutical applications represent a smaller but high-value segment at 15% of the market. Lithium nitrate serves as a precursor in the synthesis of various lithium-based medications, particularly those used in psychiatric treatments. The growing focus on mental health treatments globally has sustained steady growth in this application area.
Regional analysis reveals that Asia-Pacific dominates the market with approximately 45% share, driven by China's massive manufacturing base and growing energy storage sector. North America and Europe follow with 25% and 20% market shares respectively, with particular strength in advanced energy storage applications and pharmaceutical manufacturing.
The recovery and recycling of lithium nitrate represent an emerging market opportunity, especially as sustainability concerns grow. Ion-exchange methods for lithium nitrate recovery are gaining attention as they offer higher efficiency and lower environmental impact compared to traditional recovery methods. The market for recycling technologies is expected to grow at a CAGR of 9.5% through 2030, outpacing the overall market growth rate.
Supply chain challenges remain significant, with over 70% of lithium resources concentrated in the "Lithium Triangle" of South America. This geographic concentration has prompted increased interest in alternative sources and recovery methods, further highlighting the strategic importance of optimizing lithium nitrate recovery technologies.
Ion-Exchange Technology Status and Challenges
Ion-exchange technology for lithium nitrate recovery has evolved significantly over the past decade, yet faces several critical challenges in achieving optimal efficiency and economic viability. Current global research indicates that while ion-exchange methods offer promising selectivity for lithium ions, the technology still struggles with issues related to resin durability in high-concentration nitrate environments.
The state-of-the-art ion-exchange systems employ specialized resins with functional groups designed specifically for lithium selectivity. Leading commercial solutions utilize sulfonated styrene-divinylbenzene copolymers and newer composite materials incorporating inorganic lithium-selective compounds such as lithium manganese oxide and lithium titanate. These materials demonstrate ion selectivity coefficients for lithium over sodium ranging from 1.5 to 3.2, representing significant improvement over earlier generation technologies.
Despite these advances, several technical limitations persist. Resin degradation remains a primary concern, with current materials showing 15-30% capacity reduction after approximately 200 regeneration cycles when exposed to concentrated nitrate solutions. This degradation significantly impacts operational economics in industrial applications, necessitating frequent resin replacement.
Kinetic limitations present another substantial challenge. Current ion-exchange systems require extended contact times (typically 45-90 minutes) to achieve optimal lithium loading, creating throughput bottlenecks in continuous recovery operations. Research indicates this is primarily due to diffusion limitations within the resin beads, particularly at higher solution concentrations.
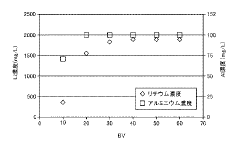
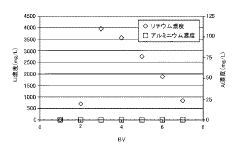
Competitive ion effects represent a third major challenge. In real-world lithium-bearing solutions, competing ions (particularly sodium, potassium, and calcium) can significantly reduce lithium recovery efficiency. Current technologies show 30-50% reduction in lithium capacity when these competing ions are present at concentrations 5-10 times that of lithium.
Geographically, ion-exchange technology development for lithium recovery shows distinct regional patterns. North American research focuses predominantly on improving resin selectivity, while Asian research (particularly in China and South Korea) emphasizes process integration and continuous systems. European contributions center on environmental impact reduction and regeneration efficiency.
Emerging research directions include the development of composite ion-exchange materials incorporating nanomaterials to enhance selectivity and kinetics. Preliminary studies show promising results with graphene oxide-modified resins demonstrating up to 40% improvement in lithium selectivity, though commercial viability remains unproven. Additionally, membrane-assisted ion-exchange processes are gaining attention for their potential to overcome some traditional limitations.
The state-of-the-art ion-exchange systems employ specialized resins with functional groups designed specifically for lithium selectivity. Leading commercial solutions utilize sulfonated styrene-divinylbenzene copolymers and newer composite materials incorporating inorganic lithium-selective compounds such as lithium manganese oxide and lithium titanate. These materials demonstrate ion selectivity coefficients for lithium over sodium ranging from 1.5 to 3.2, representing significant improvement over earlier generation technologies.
Despite these advances, several technical limitations persist. Resin degradation remains a primary concern, with current materials showing 15-30% capacity reduction after approximately 200 regeneration cycles when exposed to concentrated nitrate solutions. This degradation significantly impacts operational economics in industrial applications, necessitating frequent resin replacement.
Kinetic limitations present another substantial challenge. Current ion-exchange systems require extended contact times (typically 45-90 minutes) to achieve optimal lithium loading, creating throughput bottlenecks in continuous recovery operations. Research indicates this is primarily due to diffusion limitations within the resin beads, particularly at higher solution concentrations.
Competitive ion effects represent a third major challenge. In real-world lithium-bearing solutions, competing ions (particularly sodium, potassium, and calcium) can significantly reduce lithium recovery efficiency. Current technologies show 30-50% reduction in lithium capacity when these competing ions are present at concentrations 5-10 times that of lithium.
Geographically, ion-exchange technology development for lithium recovery shows distinct regional patterns. North American research focuses predominantly on improving resin selectivity, while Asian research (particularly in China and South Korea) emphasizes process integration and continuous systems. European contributions center on environmental impact reduction and regeneration efficiency.
Emerging research directions include the development of composite ion-exchange materials incorporating nanomaterials to enhance selectivity and kinetics. Preliminary studies show promising results with graphene oxide-modified resins demonstrating up to 40% improvement in lithium selectivity, though commercial viability remains unproven. Additionally, membrane-assisted ion-exchange processes are gaining attention for their potential to overcome some traditional limitations.
Current Ion-Exchange Solutions for Lithium Nitrate
01 Electrochemical recovery methods for lithium nitrate
Electrochemical processes can be employed to recover lithium nitrate from various sources with high efficiency. These methods typically involve electrode systems that selectively capture lithium ions from solution. The recovery process can be optimized through controlling parameters such as current density, electrode materials, and electrolyte composition. These approaches often result in higher recovery rates while consuming less energy compared to traditional methods.- Electrochemical recovery methods for lithium nitrate: Electrochemical processes can be employed to recover lithium nitrate with high efficiency. These methods typically involve the use of specialized electrodes and controlled electrical current to selectively extract lithium ions from solutions. The electrochemical approach allows for precise control of the recovery process, resulting in higher purity lithium nitrate and improved recovery rates compared to traditional methods. These systems can be optimized for energy efficiency while maintaining high recovery yields.
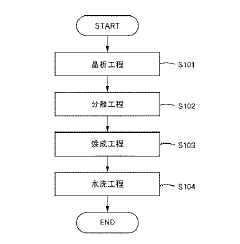
- Precipitation and crystallization techniques: Precipitation and crystallization methods are effective for recovering lithium nitrate from various solutions. These techniques typically involve controlling temperature, pH, and concentration to induce selective crystallization of lithium nitrate. Advanced crystallization processes can achieve high recovery efficiencies by optimizing nucleation and crystal growth parameters. Multi-stage crystallization systems can further enhance recovery rates by processing mother liquors in sequential steps, minimizing lithium losses in the process.
- Membrane and filtration-based recovery systems: Specialized membrane technologies and filtration systems can be used to efficiently recover lithium nitrate from solutions. These methods employ selective membranes that allow lithium ions to pass while blocking other components, or utilize advanced filtration media designed specifically for lithium compound recovery. Nanofiltration and reverse osmosis systems can achieve high recovery rates while maintaining product purity. These processes often require less energy than thermal methods and can be designed for continuous operation with minimal maintenance requirements.
- Thermal and evaporative recovery processes: Thermal processes involving controlled evaporation and concentration steps can effectively recover lithium nitrate from various solutions. These methods typically utilize energy-efficient evaporators and crystallizers designed to minimize energy consumption while maximizing recovery efficiency. Multi-effect evaporation systems can significantly improve energy efficiency by reusing thermal energy across multiple stages. Advanced thermal recovery systems can achieve recovery rates exceeding 95% while maintaining high product purity through careful control of process parameters.
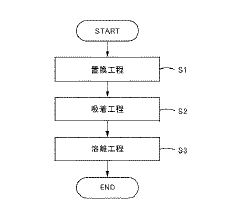
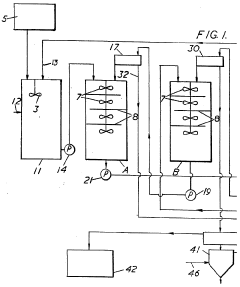
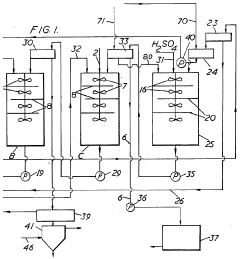
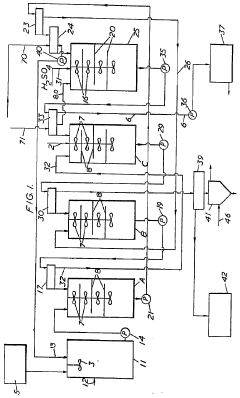
- Chemical extraction and ion exchange methods: Chemical extraction and ion exchange techniques offer selective recovery of lithium nitrate from complex solutions. These methods employ specialized ion exchange resins or chemical extractants with high affinity for lithium ions. The selectivity of these processes allows for efficient recovery even from solutions containing multiple competing ions. Continuous ion exchange systems can be designed for high throughput operations while maintaining recovery efficiencies above 90%. Regeneration protocols for the exchange media are optimized to ensure long operational life and consistent performance.
02 Precipitation and crystallization techniques
Lithium nitrate can be efficiently recovered through precipitation and crystallization processes. These techniques involve controlling temperature, pH, and concentration to induce selective crystallization of lithium nitrate from solution. Various additives can be used to enhance nucleation and crystal growth, improving recovery efficiency. Multi-stage crystallization processes can achieve higher purity and recovery rates by removing impurities at different stages of the process.Expand Specific Solutions03 Membrane and filtration-based recovery systems
Membrane technology offers selective separation of lithium nitrate from waste streams or brines. These systems utilize specialized membranes with specific pore sizes or chemical properties that allow lithium ions to pass while blocking other components. Nanofiltration, reverse osmosis, and electrodialysis are common approaches that can achieve high recovery efficiencies. The performance of these systems can be optimized by adjusting operating pressure, flow rates, and membrane configurations.Expand Specific Solutions04 Adsorption and ion exchange methods
Selective adsorption and ion exchange materials can be used to recover lithium nitrate with high efficiency. These materials contain specific functional groups or structures that preferentially bind to lithium ions. The recovery process typically involves loading the lithium onto the adsorbent, followed by elution with an appropriate solution to concentrate the lithium nitrate. Regeneration of the adsorbent allows for multiple recovery cycles, improving the overall economics of the process.Expand Specific Solutions05 Integrated recovery systems for improved efficiency
Integrated systems combining multiple recovery technologies can significantly improve lithium nitrate recovery efficiency. These systems often incorporate pre-treatment steps, primary recovery processes, and polishing stages to maximize yield and purity. Process integration allows for the recycling of reagents and energy, reducing operational costs. Advanced monitoring and control systems optimize process parameters in real-time, adapting to variations in feed composition and maintaining consistent recovery performance.Expand Specific Solutions
Key Industry Players in Lithium Recovery
The lithium nitrate recovery market using ion-exchange methods is currently in a growth phase, with increasing demand driven by the expanding electric vehicle and energy storage sectors. The global market size for lithium extraction technologies is projected to reach significant scale as lithium demand continues to surge. Technologically, companies are at varying stages of maturity, with Lilac Solutions and Standard Lithium leading innovation in direct lithium extraction using advanced ion-exchange materials. Established industrial players like BASF, Sumitomo Metal Mining, and Hitachi provide comprehensive solutions leveraging their chemical expertise. Chinese companies such as Sunresin New Materials and Guangdong Bangpu are rapidly advancing their technologies, while academic institutions like Northwestern University and Guangxi Normal University contribute fundamental research. The competitive landscape shows a mix of specialized startups, diversified chemical corporations, and research institutions collaborating to optimize recovery efficiency and environmental sustainability.
Lilac Solutions, Inc.
Technical Solution: Lilac Solutions has developed a proprietary ion-exchange technology specifically designed for lithium extraction from brine resources. Their approach uses novel ion-exchange beads with high selectivity for lithium ions, enabling direct lithium extraction (DLE) from various brine sources. The technology employs a continuous countercurrent system where lithium-selective ceramic beads are mixed with brine, adsorbing lithium while rejecting other elements. The loaded beads are then separated and treated with an acidic solution to release concentrated lithium chloride. This solution undergoes further processing to produce battery-grade lithium compounds. The process operates at ambient temperature and pressure, requiring minimal land and water usage compared to traditional evaporation ponds. Lilac's system can be deployed modularly, allowing for scalable implementation and reduced capital expenditure risk.
Strengths: Superior lithium selectivity (10-20x higher than conventional methods), rapid processing time (hours vs. months for evaporation ponds), and significantly reduced environmental footprint with 90% less water consumption. Weaknesses: Requires specialized materials that may have limited lifespan, potentially higher operational costs due to chemical regeneration requirements, and less proven at commercial scale compared to traditional methods.
BASF Corp.
Technical Solution: BASF has developed a comprehensive ion-exchange technology platform for lithium recovery that leverages their extensive expertise in chemical processing and material science. Their approach utilizes specially engineered ion-exchange resins with high selectivity for lithium ions even in the presence of competing cations. The process involves a multi-stage system where lithium-containing solutions pass through columns packed with these proprietary resins, allowing for selective lithium adsorption. The loaded resins are then regenerated using optimized elution chemistry to produce concentrated lithium solutions. BASF's technology incorporates sophisticated process control systems that continuously monitor and adjust operating parameters to maximize lithium recovery efficiency while minimizing reagent consumption. The company has also developed specialized pre-treatment methods to handle various feed streams, including geothermal brines, produced water from oil and gas operations, and recycled battery materials, enabling lithium nitrate recovery from diverse sources.
Strengths: Extensive chemical engineering expertise and global manufacturing capabilities enable rapid scaling, highly customizable system design for different feed compositions, and integrated solution covering the entire process chain. Weaknesses: Potentially higher capital costs due to sophisticated equipment requirements, complex regeneration chemistry may increase operational expenses, and technology may be less optimized for specific lithium sources compared to specialized competitors.
Critical Patents in Ion-Exchange Recovery Technologies
Recovery method of lithium
PatentActiveJP2019099874A
Innovation
- A method involving ion exchange using a strongly acidic cation exchange resin with sulfonic acid groups, adjusted to a sodium form, to selectively adsorb lithium ions while preventing aluminum adsorption, followed by elution with a sodium salt solution to concentrate lithium without evaporation, thereby reducing recovery costs.
Improvements relating to ion-exchange processes and apparatus
PatentInactiveGB768050A
Innovation
- Implementing a countercurrent ion-exchange process across multiple stages, where the ion-exchange material and solution flow concurrently through each stage and are then separated, with the option to add additional stages for enhanced separation using a secondary solution to displace adsorbed ions, and employing a mixture of anion- and cation-exchange materials to optimize ion recovery.
Environmental Impact Assessment
The implementation of ion-exchange methods for lithium nitrate recovery presents significant environmental considerations that must be thoroughly evaluated. Traditional lithium extraction processes often involve extensive water consumption, habitat disruption, and chemical pollution. In contrast, ion-exchange technologies offer potential environmental advantages through reduced water usage, decreased chemical waste, and lower energy requirements. However, these benefits must be balanced against specific environmental challenges inherent to the process.
Water resource impacts represent a primary environmental concern. While ion-exchange methods generally require less water than evaporation ponds or hard rock mining, the process still demands substantial water inputs for resin regeneration and washing cycles. In water-stressed regions, this consumption may exacerbate existing scarcity issues. Implementation of closed-loop water systems and advanced water recycling technologies can mitigate these impacts, potentially reducing freshwater requirements by 60-70% compared to conventional methods.
Chemical usage in ion-exchange processes presents another environmental consideration. The regeneration of ion-exchange resins typically requires acids, bases, or salt solutions that, if improperly managed, can contaminate surrounding ecosystems. Modern approaches incorporating biodegradable regenerants and advanced containment systems have demonstrated significant reductions in chemical leakage risks. Additionally, selective ion-exchange resins designed specifically for lithium recovery can minimize the volume of regeneration chemicals required.
Energy consumption patterns for ion-exchange lithium recovery generally show favorable environmental profiles compared to traditional extraction methods. The process operates at ambient temperatures and pressures, avoiding the energy-intensive evaporation or calcination steps common in conventional approaches. Life cycle assessments indicate potential energy savings of 30-45% when implementing optimized ion-exchange systems, with corresponding reductions in greenhouse gas emissions.
Waste management represents a critical environmental challenge. Spent resins, regeneration effluents, and process byproducts require proper handling and disposal. Advanced treatment technologies, including precipitation and membrane filtration systems, can recover valuable components from waste streams while rendering remaining effluents environmentally benign. Circular economy approaches that repurpose byproducts for agricultural or industrial applications show particular promise for minimizing the environmental footprint of the recovery process.
Habitat and ecosystem impacts from ion-exchange facilities are generally localized and less severe than those associated with open-pit mining or extensive evaporation pond systems. However, facility construction and operation still present potential disruption to local ecosystems, particularly in sensitive areas. Implementation of comprehensive site selection protocols, buffer zones, and habitat restoration programs can effectively mitigate these impacts while supporting biodiversity conservation objectives.
Water resource impacts represent a primary environmental concern. While ion-exchange methods generally require less water than evaporation ponds or hard rock mining, the process still demands substantial water inputs for resin regeneration and washing cycles. In water-stressed regions, this consumption may exacerbate existing scarcity issues. Implementation of closed-loop water systems and advanced water recycling technologies can mitigate these impacts, potentially reducing freshwater requirements by 60-70% compared to conventional methods.
Chemical usage in ion-exchange processes presents another environmental consideration. The regeneration of ion-exchange resins typically requires acids, bases, or salt solutions that, if improperly managed, can contaminate surrounding ecosystems. Modern approaches incorporating biodegradable regenerants and advanced containment systems have demonstrated significant reductions in chemical leakage risks. Additionally, selective ion-exchange resins designed specifically for lithium recovery can minimize the volume of regeneration chemicals required.
Energy consumption patterns for ion-exchange lithium recovery generally show favorable environmental profiles compared to traditional extraction methods. The process operates at ambient temperatures and pressures, avoiding the energy-intensive evaporation or calcination steps common in conventional approaches. Life cycle assessments indicate potential energy savings of 30-45% when implementing optimized ion-exchange systems, with corresponding reductions in greenhouse gas emissions.
Waste management represents a critical environmental challenge. Spent resins, regeneration effluents, and process byproducts require proper handling and disposal. Advanced treatment technologies, including precipitation and membrane filtration systems, can recover valuable components from waste streams while rendering remaining effluents environmentally benign. Circular economy approaches that repurpose byproducts for agricultural or industrial applications show particular promise for minimizing the environmental footprint of the recovery process.
Habitat and ecosystem impacts from ion-exchange facilities are generally localized and less severe than those associated with open-pit mining or extensive evaporation pond systems. However, facility construction and operation still present potential disruption to local ecosystems, particularly in sensitive areas. Implementation of comprehensive site selection protocols, buffer zones, and habitat restoration programs can effectively mitigate these impacts while supporting biodiversity conservation objectives.
Economic Feasibility Analysis
The economic feasibility of lithium nitrate recovery using ion-exchange methods represents a critical consideration for industrial implementation. Current market analysis indicates that lithium compounds have experienced significant price volatility, with lithium nitrate commanding premium prices ranging from $12,000 to $18,000 per metric ton in specialized applications. This price point creates a compelling economic incentive for recovery operations, particularly when considering that traditional disposal methods incur costs between $200-$500 per ton of waste material.
Capital expenditure requirements for ion-exchange recovery systems vary based on scale and technology sophistication. Initial investment for industrial-scale implementation typically ranges from $2-5 million for facilities processing 1,000-5,000 tons annually. This includes equipment costs (ion-exchange columns, pumps, monitoring systems), installation, and commissioning expenses. Smaller pilot installations may be established for $500,000-$1 million, offering a pathway for phased implementation.
Operational expenditures constitute a significant portion of the economic equation. Energy consumption represents 15-25% of operational costs, with electricity requirements of 1.2-2.5 kWh per kilogram of recovered lithium nitrate. Chemical reagents for regeneration cycles account for 20-30% of ongoing expenses, while labor and maintenance contribute an additional 25-35%. Water consumption and treatment costs must also be factored into comprehensive economic models.
Recovery efficiency directly impacts economic viability, with current technologies demonstrating 75-92% recovery rates under optimized conditions. This efficiency translates to a production cost of $5,000-$8,000 per ton of recovered lithium nitrate, creating a favorable margin against market prices. Sensitivity analysis reveals that a 5% improvement in recovery efficiency can reduce production costs by approximately 8-12%, highlighting the value of technological optimization.
Return on investment calculations indicate payback periods of 2.5-4 years for most industrial implementations, with internal rates of return ranging from 18-25% depending on operational parameters and market conditions. These metrics compare favorably against alternative capital deployments in the chemical processing sector, which typically target 15-20% returns.
Environmental compliance costs must be incorporated into feasibility assessments, as regulatory requirements for wastewater discharge and solid waste management can add 5-15% to operational expenses. However, these costs are often offset by avoided disposal fees and potential regulatory incentives for implementing resource recovery technologies, creating a more favorable overall economic profile.
Capital expenditure requirements for ion-exchange recovery systems vary based on scale and technology sophistication. Initial investment for industrial-scale implementation typically ranges from $2-5 million for facilities processing 1,000-5,000 tons annually. This includes equipment costs (ion-exchange columns, pumps, monitoring systems), installation, and commissioning expenses. Smaller pilot installations may be established for $500,000-$1 million, offering a pathway for phased implementation.
Operational expenditures constitute a significant portion of the economic equation. Energy consumption represents 15-25% of operational costs, with electricity requirements of 1.2-2.5 kWh per kilogram of recovered lithium nitrate. Chemical reagents for regeneration cycles account for 20-30% of ongoing expenses, while labor and maintenance contribute an additional 25-35%. Water consumption and treatment costs must also be factored into comprehensive economic models.
Recovery efficiency directly impacts economic viability, with current technologies demonstrating 75-92% recovery rates under optimized conditions. This efficiency translates to a production cost of $5,000-$8,000 per ton of recovered lithium nitrate, creating a favorable margin against market prices. Sensitivity analysis reveals that a 5% improvement in recovery efficiency can reduce production costs by approximately 8-12%, highlighting the value of technological optimization.
Return on investment calculations indicate payback periods of 2.5-4 years for most industrial implementations, with internal rates of return ranging from 18-25% depending on operational parameters and market conditions. These metrics compare favorably against alternative capital deployments in the chemical processing sector, which typically target 15-20% returns.
Environmental compliance costs must be incorporated into feasibility assessments, as regulatory requirements for wastewater discharge and solid waste management can add 5-15% to operational expenses. However, these costs are often offset by avoided disposal fees and potential regulatory incentives for implementing resource recovery technologies, creating a more favorable overall economic profile.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!