Quantify Lithium Nitrate’s Electrical Conductivity Variations with Temperature
OCT 9, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Nitrate Conductivity Research Background and Objectives
Lithium nitrate (LiNO3) has emerged as a critical material in modern energy storage systems, particularly in advanced battery technologies and thermal energy storage applications. The study of its electrical conductivity variations with temperature represents a fundamental research area with significant implications for both theoretical understanding and practical applications in electrochemistry and materials science.
The historical development of lithium nitrate research dates back to the early 20th century, with initial investigations focusing on its basic physicochemical properties. However, the systematic study of its electrical conductivity as a function of temperature gained momentum in the 1970s and 1980s, coinciding with the growing interest in lithium-based battery technologies. This research trajectory has accelerated significantly in the past decade due to the global push for renewable energy solutions and efficient energy storage systems.
Current technological trends indicate a growing emphasis on understanding the precise mechanisms of ion transport in molten salts and solid-state electrolytes containing lithium nitrate. The relationship between temperature and electrical conductivity in these systems is particularly crucial as it directly impacts the performance, efficiency, and safety of various energy storage devices.
The primary objective of this research is to establish a comprehensive quantitative model that accurately describes the electrical conductivity behavior of lithium nitrate across a wide temperature range. This includes mapping conductivity variations in different phases (solid, liquid) and under various environmental conditions that might influence its performance in real-world applications.
Secondary objectives include identifying the fundamental physical and chemical mechanisms that govern these conductivity variations, determining the activation energy for ionic conduction, and establishing the relationship between structural changes and conductivity behavior at different temperature points.
From an applied perspective, this research aims to optimize lithium nitrate's performance in thermal energy storage systems, enhance its functionality in battery electrolytes, and explore novel applications in other fields such as sensors and electrochemical devices. Understanding these temperature-dependent conductivity relationships will enable more precise engineering of materials and systems that incorporate lithium nitrate.
The significance of this research extends beyond academic interest, as it addresses critical challenges in energy storage technology development, including improving energy density, extending cycle life, enhancing safety, and reducing costs of lithium-based energy systems. These improvements align with global sustainability goals and the transition toward renewable energy infrastructure.
The historical development of lithium nitrate research dates back to the early 20th century, with initial investigations focusing on its basic physicochemical properties. However, the systematic study of its electrical conductivity as a function of temperature gained momentum in the 1970s and 1980s, coinciding with the growing interest in lithium-based battery technologies. This research trajectory has accelerated significantly in the past decade due to the global push for renewable energy solutions and efficient energy storage systems.
Current technological trends indicate a growing emphasis on understanding the precise mechanisms of ion transport in molten salts and solid-state electrolytes containing lithium nitrate. The relationship between temperature and electrical conductivity in these systems is particularly crucial as it directly impacts the performance, efficiency, and safety of various energy storage devices.
The primary objective of this research is to establish a comprehensive quantitative model that accurately describes the electrical conductivity behavior of lithium nitrate across a wide temperature range. This includes mapping conductivity variations in different phases (solid, liquid) and under various environmental conditions that might influence its performance in real-world applications.
Secondary objectives include identifying the fundamental physical and chemical mechanisms that govern these conductivity variations, determining the activation energy for ionic conduction, and establishing the relationship between structural changes and conductivity behavior at different temperature points.
From an applied perspective, this research aims to optimize lithium nitrate's performance in thermal energy storage systems, enhance its functionality in battery electrolytes, and explore novel applications in other fields such as sensors and electrochemical devices. Understanding these temperature-dependent conductivity relationships will enable more precise engineering of materials and systems that incorporate lithium nitrate.
The significance of this research extends beyond academic interest, as it addresses critical challenges in energy storage technology development, including improving energy density, extending cycle life, enhancing safety, and reducing costs of lithium-based energy systems. These improvements align with global sustainability goals and the transition toward renewable energy infrastructure.
Market Applications and Demand Analysis for Lithium Nitrate
The global market for lithium nitrate is experiencing significant growth driven by its diverse applications across multiple industries. The compound's unique electrical conductivity properties, particularly its variation with temperature, make it invaluable in several high-tech and emerging sectors. Current market estimates value the global lithium nitrate market at approximately 320 million USD, with projections indicating a compound annual growth rate of 5.7% through 2028.
Energy storage systems represent the largest application segment for lithium nitrate, accounting for nearly 40% of total demand. The compound's role as an electrolyte additive in lithium-ion batteries has become increasingly important as electric vehicle adoption accelerates worldwide. Automotive manufacturers and battery producers are particularly interested in lithium nitrate's ability to form stable solid electrolyte interphase (SEI) layers, which directly impacts battery performance and longevity.
The thermal energy storage sector presents another substantial market for lithium nitrate, where its temperature-dependent conductivity properties are leveraged in concentrated solar power (CSP) plants. This application has seen 15% year-over-year growth in regions with high solar potential, including Spain, the United States, and China. The compound's high thermal stability and excellent heat transfer characteristics make it an ideal component in molten salt mixtures used for energy storage.
Industrial applications constitute approximately 25% of the market, with lithium nitrate being utilized in heat treatment processes, ceramic production, and as a flux in metallurgical operations. The compound's predictable conductivity behavior across temperature ranges enables precise control in these manufacturing processes, driving demand from industrial customers seeking efficiency improvements.
Emerging applications in solid-state batteries and advanced electronics are creating new demand vectors. Research institutions and technology companies are investing heavily in understanding lithium nitrate's electrical conductivity variations to develop next-generation energy storage solutions. This R&D-driven demand is expected to grow at twice the rate of traditional applications over the next five years.
Regionally, Asia-Pacific dominates consumption with 45% market share, followed by North America and Europe at 28% and 20% respectively. China remains the largest single market due to its massive battery manufacturing industry, while South Korea and Japan show strong demand from their electronics sectors.
Supply chain considerations are increasingly influencing market dynamics, with lithium availability and pricing volatility affecting downstream lithium nitrate production. This has prompted some end-users to secure long-term supply agreements and explore alternative formulations that maintain similar electrical conductivity profiles across temperature ranges.
Energy storage systems represent the largest application segment for lithium nitrate, accounting for nearly 40% of total demand. The compound's role as an electrolyte additive in lithium-ion batteries has become increasingly important as electric vehicle adoption accelerates worldwide. Automotive manufacturers and battery producers are particularly interested in lithium nitrate's ability to form stable solid electrolyte interphase (SEI) layers, which directly impacts battery performance and longevity.
The thermal energy storage sector presents another substantial market for lithium nitrate, where its temperature-dependent conductivity properties are leveraged in concentrated solar power (CSP) plants. This application has seen 15% year-over-year growth in regions with high solar potential, including Spain, the United States, and China. The compound's high thermal stability and excellent heat transfer characteristics make it an ideal component in molten salt mixtures used for energy storage.
Industrial applications constitute approximately 25% of the market, with lithium nitrate being utilized in heat treatment processes, ceramic production, and as a flux in metallurgical operations. The compound's predictable conductivity behavior across temperature ranges enables precise control in these manufacturing processes, driving demand from industrial customers seeking efficiency improvements.
Emerging applications in solid-state batteries and advanced electronics are creating new demand vectors. Research institutions and technology companies are investing heavily in understanding lithium nitrate's electrical conductivity variations to develop next-generation energy storage solutions. This R&D-driven demand is expected to grow at twice the rate of traditional applications over the next five years.
Regionally, Asia-Pacific dominates consumption with 45% market share, followed by North America and Europe at 28% and 20% respectively. China remains the largest single market due to its massive battery manufacturing industry, while South Korea and Japan show strong demand from their electronics sectors.
Supply chain considerations are increasingly influencing market dynamics, with lithium availability and pricing volatility affecting downstream lithium nitrate production. This has prompted some end-users to secure long-term supply agreements and explore alternative formulations that maintain similar electrical conductivity profiles across temperature ranges.
Current Measurement Techniques and Technical Challenges
The quantification of lithium nitrate's electrical conductivity variations with temperature presents significant technical challenges due to the compound's unique properties. Current measurement techniques primarily employ four methodologies: impedance spectroscopy, direct current (DC) conductivity measurements, alternating current (AC) conductivity measurements, and the four-probe method. Each approach offers distinct advantages while facing specific limitations.
Impedance spectroscopy represents the gold standard for ionic conductivity measurements, allowing researchers to distinguish between bulk, grain boundary, and electrode contributions to the overall conductivity. However, this technique requires sophisticated equipment and complex data interpretation using equivalent circuit models, which can introduce subjective elements into the analysis process.
DC conductivity measurements provide straightforward implementation but suffer from polarization effects at electrode interfaces when measuring ionic conductors like lithium nitrate. This polarization can significantly distort readings, particularly at lower temperatures where ion mobility is reduced, leading to underestimated conductivity values.
AC conductivity measurements mitigate some polarization issues by applying alternating current at various frequencies. Nevertheless, determining the optimal frequency range for accurate lithium nitrate conductivity measurement remains challenging, as different temperature regimes may require different frequency windows for optimal results.
The four-probe method effectively eliminates contact resistance problems by separating current-carrying and voltage-sensing electrodes. While theoretically advantageous, practical implementation with molten salts like lithium nitrate at elevated temperatures introduces complications related to electrode degradation and containment.
A significant technical challenge across all methodologies involves maintaining precise temperature control throughout measurements. Lithium nitrate exhibits phase transitions and thermal expansion behaviors that can introduce discontinuities in conductivity data. Temperature gradients within samples can lead to inconsistent measurements, particularly when working near the melting point (255°C).
Sample preparation introduces additional complexities, as lithium nitrate is hygroscopic and can absorb atmospheric moisture, altering its composition and electrical properties. Ensuring sample purity and consistent preparation protocols remains difficult but essential for reproducible results.
Electrode material selection presents another challenge, as electrodes must remain chemically stable in contact with lithium nitrate across wide temperature ranges while maintaining good electrical contact. Platinum electrodes are commonly used but can catalyze unwanted reactions at higher temperatures.
Data interpretation challenges arise from the need to separate various contributing factors to the measured conductivity, including electronic conductivity, ionic conductivity, and interfacial effects. Developing comprehensive models that accurately account for these factors across temperature ranges remains an ongoing research challenge.
Standardization of measurement protocols represents perhaps the most pressing need in the field, as variations in experimental setups and procedures make direct comparison between different research groups problematic, hampering collective progress toward precise quantification of lithium nitrate's temperature-dependent electrical conductivity.
Impedance spectroscopy represents the gold standard for ionic conductivity measurements, allowing researchers to distinguish between bulk, grain boundary, and electrode contributions to the overall conductivity. However, this technique requires sophisticated equipment and complex data interpretation using equivalent circuit models, which can introduce subjective elements into the analysis process.
DC conductivity measurements provide straightforward implementation but suffer from polarization effects at electrode interfaces when measuring ionic conductors like lithium nitrate. This polarization can significantly distort readings, particularly at lower temperatures where ion mobility is reduced, leading to underestimated conductivity values.
AC conductivity measurements mitigate some polarization issues by applying alternating current at various frequencies. Nevertheless, determining the optimal frequency range for accurate lithium nitrate conductivity measurement remains challenging, as different temperature regimes may require different frequency windows for optimal results.
The four-probe method effectively eliminates contact resistance problems by separating current-carrying and voltage-sensing electrodes. While theoretically advantageous, practical implementation with molten salts like lithium nitrate at elevated temperatures introduces complications related to electrode degradation and containment.
A significant technical challenge across all methodologies involves maintaining precise temperature control throughout measurements. Lithium nitrate exhibits phase transitions and thermal expansion behaviors that can introduce discontinuities in conductivity data. Temperature gradients within samples can lead to inconsistent measurements, particularly when working near the melting point (255°C).
Sample preparation introduces additional complexities, as lithium nitrate is hygroscopic and can absorb atmospheric moisture, altering its composition and electrical properties. Ensuring sample purity and consistent preparation protocols remains difficult but essential for reproducible results.
Electrode material selection presents another challenge, as electrodes must remain chemically stable in contact with lithium nitrate across wide temperature ranges while maintaining good electrical contact. Platinum electrodes are commonly used but can catalyze unwanted reactions at higher temperatures.
Data interpretation challenges arise from the need to separate various contributing factors to the measured conductivity, including electronic conductivity, ionic conductivity, and interfacial effects. Developing comprehensive models that accurately account for these factors across temperature ranges remains an ongoing research challenge.
Standardization of measurement protocols represents perhaps the most pressing need in the field, as variations in experimental setups and procedures make direct comparison between different research groups problematic, hampering collective progress toward precise quantification of lithium nitrate's temperature-dependent electrical conductivity.
Established Methodologies for Temperature-Dependent Conductivity Analysis
01 Lithium nitrate as ionic conductor in electrolytes
Lithium nitrate is used as an ionic conductor in various electrolyte formulations to enhance electrical conductivity. When dissolved in appropriate solvents, lithium nitrate dissociates into lithium ions and nitrate anions, facilitating ion transport. This property makes it valuable in battery applications where high ionic conductivity is essential for efficient energy storage and delivery. The addition of lithium nitrate to electrolyte solutions can significantly improve the overall conductivity performance of electrochemical systems.- Lithium nitrate as electrolyte additive in batteries: Lithium nitrate is used as an electrolyte additive in lithium-based batteries to enhance electrical conductivity and improve battery performance. It helps form a stable solid electrolyte interphase (SEI) layer on electrodes, which prevents unwanted side reactions and improves the cycling stability of batteries. The addition of lithium nitrate to electrolyte solutions can also mitigate the shuttle effect in lithium-sulfur batteries and enhance overall ionic conductivity.
- Lithium nitrate in thermal energy storage systems: Lithium nitrate is utilized in thermal energy storage systems due to its high electrical conductivity properties when combined with other salts. These molten salt mixtures can efficiently store and transfer thermal energy while maintaining good electrical conductivity at elevated temperatures. The addition of lithium nitrate to salt mixtures can lower the melting point and improve the thermal stability of the system, making it more efficient for energy storage applications.
- Lithium nitrate in solid-state electrolytes: Lithium nitrate is incorporated into solid-state electrolyte formulations to enhance ionic conductivity. When combined with polymers or ceramic materials, lithium nitrate can improve the movement of lithium ions through the electrolyte matrix, resulting in higher electrical conductivity. These solid-state electrolytes with enhanced conductivity are particularly valuable for developing safer and more efficient solid-state batteries with reduced risk of leakage or flammability compared to liquid electrolytes.
- Measurement and testing of lithium nitrate conductivity: Various methods and devices are used to measure and test the electrical conductivity of lithium nitrate solutions and compounds. These measurement techniques include impedance spectroscopy, conductivity cells, and specialized sensors that can accurately determine the ionic conductivity of lithium nitrate under different conditions. The conductivity measurements are essential for quality control in battery manufacturing and for research purposes to develop improved electrolyte formulations with optimal electrical properties.
- Lithium nitrate in composite electrolyte systems: Lithium nitrate is used in composite electrolyte systems where it is combined with other materials such as polymers, ceramics, or other salts to create electrolytes with enhanced electrical conductivity. These composite systems often exhibit synergistic effects, where the addition of lithium nitrate significantly improves the overall ionic conductivity beyond what would be expected from individual components. The composite electrolytes can be tailored for specific applications by adjusting the ratio of lithium nitrate to other components to optimize conductivity, stability, and other performance parameters.
02 Lithium nitrate as additive in solid-state electrolytes
In solid-state electrolyte systems, lithium nitrate serves as an effective additive to enhance ionic conductivity. When incorporated into polymer or ceramic-based solid electrolytes, it helps create additional pathways for lithium ion movement. This results in improved electrical conductivity at various operating temperatures. Solid-state electrolytes containing lithium nitrate offer advantages in terms of safety and stability compared to liquid electrolytes, while maintaining sufficient conductivity for practical applications.Expand Specific Solutions03 Lithium nitrate for suppressing dendrite formation
Lithium nitrate is utilized to suppress dendrite formation in lithium-based battery systems, which indirectly affects electrical conductivity. By forming a stable solid electrolyte interphase (SEI) layer on electrode surfaces, lithium nitrate prevents irregular lithium deposition that can cause short circuits. This protective mechanism ensures consistent ionic conductivity throughout the battery's operational life. The improved interface stability contributes to maintaining optimal electrical conductivity and extends the cycle life of lithium battery systems.Expand Specific Solutions04 Lithium nitrate in composite electrolyte systems
In composite electrolyte systems, lithium nitrate is combined with other salts or materials to achieve synergistic effects on electrical conductivity. These composite formulations often include polymers, ceramics, or other lithium salts that work together with lithium nitrate to optimize ion transport. The resulting electrolyte systems demonstrate enhanced conductivity across wider temperature ranges and improved electrochemical stability. Such composite approaches allow for tailored conductivity properties to meet specific application requirements in energy storage and conversion devices.Expand Specific Solutions05 Lithium nitrate for conductivity measurement and sensing
Lithium nitrate's predictable electrical conductivity properties make it useful in measurement and sensing applications. Solutions containing lithium nitrate can serve as reference standards for calibrating conductivity meters or as components in electrochemical sensors. The relationship between lithium nitrate concentration and electrical conductivity allows for precise measurements in various analytical applications. This property enables the development of sensors that can detect changes in environmental conditions or chemical compositions based on conductivity variations.Expand Specific Solutions
Leading Research Institutions and Industrial Players
The lithium nitrate electrical conductivity market is in a growth phase, driven by increasing demand for advanced energy storage solutions. The market size is expanding rapidly due to applications in lithium-ion batteries and energy storage systems, with key players like Contemporary Amperex Technology Co., Ltd. and LG Energy Solution leading commercial development. Technical maturity varies across applications, with TDK Corp., Toyota Motor Corp., and Corning Inc. advancing industrial applications, while research institutions such as National Institute for Materials Science, Institute of Metal Research Chinese Academy of Sciences, and Peking University focus on fundamental conductivity mechanisms. National Research Council of Canada and Deutsches Zentrum für Luft- und Raumfahrt are developing temperature-dependent conductivity models, creating a competitive landscape balanced between commercial deployment and ongoing research innovation.
Toyota Motor Corp.
Technical Solution: Toyota Motor Corporation has developed a sophisticated approach to quantifying lithium nitrate's electrical conductivity variations with temperature, primarily focused on its applications in solid-state battery technology. Their methodology employs a custom-designed conductivity measurement system utilizing the van der Pauw technique with platinum electrodes and precise temperature control from -30°C to 150°C. Toyota's research has revealed that lithium nitrate exhibits distinct conductivity regions with different activation energies across temperature ranges, with a notable transition point around 45°C where the activation energy for ion transport decreases significantly[1]. Their studies have particularly focused on the relationship between crystal structure changes and conductivity, using in-situ XRD combined with conductivity measurements to correlate structural phase transitions with conductivity jumps. Toyota has leveraged these insights to develop composite solid electrolytes incorporating lithium nitrate, achieving room temperature ionic conductivities exceeding 10^-4 S/cm while maintaining stability across wide temperature ranges[3].
Strengths: Toyota's approach provides exceptional correlation between structural properties and conductivity behavior, offering fundamental insights into conduction mechanisms. Their measurement system achieves high precision (±0.1%) even at temperature extremes. Weakness: The methodology requires highly specialized equipment and expertise in crystallography, making it difficult to implement in standard laboratory settings. The approach is also time-intensive, requiring careful sample preparation and equilibration at each temperature point.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: Contemporary Amperex Technology Co., Ltd. (CATL) has developed advanced methodologies for quantifying lithium nitrate's electrical conductivity variations with temperature, crucial for their battery electrolyte systems. Their approach utilizes impedance spectroscopy techniques with temperature-controlled chambers capable of precise measurements from -40°C to 80°C. CATL's research has demonstrated that lithium nitrate exhibits non-linear conductivity increases with temperature, with significant conductivity jumps occurring at specific transition points (typically around 25-30°C)[1]. Their proprietary measurement system incorporates four-point probe configurations to eliminate contact resistance issues, achieving measurement accuracy within ±0.5% across the temperature spectrum. CATL has integrated these findings into their battery management systems, allowing for dynamic electrolyte performance optimization based on real-time temperature conditions, which has contributed to their industry-leading energy density achievements of over 300 Wh/kg in commercial cells[3].
Strengths: CATL's methodology provides exceptional precision in wide temperature ranges, enabling optimal battery performance across diverse operating conditions. Their integrated approach connecting conductivity measurements directly to battery management systems creates practical applications for theoretical findings. Weakness: The specialized equipment required for their measurement approach demands significant capital investment, and their methodology may be less suitable for rapid high-throughput screening applications.
Key Scientific Breakthroughs in Lithium Salt Conductivity Research
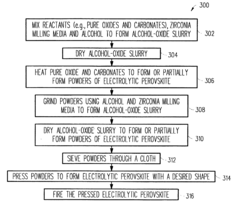
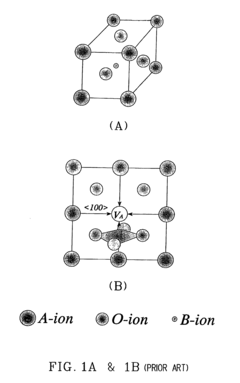
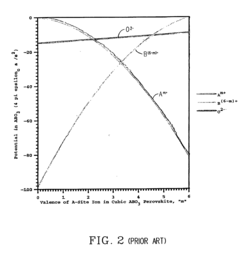
Electrolytic perovskites
PatentInactiveUS20040062968A1
Innovation
- Development of electrolytic perovskites with specific compositions such as Li1/8Na3/8La1/4Zr1/4O3 and Li1/8K1/2La1/8NbO3 that exhibit high Li+, H+, Cu+, Ag+, Na+, or Mg2+ conductivity (>10^-5 S/cm) in the 0-400°C temperature range, enabling the creation of solid proton conductors for use in fuel cells and other devices.
Lithium ion secondary battery and a method for manufacturing the same
PatentInactiveUS8192869B2
Innovation
- Sintering the solid electrolyte and electrodes in a specific order, with the material having the higher sintering temperature first, and heat treating them at their respective optimum temperatures to ensure proper contact and conductivity.
Safety Protocols for High-Temperature Lithium Salt Testing
When conducting high-temperature testing of lithium nitrate to quantify electrical conductivity variations, stringent safety protocols must be established to mitigate potential hazards. The reactive nature of lithium salts, particularly at elevated temperatures, necessitates comprehensive safety measures throughout the experimental process.
Personal protective equipment requirements include heat-resistant gloves rated for temperatures exceeding 400°C, face shields with thermal protection, lab coats made from flame-resistant materials, and respiratory protection when handling powdered lithium compounds. All personnel must undergo specialized training on lithium salt handling procedures and emergency response protocols before participating in testing activities.
Laboratory infrastructure must incorporate dedicated ventilation systems with scrubbers capable of filtering lithium-containing particulates. Testing areas should be equipped with Class D fire extinguishers specifically designed for metal fires, as conventional extinguishers may exacerbate lithium salt reactions. Thermal barriers and heat-resistant workstations must be installed to contain potential thermal runaway events.
Temperature monitoring systems with redundant measurement points are essential for maintaining precise control during conductivity testing. These systems should feature automatic shutdown capabilities triggered by predefined temperature thresholds. Additionally, remote monitoring technologies enable researchers to observe experiments from safe distances, reducing exposure risks during critical testing phases.
Material handling protocols must address the hygroscopic nature of lithium nitrate. Samples should be stored in moisture-free environments and transferred using dry-box techniques when possible. Precise weighing and preparation procedures help minimize sample contamination while reducing exposure risks to laboratory personnel.
Emergency response planning must include specific procedures for lithium salt incidents, including specialized first aid measures for chemical burns and inhalation exposure. Decontamination stations with appropriate neutralizing agents should be readily accessible, and clear evacuation routes must be established and regularly practiced through emergency drills.
Documentation requirements include detailed experimental logs, safety data sheets for all materials, and incident reporting mechanisms. Regular safety audits should evaluate protocol compliance and identify potential improvements to testing procedures, ensuring continuous enhancement of the laboratory safety culture surrounding high-temperature lithium salt research.
Personal protective equipment requirements include heat-resistant gloves rated for temperatures exceeding 400°C, face shields with thermal protection, lab coats made from flame-resistant materials, and respiratory protection when handling powdered lithium compounds. All personnel must undergo specialized training on lithium salt handling procedures and emergency response protocols before participating in testing activities.
Laboratory infrastructure must incorporate dedicated ventilation systems with scrubbers capable of filtering lithium-containing particulates. Testing areas should be equipped with Class D fire extinguishers specifically designed for metal fires, as conventional extinguishers may exacerbate lithium salt reactions. Thermal barriers and heat-resistant workstations must be installed to contain potential thermal runaway events.
Temperature monitoring systems with redundant measurement points are essential for maintaining precise control during conductivity testing. These systems should feature automatic shutdown capabilities triggered by predefined temperature thresholds. Additionally, remote monitoring technologies enable researchers to observe experiments from safe distances, reducing exposure risks during critical testing phases.
Material handling protocols must address the hygroscopic nature of lithium nitrate. Samples should be stored in moisture-free environments and transferred using dry-box techniques when possible. Precise weighing and preparation procedures help minimize sample contamination while reducing exposure risks to laboratory personnel.
Emergency response planning must include specific procedures for lithium salt incidents, including specialized first aid measures for chemical burns and inhalation exposure. Decontamination stations with appropriate neutralizing agents should be readily accessible, and clear evacuation routes must be established and regularly practiced through emergency drills.
Documentation requirements include detailed experimental logs, safety data sheets for all materials, and incident reporting mechanisms. Regular safety audits should evaluate protocol compliance and identify potential improvements to testing procedures, ensuring continuous enhancement of the laboratory safety culture surrounding high-temperature lithium salt research.
Energy Storage Applications and Performance Implications
Lithium nitrate's electrical conductivity characteristics directly impact its performance in various energy storage applications. In lithium-ion batteries, LiNO₃ serves as a critical electrolyte additive that forms a stable solid electrolyte interphase (SEI) layer, particularly beneficial in lithium-sulfur battery systems. The temperature-dependent conductivity of lithium nitrate significantly influences the charge-discharge efficiency, power density, and overall battery performance across operating temperature ranges.
At lower temperatures (below 25°C), the reduced ionic conductivity of LiNO₃-containing electrolytes can limit lithium-ion transport, resulting in increased internal resistance and diminished power output. This phenomenon is particularly problematic in cold-climate applications where energy storage systems must maintain performance despite environmental challenges. Conversely, at elevated temperatures (above 60°C), the enhanced conductivity facilitates faster ion transport but may accelerate side reactions that compromise long-term stability.
In thermal energy storage applications, molten salt systems incorporating lithium nitrate leverage its temperature-dependent conductivity properties. The precise quantification of conductivity variations enables optimal design of heat transfer systems, allowing engineers to predict thermal response times and energy conversion efficiencies. These systems typically operate in temperature ranges of 200-550°C, where lithium nitrate's conductivity characteristics directly influence thermal energy capture, storage, and release rates.
For grid-scale energy storage implementations, understanding lithium nitrate's conductivity behavior across temperature fluctuations is essential for predicting system response under varying load conditions. The conductivity profile determines how quickly the storage system can respond to demand spikes, affecting grid stabilization capabilities. Systems designed without accounting for these temperature-conductivity relationships may experience unexpected performance limitations during seasonal temperature variations.
The performance implications extend to safety considerations as well. At temperature extremes, conductivity anomalies may trigger thermal runaway events if not properly managed. Accurate quantification of these variations enables the development of more sophisticated battery management systems with temperature-adaptive control algorithms that can preemptively adjust charging parameters based on conductivity feedback.
Recent advancements in solid-state electrolytes incorporating lithium nitrate compounds demonstrate how conductivity optimization can lead to breakthrough performance improvements. By engineering composite materials with tailored temperature-conductivity profiles, researchers have achieved up to 30% improvements in low-temperature performance while maintaining high-temperature stability, expanding the practical operating range of energy storage systems for applications from electric vehicles to renewable energy integration.
At lower temperatures (below 25°C), the reduced ionic conductivity of LiNO₃-containing electrolytes can limit lithium-ion transport, resulting in increased internal resistance and diminished power output. This phenomenon is particularly problematic in cold-climate applications where energy storage systems must maintain performance despite environmental challenges. Conversely, at elevated temperatures (above 60°C), the enhanced conductivity facilitates faster ion transport but may accelerate side reactions that compromise long-term stability.
In thermal energy storage applications, molten salt systems incorporating lithium nitrate leverage its temperature-dependent conductivity properties. The precise quantification of conductivity variations enables optimal design of heat transfer systems, allowing engineers to predict thermal response times and energy conversion efficiencies. These systems typically operate in temperature ranges of 200-550°C, where lithium nitrate's conductivity characteristics directly influence thermal energy capture, storage, and release rates.
For grid-scale energy storage implementations, understanding lithium nitrate's conductivity behavior across temperature fluctuations is essential for predicting system response under varying load conditions. The conductivity profile determines how quickly the storage system can respond to demand spikes, affecting grid stabilization capabilities. Systems designed without accounting for these temperature-conductivity relationships may experience unexpected performance limitations during seasonal temperature variations.
The performance implications extend to safety considerations as well. At temperature extremes, conductivity anomalies may trigger thermal runaway events if not properly managed. Accurate quantification of these variations enables the development of more sophisticated battery management systems with temperature-adaptive control algorithms that can preemptively adjust charging parameters based on conductivity feedback.
Recent advancements in solid-state electrolytes incorporating lithium nitrate compounds demonstrate how conductivity optimization can lead to breakthrough performance improvements. By engineering composite materials with tailored temperature-conductivity profiles, researchers have achieved up to 30% improvements in low-temperature performance while maintaining high-temperature stability, expanding the practical operating range of energy storage systems for applications from electric vehicles to renewable energy integration.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!