Lithium Nitrate vs Lithium Chlorate: Safety and Reactivity Comparison
OCT 9, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Salt Chemistry Background and Research Objectives
Lithium salts have been integral to various industrial and technological applications for decades, with their unique chemical properties making them valuable in energy storage, pharmaceuticals, and materials science. The evolution of lithium salt chemistry can be traced back to the early 19th century when lithium was first isolated as an element. Since then, understanding of lithium compounds has expanded significantly, particularly regarding their reactivity patterns and safety profiles.
The comparative study of lithium nitrate (LiNO₃) and lithium chlorate (LiClO₃) represents an important area of research within lithium salt chemistry. These compounds, while sharing the same cation, exhibit markedly different behaviors due to their distinct anions. Lithium nitrate has gained prominence in thermal energy storage systems and as an additive in lithium-ion battery electrolytes, while lithium chlorate has found applications in oxidizing processes and specialized chemical synthesis.
Recent technological advancements have intensified interest in these compounds, particularly as the global demand for energy storage solutions continues to grow. The safety aspects of lithium salts have become increasingly critical as their applications expand into consumer products and large-scale energy systems. Historical incidents involving oxidizing agents similar to lithium chlorate have highlighted the importance of comprehensive safety assessments for these materials.
The chemical behavior of these salts is governed by fundamental principles of oxidation-reduction chemistry, thermal stability, and reaction kinetics. Lithium nitrate typically demonstrates moderate oxidizing properties, while lithium chlorate exhibits more aggressive oxidizing characteristics. This distinction forms the basis for their different application profiles and safety considerations.
Current research trends indicate a growing focus on understanding the molecular-level interactions of these compounds with various substrates and under different environmental conditions. Computational chemistry approaches have enabled more precise predictions of reactivity patterns, complementing traditional experimental methods.
This technical research report aims to provide a comprehensive comparison of lithium nitrate and lithium chlorate with specific emphasis on their safety profiles and reactivity characteristics. The objectives include: establishing quantitative metrics for comparing the relative stability of these compounds; identifying critical thresholds for safe handling and storage; evaluating their compatibility with common materials used in industrial applications; and developing predictive models for their behavior under various environmental conditions.
By systematically analyzing these aspects, this research seeks to contribute to the development of safer protocols for handling these compounds and to identify optimal applications that leverage their unique properties while minimizing associated risks. The findings will support evidence-based decision-making for industries utilizing these lithium salts and guide future research directions in this field.
The comparative study of lithium nitrate (LiNO₃) and lithium chlorate (LiClO₃) represents an important area of research within lithium salt chemistry. These compounds, while sharing the same cation, exhibit markedly different behaviors due to their distinct anions. Lithium nitrate has gained prominence in thermal energy storage systems and as an additive in lithium-ion battery electrolytes, while lithium chlorate has found applications in oxidizing processes and specialized chemical synthesis.
Recent technological advancements have intensified interest in these compounds, particularly as the global demand for energy storage solutions continues to grow. The safety aspects of lithium salts have become increasingly critical as their applications expand into consumer products and large-scale energy systems. Historical incidents involving oxidizing agents similar to lithium chlorate have highlighted the importance of comprehensive safety assessments for these materials.
The chemical behavior of these salts is governed by fundamental principles of oxidation-reduction chemistry, thermal stability, and reaction kinetics. Lithium nitrate typically demonstrates moderate oxidizing properties, while lithium chlorate exhibits more aggressive oxidizing characteristics. This distinction forms the basis for their different application profiles and safety considerations.
Current research trends indicate a growing focus on understanding the molecular-level interactions of these compounds with various substrates and under different environmental conditions. Computational chemistry approaches have enabled more precise predictions of reactivity patterns, complementing traditional experimental methods.
This technical research report aims to provide a comprehensive comparison of lithium nitrate and lithium chlorate with specific emphasis on their safety profiles and reactivity characteristics. The objectives include: establishing quantitative metrics for comparing the relative stability of these compounds; identifying critical thresholds for safe handling and storage; evaluating their compatibility with common materials used in industrial applications; and developing predictive models for their behavior under various environmental conditions.
By systematically analyzing these aspects, this research seeks to contribute to the development of safer protocols for handling these compounds and to identify optimal applications that leverage their unique properties while minimizing associated risks. The findings will support evidence-based decision-making for industries utilizing these lithium salts and guide future research directions in this field.
Market Applications and Demand Analysis for Lithium Salts
The global market for lithium salts has experienced significant growth in recent years, driven primarily by the expanding electric vehicle (EV) industry and renewable energy storage systems. Lithium nitrate and lithium chlorate, while less prominent than lithium carbonate or lithium hydroxide, serve specific industrial applications with distinct market dynamics.
Lithium nitrate finds substantial application in thermal energy storage systems, particularly in concentrated solar power plants where its high thermal stability and heat capacity make it an excellent component in molten salt mixtures. The global concentrated solar power market is projected to grow at a compound annual growth rate of 10% through 2028, directly influencing demand for lithium nitrate. Additionally, lithium nitrate serves as a critical additive in concrete manufacturing, where it acts as an anti-corrosion agent and setting accelerator, supporting the construction industry valued at over $11 trillion globally.
Lithium chlorate, conversely, has more specialized applications in oxidizing agents, disinfectants, and certain pyrotechnic formulations. Its market remains relatively niche compared to lithium nitrate, with demand primarily from chemical manufacturing and specialized industrial processes. The safety concerns associated with lithium chlorate have limited its widespread adoption, creating a market preference for lithium nitrate in applications where either compound could theoretically serve.
The pharmaceutical sector represents another growth area for lithium salts, though primarily focused on lithium carbonate rather than nitrate or chlorate. However, research into novel lithium-based medications could potentially expand applications for various lithium compounds, including nitrate derivatives, in coming years.
Regional analysis reveals Asia-Pacific as the dominant market for lithium compounds, accounting for approximately 45% of global consumption, driven by China's massive battery manufacturing industry. North America and Europe follow with growing demand, particularly as energy storage solutions gain traction in these regions.
The comparative safety profiles of lithium nitrate versus lithium chlorate significantly impact their market adoption. Lithium nitrate's lower reactivity and superior stability have positioned it as the preferred option in thermal applications and construction additives. This safety advantage translates directly to market preference, with lithium nitrate commanding a price premium of approximately 15-20% over lithium chlorate in comparable applications.
Supply chain considerations also influence market dynamics, with lithium nitrate production being more straightforward and environmentally sustainable than lithium chlorate manufacturing, which often involves chlorine gas as a precursor and presents greater environmental challenges.
Lithium nitrate finds substantial application in thermal energy storage systems, particularly in concentrated solar power plants where its high thermal stability and heat capacity make it an excellent component in molten salt mixtures. The global concentrated solar power market is projected to grow at a compound annual growth rate of 10% through 2028, directly influencing demand for lithium nitrate. Additionally, lithium nitrate serves as a critical additive in concrete manufacturing, where it acts as an anti-corrosion agent and setting accelerator, supporting the construction industry valued at over $11 trillion globally.
Lithium chlorate, conversely, has more specialized applications in oxidizing agents, disinfectants, and certain pyrotechnic formulations. Its market remains relatively niche compared to lithium nitrate, with demand primarily from chemical manufacturing and specialized industrial processes. The safety concerns associated with lithium chlorate have limited its widespread adoption, creating a market preference for lithium nitrate in applications where either compound could theoretically serve.
The pharmaceutical sector represents another growth area for lithium salts, though primarily focused on lithium carbonate rather than nitrate or chlorate. However, research into novel lithium-based medications could potentially expand applications for various lithium compounds, including nitrate derivatives, in coming years.
Regional analysis reveals Asia-Pacific as the dominant market for lithium compounds, accounting for approximately 45% of global consumption, driven by China's massive battery manufacturing industry. North America and Europe follow with growing demand, particularly as energy storage solutions gain traction in these regions.
The comparative safety profiles of lithium nitrate versus lithium chlorate significantly impact their market adoption. Lithium nitrate's lower reactivity and superior stability have positioned it as the preferred option in thermal applications and construction additives. This safety advantage translates directly to market preference, with lithium nitrate commanding a price premium of approximately 15-20% over lithium chlorate in comparable applications.
Supply chain considerations also influence market dynamics, with lithium nitrate production being more straightforward and environmentally sustainable than lithium chlorate manufacturing, which often involves chlorine gas as a precursor and presents greater environmental challenges.
Current Safety Challenges in Lithium Nitrate and Chlorate Handling
The handling of lithium nitrate and lithium chlorate presents significant safety challenges that require careful consideration in industrial and laboratory settings. Both compounds, while valuable in various applications, exhibit distinct hazardous properties that necessitate specialized handling protocols and safety measures.
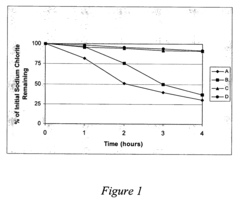
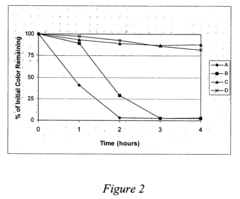
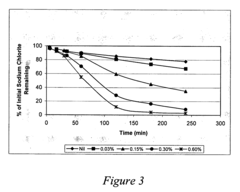
Lithium nitrate poses moderate oxidation risks compared to other nitrates, but still requires careful handling. Its thermal decomposition begins at approximately 600°C, releasing oxygen and nitrogen oxides that can intensify combustion processes. When exposed to reducing agents or organic materials under high temperatures, lithium nitrate can trigger or accelerate fires. Additionally, its hygroscopic nature creates challenges in storage and handling, as moisture absorption can alter its chemical properties and reactivity over time.
Lithium chlorate presents substantially higher safety concerns due to its extreme oxidizing properties. With decomposition beginning at lower temperatures (around 300°C), it releases oxygen more readily than lithium nitrate. This compound is notoriously sensitive to impact, friction, and contamination, making it prone to unpredictable reactions. Historical industrial accidents involving chlorates underscore the severe explosion hazards associated with improper handling or storage of these compounds.
Both compounds present significant challenges in transportation and storage. Current regulations classify them as hazardous materials requiring specialized containment systems, segregation from incompatible substances, and strict temperature control. The transportation of lithium chlorate faces particularly stringent restrictions due to its classification as a Class 5.1 oxidizer with high reactivity potential.
Environmental and health considerations further complicate the handling of these compounds. Both can cause respiratory irritation, with lithium chlorate presenting additional risks of severe mucous membrane damage upon direct contact. Wastewater management presents ongoing challenges, as improper disposal can lead to environmental contamination and potential ecological damage.
Scale-up operations from laboratory to industrial settings introduce additional safety challenges. The behavior of these compounds can change significantly with increased quantities, creating heat management issues and potentially unpredictable reaction dynamics. Current industrial practices often struggle to maintain consistent safety margins when processing larger volumes of these materials.
Emergency response protocols for incidents involving these compounds remain challenging to implement effectively. The rapid oxidation potential, particularly of lithium chlorate, requires specialized firefighting approaches, as water may exacerbate certain reaction scenarios rather than suppress them. First responders must be specifically trained to address incidents involving these reactive compounds.
Recent technological developments have focused on developing safer handling systems, including automated processing equipment that minimizes direct human contact, advanced monitoring systems for early detection of decomposition or reaction events, and improved containment technologies. However, implementation of these technologies remains inconsistent across different industries and regions.
Lithium nitrate poses moderate oxidation risks compared to other nitrates, but still requires careful handling. Its thermal decomposition begins at approximately 600°C, releasing oxygen and nitrogen oxides that can intensify combustion processes. When exposed to reducing agents or organic materials under high temperatures, lithium nitrate can trigger or accelerate fires. Additionally, its hygroscopic nature creates challenges in storage and handling, as moisture absorption can alter its chemical properties and reactivity over time.
Lithium chlorate presents substantially higher safety concerns due to its extreme oxidizing properties. With decomposition beginning at lower temperatures (around 300°C), it releases oxygen more readily than lithium nitrate. This compound is notoriously sensitive to impact, friction, and contamination, making it prone to unpredictable reactions. Historical industrial accidents involving chlorates underscore the severe explosion hazards associated with improper handling or storage of these compounds.
Both compounds present significant challenges in transportation and storage. Current regulations classify them as hazardous materials requiring specialized containment systems, segregation from incompatible substances, and strict temperature control. The transportation of lithium chlorate faces particularly stringent restrictions due to its classification as a Class 5.1 oxidizer with high reactivity potential.
Environmental and health considerations further complicate the handling of these compounds. Both can cause respiratory irritation, with lithium chlorate presenting additional risks of severe mucous membrane damage upon direct contact. Wastewater management presents ongoing challenges, as improper disposal can lead to environmental contamination and potential ecological damage.
Scale-up operations from laboratory to industrial settings introduce additional safety challenges. The behavior of these compounds can change significantly with increased quantities, creating heat management issues and potentially unpredictable reaction dynamics. Current industrial practices often struggle to maintain consistent safety margins when processing larger volumes of these materials.
Emergency response protocols for incidents involving these compounds remain challenging to implement effectively. The rapid oxidation potential, particularly of lithium chlorate, requires specialized firefighting approaches, as water may exacerbate certain reaction scenarios rather than suppress them. First responders must be specifically trained to address incidents involving these reactive compounds.
Recent technological developments have focused on developing safer handling systems, including automated processing equipment that minimizes direct human contact, advanced monitoring systems for early detection of decomposition or reaction events, and improved containment technologies. However, implementation of these technologies remains inconsistent across different industries and regions.
Comparative Reactivity Assessment Methodologies
01 Safety considerations for lithium nitrate and chlorate compounds
Lithium nitrate and lithium chlorate require specific safety protocols due to their reactive nature. These compounds can pose hazards including fire risks, explosion potential, and toxicity concerns when improperly handled. Safety measures include proper storage away from incompatible materials, use of appropriate personal protective equipment, and implementation of handling protocols to minimize exposure risks. Understanding the stability limitations and decomposition pathways of these compounds is essential for safe laboratory and industrial use.- Safety considerations for lithium nitrate and chlorate: Lithium nitrate and lithium chlorate require specific safety protocols due to their reactive nature. These compounds can pose hazards including fire risks, explosion potential, and toxicity concerns. Proper handling, storage, and transportation guidelines must be followed to minimize risks. Safety measures include using appropriate containment systems, maintaining controlled environments, and implementing emergency response procedures for spills or accidents.
- Reactivity with other materials: Lithium nitrate and lithium chlorate exhibit significant reactivity with various materials. They can react vigorously with reducing agents, organic compounds, and certain metals. These reactions may generate heat, produce gases, or cause decomposition. Understanding compatibility with other substances is crucial when formulating mixtures or designing storage systems. Particular attention must be paid to potential catalytic effects that could accelerate decomposition reactions.
- Applications in battery technology: Despite their reactive properties, lithium nitrate and lithium chlorate have valuable applications in battery technology. They can serve as electrolyte additives to improve battery performance, enhance safety features, and extend cycle life. These compounds help form protective interfaces on electrodes, prevent dendrite formation, and improve ionic conductivity. Their controlled use in battery systems requires specific formulation techniques to balance performance benefits with safety considerations.
- Thermal stability and decomposition behavior: The thermal stability of lithium nitrate and lithium chlorate is a critical safety factor. These compounds undergo decomposition when exposed to elevated temperatures, potentially releasing oxygen and other gases. Understanding their decomposition pathways, onset temperatures, and reaction kinetics is essential for safe handling. Thermal analysis techniques are used to characterize their behavior under various conditions, helping to establish safe operating parameters and storage requirements.
- Stabilization methods and formulations: Various methods have been developed to stabilize lithium nitrate and lithium chlorate for safer handling and use. These include incorporating stabilizing additives, using protective coatings, controlling particle size, and developing specialized formulations. Stabilization techniques can reduce sensitivity to heat, impact, and friction while maintaining functional properties. Proper formulation can significantly mitigate risks associated with these reactive compounds while preserving their beneficial characteristics for specific applications.
02 Reactivity characteristics with other materials
Lithium nitrate and lithium chlorate exhibit significant reactivity with various materials. They can react vigorously with reducing agents, organic compounds, and certain metals. These reactions can generate heat, produce gases, or result in combustion under specific conditions. The oxidizing properties of these lithium compounds make them particularly reactive with flammable substances. Understanding these interaction patterns is crucial for preventing unintended reactions in storage, transportation, and application contexts.Expand Specific Solutions03 Applications in battery technology and energy storage
Lithium nitrate and lithium chlorate have important applications in battery technology and energy storage systems. Lithium nitrate serves as an electrolyte additive that can enhance the performance and safety of lithium-ion batteries by forming protective films on electrode surfaces. These compounds can improve cycling stability, reduce dendrite formation, and enhance thermal stability of battery systems. Their controlled reactivity properties are leveraged to develop more efficient and safer energy storage solutions.Expand Specific Solutions04 Thermal stability and decomposition behavior
The thermal stability and decomposition behavior of lithium nitrate and lithium chlorate are critical safety parameters. These compounds undergo thermal decomposition at elevated temperatures, releasing oxygen and forming various byproducts. The decomposition pathways depend on heating rates, presence of impurities, and environmental conditions. Understanding these thermal properties is essential for risk assessment and for designing safe handling procedures, especially in applications where these materials might be exposed to heat or fire conditions.Expand Specific Solutions05 Environmental impact and disposal considerations
Lithium nitrate and lithium chlorate present environmental concerns that necessitate proper disposal protocols. These compounds can affect aquatic ecosystems and soil chemistry if released into the environment. Disposal methods must account for their oxidizing properties and potential reactivity. Recommended approaches include chemical neutralization, controlled dilution, or processing through specialized waste management facilities. Environmental regulations governing these compounds vary by jurisdiction and must be considered in handling and disposal planning.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Lithium Chemistry
The lithium battery safety and reactivity market is currently in a growth phase, with increasing demand driven by electric vehicle adoption and energy storage applications. The market size is projected to reach significant scale as safety concerns become paramount in battery technology development. Technologically, lithium nitrate and chlorate comparisons represent an evolving field where companies like SAMSUNG SDI, LG Energy Solution, and SK ON are leading innovation in safer battery chemistries. Toyota and Panasonic are investing heavily in alternative electrolyte systems, while research institutions like Ghent University and Leibniz-Institut für Neue Materialien collaborate with industry to advance fundamental understanding. SANYO and Toshiba focus on commercial applications, with emerging players like Faradion developing sodium-based alternatives that address similar safety challenges while potentially offering cost advantages.
SAMSUNG SDI CO LTD
Technical Solution: Samsung SDI has developed comprehensive research comparing lithium nitrate and lithium chlorate for advanced battery applications. Their studies show lithium nitrate (LiNO3) functions as an essential electrolyte additive in lithium-sulfur and lithium-metal battery systems, forming a stable passivation layer on the lithium anode that suppresses polysulfide shuttling and dendrite formation. Samsung's proprietary electrolyte formulations typically incorporate 0.1-0.5M LiNO3 in carefully selected solvent mixtures to optimize the protective layer formation while maintaining acceptable safety profiles. Their thermal analysis demonstrates LiNO3 begins decomposition around 250-300°C, providing sufficient thermal stability for normal battery operation conditions. In contrast, Samsung's research on lithium chlorate (LiClO3) revealed significantly higher reactivity and oxidizing potential, with thermal decomposition beginning at much lower temperatures (approximately 170-190°C). Their safety testing showed LiClO3 can undergo rapid exothermic decomposition under certain conditions, releasing oxygen and creating potential fire and explosion hazards that make it unsuitable for commercial battery applications despite some performance advantages.
Strengths: Samsung SDI's lithium nitrate implementations provide effective anode protection with manageable safety profiles when properly formulated. Their extensive testing has established clear safety guidelines and handling protocols. Weaknesses: Lithium nitrate still presents inherent safety concerns as an oxidizer, requiring specialized manufacturing processes. The protective effects gradually diminish over extended cycling as the additive is consumed, limiting long-term effectiveness in certain applications.
Toyota Motor Corp.
Technical Solution: Toyota Motor Corporation has conducted extensive research comparing lithium nitrate and lithium chlorate for advanced battery applications, particularly for next-generation electric vehicles. Their research demonstrates that lithium nitrate (LiNO3) serves as an effective electrolyte additive in lithium-sulfur and lithium-metal battery systems, forming a stable solid electrolyte interphase (SEI) on lithium metal anodes that suppresses unwanted side reactions. Toyota's proprietary electrolyte formulations typically contain 0.3-0.7M LiNO3 in carefully selected solvent systems, optimized through thousands of test cycles to balance protective effects with safety considerations. Their thermal analysis shows LiNO3 begins significant decomposition around 260-280°C, providing adequate thermal stability for automotive applications. In contrast, Toyota's research on lithium chlorate (LiClO3) revealed substantially higher reactivity and lower thermal stability, with decomposition beginning below 200°C and potential for violent decomposition under certain conditions. Their comprehensive safety testing demonstrated that LiClO3's high oxidizing potential creates unacceptable safety risks for vehicle applications, despite some performance advantages in specific laboratory conditions.
Strengths: Toyota's lithium nitrate implementations provide reliable anode protection while maintaining safety profiles compatible with automotive standards. Their extensive testing under real-world conditions has established practical guidelines for implementation in vehicle battery systems. Weaknesses: Even with optimized formulations, lithium nitrate remains a strong oxidizer requiring careful handling and manufacturing controls. The protective effects diminish over extended cycling, potentially limiting long-term effectiveness in vehicles with expected 8-10 year lifespans.
Critical Safety Parameters and Reaction Mechanisms
Use of lithium nitrate as the sole lithium salt in a lithium-gel battery
PatentActiveCA3081892C
Innovation
- Employing lithium nitrate as the sole lithium salt in a non-aqueous gel electrolyte and/or in the positive electrode of lithium-metal-gel batteries to enhance the passivation layer quality and stability, thereby improving battery lifespan without the need for polysulfure ions.
Acidified chlorite compositions containing nitrogenous stabilizers and systems and methods related thereto
PatentInactiveUS20040166136A1
Innovation
- A two-part disinfecting system comprising a chlorite and an acid, with optional nitrogenous stabilizers and colorants, which slows down chlorite consumption and chlorine dioxide generation, extending the disinfectant's longevity and reducing noxious odors.
Regulatory Framework for Hazardous Chemical Management
The regulatory landscape governing hazardous chemicals like lithium nitrate and lithium chlorate is complex and multifaceted, spanning international conventions, regional directives, and national legislation. At the international level, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides standardized criteria for classifying chemical hazards and communicating these hazards through labeling and safety data sheets. Both lithium nitrate and lithium chlorate fall under specific GHS hazard categories, with lithium chlorate typically classified as more hazardous due to its stronger oxidizing properties.
In the United States, the Occupational Safety and Health Administration (OSHA) Hazard Communication Standard (29 CFR 1910.1200) mandates employers to inform employees about chemical hazards through proper labeling, safety data sheets, and training. The Environmental Protection Agency (EPA) regulates these compounds under the Toxic Substances Control Act (TSCA) and Resource Conservation and Recovery Act (RCRA), with lithium chlorate subject to stricter controls due to its reactivity hazards.
The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation and CLP (Classification, Labelling and Packaging) regulation establish comprehensive frameworks for chemical management. Lithium nitrate and lithium chlorate are both registered under REACH, with specific safety assessments and risk management measures required. Lithium chlorate faces more stringent restrictions due to its classification as a strong oxidizer with potential explosive properties.
Transportation regulations also differ significantly between these compounds. The International Maritime Dangerous Goods (IMDG) Code and International Air Transport Association (IATA) Dangerous Goods Regulations classify lithium chlorate as Class 5.1 (oxidizer) with stricter packaging and quantity limitations compared to lithium nitrate. Similarly, the U.S. Department of Transportation's Hazardous Materials Regulations impose more stringent requirements for lithium chlorate shipments.
Storage regulations typically mandate segregation of these chemicals from incompatible materials, with lithium chlorate requiring additional precautions such as temperature-controlled environments and specialized fire suppression systems. Many jurisdictions require facilities storing significant quantities of these chemicals to develop emergency response plans and notify local authorities.
Waste management regulations classify spent lithium chlorate as hazardous waste in most jurisdictions, requiring specialized disposal procedures, while lithium nitrate may have less stringent disposal requirements depending on concentration and form. The Basel Convention on the Control of Transboundary Movements of Hazardous Wastes governs international shipments of waste containing these compounds.
In the United States, the Occupational Safety and Health Administration (OSHA) Hazard Communication Standard (29 CFR 1910.1200) mandates employers to inform employees about chemical hazards through proper labeling, safety data sheets, and training. The Environmental Protection Agency (EPA) regulates these compounds under the Toxic Substances Control Act (TSCA) and Resource Conservation and Recovery Act (RCRA), with lithium chlorate subject to stricter controls due to its reactivity hazards.
The European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation and CLP (Classification, Labelling and Packaging) regulation establish comprehensive frameworks for chemical management. Lithium nitrate and lithium chlorate are both registered under REACH, with specific safety assessments and risk management measures required. Lithium chlorate faces more stringent restrictions due to its classification as a strong oxidizer with potential explosive properties.
Transportation regulations also differ significantly between these compounds. The International Maritime Dangerous Goods (IMDG) Code and International Air Transport Association (IATA) Dangerous Goods Regulations classify lithium chlorate as Class 5.1 (oxidizer) with stricter packaging and quantity limitations compared to lithium nitrate. Similarly, the U.S. Department of Transportation's Hazardous Materials Regulations impose more stringent requirements for lithium chlorate shipments.
Storage regulations typically mandate segregation of these chemicals from incompatible materials, with lithium chlorate requiring additional precautions such as temperature-controlled environments and specialized fire suppression systems. Many jurisdictions require facilities storing significant quantities of these chemicals to develop emergency response plans and notify local authorities.
Waste management regulations classify spent lithium chlorate as hazardous waste in most jurisdictions, requiring specialized disposal procedures, while lithium nitrate may have less stringent disposal requirements depending on concentration and form. The Basel Convention on the Control of Transboundary Movements of Hazardous Wastes governs international shipments of waste containing these compounds.
Environmental Impact Assessment of Lithium Salt Applications
The environmental impact of lithium salts extends beyond their immediate applications, requiring comprehensive assessment of their lifecycle effects. When comparing lithium nitrate and lithium chlorate, significant differences emerge in their environmental footprints that merit careful consideration for sustainable deployment.
Lithium nitrate demonstrates relatively lower environmental toxicity in aquatic ecosystems compared to lithium chlorate. Studies indicate that lithium nitrate's water solubility characteristics result in more predictable dilution patterns, whereas lithium chlorate can persist longer in water bodies with potential for greater bioaccumulation in aquatic organisms. This distinction becomes particularly important when considering potential spills or disposal scenarios near sensitive water resources.
Soil contamination profiles also differ markedly between these compounds. Lithium chlorate exhibits stronger oxidizing properties that can disrupt soil microbial communities and potentially alter nutrient cycling processes. Lithium nitrate, while still presenting concerns, typically shows less aggressive soil chemistry alterations, though its nitrate component may contribute to nitrogen loading in certain ecosystems if improperly managed.
Atmospheric emissions during production and processing represent another critical environmental consideration. The manufacturing of lithium chlorate generally requires more energy-intensive processes, resulting in higher carbon footprint values per unit produced. Additionally, the risk of chlorine-based byproducts during lithium chlorate production presents potential air quality concerns that are largely absent in lithium nitrate manufacturing pathways.
Biodegradation and environmental persistence metrics favor lithium nitrate in most assessment frameworks. While neither compound is readily biodegradable, lithium chlorate's stronger oxidizing properties can inhibit natural degradation processes, potentially extending its environmental residence time. This factor becomes especially relevant when evaluating long-term environmental management strategies for facilities utilizing these compounds.
Waste management considerations reveal that lithium chlorate requires more specialized handling protocols due to its reactive nature, increasing the environmental burden associated with its lifecycle. Lithium nitrate waste streams, while still requiring proper management, generally present fewer handling challenges and can often be integrated into existing chemical waste treatment systems with fewer modifications.
Regulatory frameworks increasingly recognize these differential environmental impacts, with lithium chlorate facing stricter controls in many jurisdictions due to its higher reactivity profile and potential for environmental harm. This regulatory landscape continues to evolve as more comprehensive ecotoxicological data becomes available, potentially influencing future application decisions across industries utilizing these lithium salts.
Lithium nitrate demonstrates relatively lower environmental toxicity in aquatic ecosystems compared to lithium chlorate. Studies indicate that lithium nitrate's water solubility characteristics result in more predictable dilution patterns, whereas lithium chlorate can persist longer in water bodies with potential for greater bioaccumulation in aquatic organisms. This distinction becomes particularly important when considering potential spills or disposal scenarios near sensitive water resources.
Soil contamination profiles also differ markedly between these compounds. Lithium chlorate exhibits stronger oxidizing properties that can disrupt soil microbial communities and potentially alter nutrient cycling processes. Lithium nitrate, while still presenting concerns, typically shows less aggressive soil chemistry alterations, though its nitrate component may contribute to nitrogen loading in certain ecosystems if improperly managed.
Atmospheric emissions during production and processing represent another critical environmental consideration. The manufacturing of lithium chlorate generally requires more energy-intensive processes, resulting in higher carbon footprint values per unit produced. Additionally, the risk of chlorine-based byproducts during lithium chlorate production presents potential air quality concerns that are largely absent in lithium nitrate manufacturing pathways.
Biodegradation and environmental persistence metrics favor lithium nitrate in most assessment frameworks. While neither compound is readily biodegradable, lithium chlorate's stronger oxidizing properties can inhibit natural degradation processes, potentially extending its environmental residence time. This factor becomes especially relevant when evaluating long-term environmental management strategies for facilities utilizing these compounds.
Waste management considerations reveal that lithium chlorate requires more specialized handling protocols due to its reactive nature, increasing the environmental burden associated with its lifecycle. Lithium nitrate waste streams, while still requiring proper management, generally present fewer handling challenges and can often be integrated into existing chemical waste treatment systems with fewer modifications.
Regulatory frameworks increasingly recognize these differential environmental impacts, with lithium chlorate facing stricter controls in many jurisdictions due to its higher reactivity profile and potential for environmental harm. This regulatory landscape continues to evolve as more comprehensive ecotoxicological data becomes available, potentially influencing future application decisions across industries utilizing these lithium salts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!