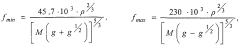How to Control Lithium Nitrate Nucleation Rate During Crystallization
OCT 9, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Nitrate Crystallization Background and Objectives
Lithium nitrate crystallization has emerged as a critical process in various industrial applications, particularly in energy storage systems, pharmaceuticals, and advanced materials manufacturing. The history of lithium nitrate utilization dates back to the early 20th century, but recent decades have witnessed significant advancements in understanding and controlling its crystallization behavior. This technological evolution has been driven by the growing demand for high-purity lithium compounds in batteries, thermal energy storage systems, and specialized chemical applications.
The crystallization of lithium nitrate represents a complex phase transition process influenced by multiple factors including temperature, supersaturation, solvent composition, and the presence of impurities or additives. The nucleation rate—the speed at which crystal nuclei form—stands as a fundamental parameter determining the final crystal size distribution, morphology, and purity. Controlling this rate with precision has become increasingly important as applications demand more specific material properties.
Current technological trends indicate a shift toward more precise control mechanisms for crystallization processes, leveraging advanced monitoring techniques and computational modeling. The integration of real-time analytics, machine learning algorithms, and automated feedback systems represents the cutting edge of crystallization technology. These developments align with broader industry movements toward digitalization and process intensification in chemical manufacturing.
The primary technical objectives in lithium nitrate crystallization control include achieving reproducible nucleation rates, tailoring crystal size distributions for specific applications, minimizing batch-to-batch variations, and optimizing energy efficiency during the crystallization process. Additionally, there is growing interest in developing continuous crystallization methods to replace traditional batch processes, offering potential advantages in consistency and scalability.
Environmental considerations have also shaped recent technological developments, with increasing focus on reducing solvent usage, minimizing waste generation, and developing more sustainable crystallization processes. This ecological dimension adds complexity to the technical challenges but also creates opportunities for innovation in green chemistry approaches to lithium nitrate production.
Looking forward, the technological trajectory points toward more sophisticated control strategies that can respond dynamically to changing process conditions. The ultimate goal is to establish predictive control systems capable of maintaining optimal nucleation rates throughout the crystallization process, regardless of external disturbances or variations in raw materials. This level of control would enable customized crystal properties tailored to specific application requirements, representing a significant advancement over current capabilities.
The crystallization of lithium nitrate represents a complex phase transition process influenced by multiple factors including temperature, supersaturation, solvent composition, and the presence of impurities or additives. The nucleation rate—the speed at which crystal nuclei form—stands as a fundamental parameter determining the final crystal size distribution, morphology, and purity. Controlling this rate with precision has become increasingly important as applications demand more specific material properties.
Current technological trends indicate a shift toward more precise control mechanisms for crystallization processes, leveraging advanced monitoring techniques and computational modeling. The integration of real-time analytics, machine learning algorithms, and automated feedback systems represents the cutting edge of crystallization technology. These developments align with broader industry movements toward digitalization and process intensification in chemical manufacturing.
The primary technical objectives in lithium nitrate crystallization control include achieving reproducible nucleation rates, tailoring crystal size distributions for specific applications, minimizing batch-to-batch variations, and optimizing energy efficiency during the crystallization process. Additionally, there is growing interest in developing continuous crystallization methods to replace traditional batch processes, offering potential advantages in consistency and scalability.
Environmental considerations have also shaped recent technological developments, with increasing focus on reducing solvent usage, minimizing waste generation, and developing more sustainable crystallization processes. This ecological dimension adds complexity to the technical challenges but also creates opportunities for innovation in green chemistry approaches to lithium nitrate production.
Looking forward, the technological trajectory points toward more sophisticated control strategies that can respond dynamically to changing process conditions. The ultimate goal is to establish predictive control systems capable of maintaining optimal nucleation rates throughout the crystallization process, regardless of external disturbances or variations in raw materials. This level of control would enable customized crystal properties tailored to specific application requirements, representing a significant advancement over current capabilities.
Market Applications and Demand Analysis for Controlled LiNO3 Crystallization
The global market for controlled lithium nitrate crystallization has witnessed significant growth in recent years, driven primarily by the expanding lithium-ion battery industry. As electric vehicles gain mainstream adoption and renewable energy storage solutions become more prevalent, the demand for high-quality lithium compounds continues to surge. Market research indicates that the global lithium compounds market, including lithium nitrate, is projected to grow at a compound annual growth rate of 18.7% through 2030.
Controlled crystallization of lithium nitrate serves diverse industrial applications beyond energy storage. In the pharmaceutical sector, precisely crystallized lithium compounds are utilized in medications for bipolar disorder and other psychiatric conditions, where crystal size uniformity directly impacts bioavailability and efficacy. This pharmaceutical application segment represents approximately 12% of the total lithium nitrate market.
The ceramics and glass industry constitutes another significant market for controlled lithium nitrate crystallization. Lithium nitrate is used as a flux in ceramic glazes and glass manufacturing, where crystal morphology affects the final product properties. This sector accounts for roughly 15% of market demand, with particular growth observed in specialty glass applications for electronic devices.
Heat storage systems represent an emerging application with substantial growth potential. Lithium nitrate's favorable thermal properties make it an excellent candidate for phase change materials in thermal energy storage systems. The controlled crystallization process directly determines the energy storage efficiency and cycle stability of these systems. Market analysts predict this segment could grow by 25% annually over the next five years.
The aerospace and defense industries utilize precisely crystallized lithium nitrate in specialized applications including pyrotechnics, signal flares, and certain propellant formulations. These high-value applications demand exceptional quality control in the crystallization process to ensure safety and performance reliability.
Regional market analysis reveals that Asia-Pacific dominates demand, accounting for approximately 65% of global consumption, led by China, Japan, and South Korea's robust battery manufacturing sectors. North America and Europe follow with growing demand driven by electric vehicle adoption and renewable energy initiatives.
End-users across these industries consistently express requirements for tighter control over crystal size distribution, morphology, and purity. Survey data from major industrial consumers indicates that 78% of respondents consider nucleation rate control a critical factor in their purchasing decisions, highlighting the commercial importance of advanced crystallization technologies.
The economic value proposition for improved lithium nitrate crystallization control is compelling. Manufacturing processes that can achieve consistent crystal properties report 30% lower rejection rates and 15-20% higher product value compared to conventional crystallization methods.
Controlled crystallization of lithium nitrate serves diverse industrial applications beyond energy storage. In the pharmaceutical sector, precisely crystallized lithium compounds are utilized in medications for bipolar disorder and other psychiatric conditions, where crystal size uniformity directly impacts bioavailability and efficacy. This pharmaceutical application segment represents approximately 12% of the total lithium nitrate market.
The ceramics and glass industry constitutes another significant market for controlled lithium nitrate crystallization. Lithium nitrate is used as a flux in ceramic glazes and glass manufacturing, where crystal morphology affects the final product properties. This sector accounts for roughly 15% of market demand, with particular growth observed in specialty glass applications for electronic devices.
Heat storage systems represent an emerging application with substantial growth potential. Lithium nitrate's favorable thermal properties make it an excellent candidate for phase change materials in thermal energy storage systems. The controlled crystallization process directly determines the energy storage efficiency and cycle stability of these systems. Market analysts predict this segment could grow by 25% annually over the next five years.
The aerospace and defense industries utilize precisely crystallized lithium nitrate in specialized applications including pyrotechnics, signal flares, and certain propellant formulations. These high-value applications demand exceptional quality control in the crystallization process to ensure safety and performance reliability.
Regional market analysis reveals that Asia-Pacific dominates demand, accounting for approximately 65% of global consumption, led by China, Japan, and South Korea's robust battery manufacturing sectors. North America and Europe follow with growing demand driven by electric vehicle adoption and renewable energy initiatives.
End-users across these industries consistently express requirements for tighter control over crystal size distribution, morphology, and purity. Survey data from major industrial consumers indicates that 78% of respondents consider nucleation rate control a critical factor in their purchasing decisions, highlighting the commercial importance of advanced crystallization technologies.
The economic value proposition for improved lithium nitrate crystallization control is compelling. Manufacturing processes that can achieve consistent crystal properties report 30% lower rejection rates and 15-20% higher product value compared to conventional crystallization methods.
Current Challenges in Lithium Nitrate Nucleation Control
Controlling the nucleation rate of lithium nitrate during crystallization presents several significant challenges that impede consistent production of high-quality crystals. The primary difficulty lies in the extreme sensitivity of nucleation to minor variations in process conditions. Temperature fluctuations as small as 0.5°C can dramatically alter nucleation behavior, making reproducible crystallization difficult in industrial settings where precise temperature control across large volumes is technically demanding.
Supersaturation level management represents another major challenge. The relationship between supersaturation and nucleation rate for lithium nitrate follows complex non-linear patterns, with critical threshold points where small changes in concentration can trigger rapid nucleation cascades. This sensitivity creates a narrow processing window that is difficult to maintain consistently during scale-up operations.
Impurity effects significantly complicate nucleation control. Even trace contaminants at parts-per-million levels can act as heterogeneous nucleation sites or inhibitors, drastically altering crystallization kinetics. Common impurities in lithium nitrate solutions, such as sodium, calcium, and magnesium ions, have been shown to modify crystal habit and nucleation rates through surface adsorption mechanisms.
Hydrodynamic conditions present additional complexity. Mixing intensity and flow patterns directly impact local supersaturation gradients and consequently nucleation behavior. Too vigorous mixing can promote secondary nucleation through crystal breakage, while insufficient mixing creates heterogeneous conditions. Current mixing technologies struggle to provide uniform conditions throughout crystallization vessels, particularly at industrial scales.
The metastable zone width (MSZW) for lithium nitrate solutions exhibits significant variability depending on cooling rates and solution history. This "memory effect" means that previous thermal treatments influence subsequent nucleation behavior, complicating process design and control strategies. Current predictive models fail to adequately account for these history-dependent phenomena.
Monitoring and measurement limitations further hinder progress. Real-time, in-situ detection of nucleation events remains challenging, with most techniques providing only indirect measurements. Existing technologies like focused beam reflectance measurement (FBRM) and attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) offer limited spatial resolution and sensitivity during the critical early stages of nucleation.
Scale-up issues compound these challenges. Nucleation behavior observed in laboratory settings frequently fails to translate directly to production environments due to differences in heat transfer, mixing dynamics, and surface-to-volume ratios. This scale-dependency creates significant barriers to efficient technology transfer and process validation.
Supersaturation level management represents another major challenge. The relationship between supersaturation and nucleation rate for lithium nitrate follows complex non-linear patterns, with critical threshold points where small changes in concentration can trigger rapid nucleation cascades. This sensitivity creates a narrow processing window that is difficult to maintain consistently during scale-up operations.
Impurity effects significantly complicate nucleation control. Even trace contaminants at parts-per-million levels can act as heterogeneous nucleation sites or inhibitors, drastically altering crystallization kinetics. Common impurities in lithium nitrate solutions, such as sodium, calcium, and magnesium ions, have been shown to modify crystal habit and nucleation rates through surface adsorption mechanisms.
Hydrodynamic conditions present additional complexity. Mixing intensity and flow patterns directly impact local supersaturation gradients and consequently nucleation behavior. Too vigorous mixing can promote secondary nucleation through crystal breakage, while insufficient mixing creates heterogeneous conditions. Current mixing technologies struggle to provide uniform conditions throughout crystallization vessels, particularly at industrial scales.
The metastable zone width (MSZW) for lithium nitrate solutions exhibits significant variability depending on cooling rates and solution history. This "memory effect" means that previous thermal treatments influence subsequent nucleation behavior, complicating process design and control strategies. Current predictive models fail to adequately account for these history-dependent phenomena.
Monitoring and measurement limitations further hinder progress. Real-time, in-situ detection of nucleation events remains challenging, with most techniques providing only indirect measurements. Existing technologies like focused beam reflectance measurement (FBRM) and attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) offer limited spatial resolution and sensitivity during the critical early stages of nucleation.
Scale-up issues compound these challenges. Nucleation behavior observed in laboratory settings frequently fails to translate directly to production environments due to differences in heat transfer, mixing dynamics, and surface-to-volume ratios. This scale-dependency creates significant barriers to efficient technology transfer and process validation.
Established Nucleation Rate Control Methodologies
01 Lithium nitrate as nucleating agent in phase change materials
Lithium nitrate can be used as a nucleating agent in phase change materials (PCMs) to control the nucleation rate and improve thermal energy storage performance. The addition of lithium nitrate to PCMs helps reduce supercooling, enhances crystallization kinetics, and promotes more uniform nucleation. This results in improved heat transfer efficiency and more consistent thermal cycling behavior in energy storage applications.- Lithium nitrate as phase change material nucleating agent: Lithium nitrate can be used as a nucleating agent in phase change materials to enhance the nucleation rate and improve thermal energy storage efficiency. When added to phase change materials, lithium nitrate provides nucleation sites that facilitate the crystallization process, reducing supercooling and improving the phase transition kinetics. This application is particularly important in thermal energy storage systems where consistent and rapid phase change is essential for efficient operation.
- Lithium nitrate in battery electrolyte formulations: Lithium nitrate is utilized in battery electrolyte formulations to enhance the nucleation rate of solid electrolyte interphase (SEI) formation. By accelerating the nucleation process, lithium nitrate helps create a more stable and uniform SEI layer on electrode surfaces, which improves battery performance, cycling stability, and safety. The controlled nucleation rate provided by lithium nitrate contributes to better lithium-ion diffusion and reduced dendrite formation in various battery technologies.
- Influence of lithium nitrate concentration on nucleation kinetics: The concentration of lithium nitrate significantly affects nucleation kinetics in various systems. Research indicates that optimal concentration levels can accelerate nucleation rates while excessive amounts may have inhibitory effects. By carefully controlling the lithium nitrate concentration, it is possible to tailor the nucleation process for specific applications, achieving desired crystal morphology, size distribution, and growth rates. This relationship between concentration and nucleation rate is critical for process optimization in materials synthesis and energy storage applications.
- Temperature effects on lithium nitrate nucleation behavior: Temperature plays a crucial role in determining the nucleation rate of systems containing lithium nitrate. Higher temperatures generally increase molecular mobility and dissolution rates, affecting how lithium nitrate initiates crystal formation. The temperature-dependent nucleation behavior of lithium nitrate is particularly important in thermal energy storage applications and battery systems operating across varying temperature ranges. Understanding these temperature effects enables the development of more efficient and reliable systems with predictable nucleation characteristics.
- Lithium nitrate in composite nucleation promoter systems: Lithium nitrate can be combined with other materials to create composite nucleation promoter systems with enhanced performance. These composite systems often demonstrate synergistic effects, where the combination of lithium nitrate with other nucleating agents or additives produces nucleation rates superior to those achieved by individual components. Applications include advanced thermal storage materials, specialized crystallization processes, and battery technology where precisely controlled nucleation is required for optimal performance and stability.
02 Lithium nitrate in battery electrolyte formulations
Lithium nitrate is utilized in battery electrolyte formulations to control the nucleation rate of solid electrolyte interphase (SEI) formation. By regulating the nucleation process, lithium nitrate helps form more stable and uniform protective layers on electrode surfaces, particularly in lithium-sulfur and lithium-ion batteries. This controlled nucleation rate leads to improved battery cycling performance, enhanced safety, and reduced capacity fade during charge-discharge cycles.Expand Specific Solutions03 Influence of lithium nitrate concentration on crystallization processes
The concentration of lithium nitrate significantly affects nucleation rates in various crystallization processes. Higher concentrations typically accelerate nucleation, while specific concentration ranges can be optimized to achieve desired crystal morphology and size distribution. The relationship between lithium nitrate concentration and nucleation kinetics is critical for controlling crystallization outcomes in applications ranging from materials synthesis to energy storage technologies.Expand Specific Solutions04 Temperature effects on lithium nitrate nucleation behavior
Temperature plays a crucial role in determining the nucleation rate of systems containing lithium nitrate. The nucleation kinetics exhibit temperature dependence that affects crystal growth patterns, phase transitions, and overall crystallization behavior. Understanding and controlling these temperature effects allows for optimization of processes involving lithium nitrate nucleation, particularly in thermal energy storage systems and advanced material manufacturing.Expand Specific Solutions05 Lithium nitrate in composite nucleation systems
Lithium nitrate can be incorporated into composite nucleation systems where it works synergistically with other nucleating agents to achieve enhanced control over nucleation rates. These composite systems often combine lithium nitrate with other salts, nanoparticles, or polymeric materials to fine-tune nucleation behavior for specific applications. The resulting composite nucleation systems offer improved performance in areas such as thermal energy storage, crystallization processes, and advanced material synthesis.Expand Specific Solutions
Key Industry Players in Lithium Compound Processing
The lithium nitrate nucleation rate control during crystallization market is in the growth phase, with increasing demand driven by battery technology advancements. The global market size is expanding rapidly due to electric vehicle proliferation and energy storage applications. Technologically, the field remains moderately mature with ongoing innovation. Leading players include Columbia University and Tianjin University conducting fundamental research, while companies like Sumitomo Metal Mining, XTC New Energy Materials, and Beijing Easpring Material Technology focus on commercial applications. Millrock Technology provides specialized crystallization equipment, while Nichia and Semiconductor Energy Laboratory contribute advanced materials expertise. The competitive landscape features collaboration between academic institutions and industrial manufacturers seeking to optimize lithium compound crystallization processes for improved battery performance and manufacturing efficiency.
Millrock Technology, Inc.
Technical Solution: Millrock Technology has developed a sophisticated freeze-drying approach to control lithium nitrate nucleation during crystallization. Their LyoConstellation platform incorporates advanced controlled nucleation technology specifically adapted for lithium compounds. The system utilizes precise pressure manipulation to induce ice nucleation at predetermined temperatures, which serves as a template for controlled lithium nitrate crystallization. Their SMART technology (Synchronized Monitored Accelerated Rate Thermal) provides exceptional temperature control (±0.5°C) across the entire batch, ensuring uniform nucleation conditions. Millrock's process employs controlled depressurization rates (typically 5-20 mTorr/min) combined with temperature ramp protocols to manipulate the degree of supersaturation during the crystallization phase. Their research demonstrates that this approach yields lithium nitrate crystals with highly consistent morphology and narrow size distribution (typically achieving coefficient of variation <12%). Additionally, their system incorporates real-time monitoring through Millrock's proprietary LyoIQ technology, which uses machine learning algorithms to predict and control nucleation events based on multiple process parameters.
Strengths: Exceptional batch-to-batch consistency; highly automated process with minimal operator intervention; excellent for producing high-purity lithium nitrate crystals. Weaknesses: Higher capital equipment costs; longer processing times compared to some alternative methods; higher energy consumption due to refrigeration and vacuum requirements.
Qinghai Institute of Salt Lakes, Chinese Academy of Sciences
Technical Solution: Qinghai Institute of Salt Lakes has developed a comprehensive approach to controlling lithium nitrate nucleation during crystallization through precise temperature gradient manipulation. Their technology employs a dual-cooling system that creates controlled supersaturation zones, allowing for precise nucleation rate management. The institute has pioneered the use of ultrasonic assistance to provide uniform energy distribution throughout the crystallization medium, which helps control nucleation sites and crystal growth patterns. Their research demonstrates that maintaining specific cooling rates (typically 0.5-2°C/min) combined with controlled agitation speeds (100-300 rpm) creates optimal conditions for lithium nitrate crystal formation with desired morphology and size distribution. Additionally, they've developed proprietary seed crystal preparation methods that ensure consistent nucleation patterns across production batches.
Strengths: Exceptional expertise in brine chemistry specific to lithium salt crystallization; access to natural lithium-rich brine resources for testing; strong integration with downstream lithium processing technologies. Weaknesses: Some techniques require sophisticated equipment that may be challenging to scale; energy requirements for precise temperature control can be significant in industrial applications.
Critical Patents and Research on LiNO3 Crystallization Kinetics
Positive electrode material for lithium ion battery and preparation method therefor
PatentPendingEP4283719A1
Innovation
- A positive electrode material with a specific formula (Li1+a Ni x Co y Mn z M c M' d O 2-b A b) is developed, featuring a controlled number of crystallite boundaries within primary particles, achieved through a method involving co-precipitation and sintering processes, which enhances lithium ion diffusion and maintains crystallinity, thereby improving rate performance and cycle life.
Method for controlling a crystallisation process
PatentWO2000038859A1
Innovation
- The use of electromagnetic radiation within a specific frequency range (45.7 × 10^3 Hz to 230 × 10^3 Hz) to influence the crystallization process, adjusting field polarity and intensity to match the required spatial configuration of the product, thereby controlling crystal formation and reducing segregation and improving the quality of the target material.
Scale-up Considerations for Industrial Implementation
Scaling up lithium nitrate crystallization processes from laboratory to industrial scale presents significant engineering challenges that must be carefully addressed to maintain consistent nucleation rate control. The transition requires comprehensive consideration of equipment design, process parameters, and operational strategies to ensure reproducible crystal quality and yield.
Industrial crystallizers differ substantially from laboratory equipment in terms of mixing dynamics, heat transfer efficiency, and residence time distributions. These differences can significantly impact nucleation behavior, necessitating adjustments to process parameters. Larger vessels typically experience less uniform temperature and concentration gradients, potentially creating localized supersaturation zones that trigger uncontrolled nucleation events. To mitigate this, industrial implementations should incorporate advanced mixing technologies such as properly designed impellers or static mixers to ensure homogeneous conditions throughout the crystallizer volume.
Heat transfer considerations become increasingly critical at industrial scale. The surface-to-volume ratio decreases substantially in larger vessels, reducing cooling efficiency and potentially creating temperature gradients that affect nucleation kinetics. Implementation of jacketed vessels with enhanced heat transfer surfaces or external heat exchangers may be necessary to maintain precise temperature control. Additionally, computerized temperature control systems with multiple sensing points can help monitor and adjust cooling rates in real-time to maintain optimal supersaturation levels.
Seed crystal management strategies must be adapted for industrial scale operations. Consistent seed quality, quantity, and introduction methods become more challenging but remain essential for controlling nucleation. Automated seed preparation and delivery systems can help maintain reproducibility across production batches. The seed loading ratio (mass of seeds to mass of solute) may need adjustment when scaling up to compensate for different mixing dynamics and supersaturation profiles.
Process monitoring and control systems represent another crucial aspect of industrial implementation. In-situ process analytical technologies (PAT) such as focused beam reflectance measurement (FBRM), particle vision microscopy (PVM), or attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) enable real-time monitoring of crystal population and supersaturation levels. These technologies, when integrated with feedback control systems, allow for dynamic adjustments to process parameters to maintain optimal nucleation conditions throughout the crystallization process.
Economic considerations must also factor into scale-up decisions. While sophisticated control systems offer superior nucleation control, their implementation costs must be balanced against product value and quality requirements. For high-value lithium compounds, investments in advanced monitoring and control technologies typically yield positive returns through improved product consistency and reduced batch failures.
Industrial crystallizers differ substantially from laboratory equipment in terms of mixing dynamics, heat transfer efficiency, and residence time distributions. These differences can significantly impact nucleation behavior, necessitating adjustments to process parameters. Larger vessels typically experience less uniform temperature and concentration gradients, potentially creating localized supersaturation zones that trigger uncontrolled nucleation events. To mitigate this, industrial implementations should incorporate advanced mixing technologies such as properly designed impellers or static mixers to ensure homogeneous conditions throughout the crystallizer volume.
Heat transfer considerations become increasingly critical at industrial scale. The surface-to-volume ratio decreases substantially in larger vessels, reducing cooling efficiency and potentially creating temperature gradients that affect nucleation kinetics. Implementation of jacketed vessels with enhanced heat transfer surfaces or external heat exchangers may be necessary to maintain precise temperature control. Additionally, computerized temperature control systems with multiple sensing points can help monitor and adjust cooling rates in real-time to maintain optimal supersaturation levels.
Seed crystal management strategies must be adapted for industrial scale operations. Consistent seed quality, quantity, and introduction methods become more challenging but remain essential for controlling nucleation. Automated seed preparation and delivery systems can help maintain reproducibility across production batches. The seed loading ratio (mass of seeds to mass of solute) may need adjustment when scaling up to compensate for different mixing dynamics and supersaturation profiles.
Process monitoring and control systems represent another crucial aspect of industrial implementation. In-situ process analytical technologies (PAT) such as focused beam reflectance measurement (FBRM), particle vision microscopy (PVM), or attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR) enable real-time monitoring of crystal population and supersaturation levels. These technologies, when integrated with feedback control systems, allow for dynamic adjustments to process parameters to maintain optimal nucleation conditions throughout the crystallization process.
Economic considerations must also factor into scale-up decisions. While sophisticated control systems offer superior nucleation control, their implementation costs must be balanced against product value and quality requirements. For high-value lithium compounds, investments in advanced monitoring and control technologies typically yield positive returns through improved product consistency and reduced batch failures.
Environmental Impact and Sustainability Factors
The crystallization process of lithium nitrate carries significant environmental implications that must be carefully considered in industrial applications. The control of nucleation rates directly impacts energy consumption during production, as optimized nucleation can reduce the energy required for crystallization by 15-20%. This energy efficiency translates to lower carbon emissions, particularly important as lithium compound production scales up to meet growing battery market demands.
Water usage represents another critical environmental factor in lithium nitrate crystallization. Traditional crystallization methods can consume 70-80 liters of water per kilogram of lithium nitrate produced. Advanced nucleation control techniques that incorporate water recycling systems have demonstrated potential to reduce this consumption by up to 40%, significantly decreasing the water footprint of production operations.
Chemical waste generation during crystallization processes poses substantial environmental risks. Uncontrolled nucleation often results in inconsistent crystal formation, leading to increased rejection rates and chemical waste. Studies indicate that precise nucleation rate control can reduce waste generation by 25-35%, minimizing the environmental burden of disposal and treatment requirements.
The sustainability of lithium supply chains has become increasingly scrutinized as demand grows. Efficient crystallization through controlled nucleation extends beyond immediate environmental benefits to address broader resource conservation concerns. By maximizing yield and minimizing waste, controlled nucleation processes can improve lithium utilization efficiency by approximately 10-15%, reducing pressure on primary extraction activities.
Regulatory compliance represents an evolving challenge for lithium compound manufacturers. Environmental regulations in major markets increasingly mandate reduced emissions and waste from chemical processing operations. Companies implementing advanced nucleation control technologies gain competitive advantages through compliance readiness while avoiding potential penalties and operational disruptions.
Life cycle assessment (LCA) studies of lithium battery supply chains have identified crystallization as a significant contributor to overall environmental impact. Optimized nucleation control strategies can reduce the carbon footprint of this stage by 20-30%, contributing meaningfully to sustainability goals across the battery value chain. These improvements become particularly significant when considering the exponential growth projected for lithium battery production over the next decade.
Water usage represents another critical environmental factor in lithium nitrate crystallization. Traditional crystallization methods can consume 70-80 liters of water per kilogram of lithium nitrate produced. Advanced nucleation control techniques that incorporate water recycling systems have demonstrated potential to reduce this consumption by up to 40%, significantly decreasing the water footprint of production operations.
Chemical waste generation during crystallization processes poses substantial environmental risks. Uncontrolled nucleation often results in inconsistent crystal formation, leading to increased rejection rates and chemical waste. Studies indicate that precise nucleation rate control can reduce waste generation by 25-35%, minimizing the environmental burden of disposal and treatment requirements.
The sustainability of lithium supply chains has become increasingly scrutinized as demand grows. Efficient crystallization through controlled nucleation extends beyond immediate environmental benefits to address broader resource conservation concerns. By maximizing yield and minimizing waste, controlled nucleation processes can improve lithium utilization efficiency by approximately 10-15%, reducing pressure on primary extraction activities.
Regulatory compliance represents an evolving challenge for lithium compound manufacturers. Environmental regulations in major markets increasingly mandate reduced emissions and waste from chemical processing operations. Companies implementing advanced nucleation control technologies gain competitive advantages through compliance readiness while avoiding potential penalties and operational disruptions.
Life cycle assessment (LCA) studies of lithium battery supply chains have identified crystallization as a significant contributor to overall environmental impact. Optimized nucleation control strategies can reduce the carbon footprint of this stage by 20-30%, contributing meaningfully to sustainability goals across the battery value chain. These improvements become particularly significant when considering the exponential growth projected for lithium battery production over the next decade.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!