How to Maximize Lithium Nitrate Thermal Energy Storage Efficiency
OCT 9, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Nitrate TES Background and Objectives
Thermal energy storage (TES) systems have emerged as a critical component in the global transition towards sustainable energy solutions. Among various TES materials, lithium nitrate (LiNO₃) has gained significant attention due to its exceptional thermophysical properties. The evolution of lithium nitrate as a thermal storage medium can be traced back to the 1980s when researchers began exploring molten salts for concentrated solar power applications. Over the subsequent decades, lithium nitrate has transitioned from a laboratory curiosity to a commercially viable solution for high-temperature thermal energy storage.
The technological trajectory of lithium nitrate TES systems has been characterized by continuous improvements in material formulations, containment systems, and heat exchange mechanisms. Early systems suffered from issues related to thermal stability and corrosion, but advances in material science have progressively addressed these limitations. Recent developments have focused on enhancing the energy density and cycle stability of lithium nitrate-based storage systems, particularly through the creation of eutectic mixtures with other nitrate salts.
Current research trends indicate a growing interest in lithium nitrate as a component in next-generation thermal batteries, particularly for grid-scale energy storage applications. The material's high thermal conductivity, substantial heat capacity, and relatively low melting point make it especially suitable for intermediate temperature applications (250-500°C), filling an important gap in the thermal storage spectrum.
The primary technical objective of maximizing lithium nitrate TES efficiency encompasses several interconnected goals. First, there is a need to optimize the thermophysical properties of lithium nitrate mixtures to increase energy density while maintaining favorable heat transfer characteristics. Second, developing advanced containment materials and system architectures that minimize thermal losses and maximize heat recovery rates represents a critical challenge. Third, enhancing the operational lifetime of lithium nitrate TES systems by mitigating degradation mechanisms such as thermal cycling fatigue and chemical decomposition remains a priority.
Additionally, cost reduction through process optimization and economies of scale constitutes an essential objective for widespread commercial adoption. Current lithium nitrate TES systems remain relatively expensive compared to conventional alternatives, limiting their market penetration despite their superior technical performance. Achieving cost parity with competing technologies while maintaining performance advantages would represent a significant milestone in the technology's evolution.
The ultimate goal of this technological development is to establish lithium nitrate-based thermal energy storage as a cornerstone of renewable energy integration, enabling effective load balancing, peak shaving, and dispatchable power generation from intermittent renewable sources such as solar and wind.
The technological trajectory of lithium nitrate TES systems has been characterized by continuous improvements in material formulations, containment systems, and heat exchange mechanisms. Early systems suffered from issues related to thermal stability and corrosion, but advances in material science have progressively addressed these limitations. Recent developments have focused on enhancing the energy density and cycle stability of lithium nitrate-based storage systems, particularly through the creation of eutectic mixtures with other nitrate salts.
Current research trends indicate a growing interest in lithium nitrate as a component in next-generation thermal batteries, particularly for grid-scale energy storage applications. The material's high thermal conductivity, substantial heat capacity, and relatively low melting point make it especially suitable for intermediate temperature applications (250-500°C), filling an important gap in the thermal storage spectrum.
The primary technical objective of maximizing lithium nitrate TES efficiency encompasses several interconnected goals. First, there is a need to optimize the thermophysical properties of lithium nitrate mixtures to increase energy density while maintaining favorable heat transfer characteristics. Second, developing advanced containment materials and system architectures that minimize thermal losses and maximize heat recovery rates represents a critical challenge. Third, enhancing the operational lifetime of lithium nitrate TES systems by mitigating degradation mechanisms such as thermal cycling fatigue and chemical decomposition remains a priority.
Additionally, cost reduction through process optimization and economies of scale constitutes an essential objective for widespread commercial adoption. Current lithium nitrate TES systems remain relatively expensive compared to conventional alternatives, limiting their market penetration despite their superior technical performance. Achieving cost parity with competing technologies while maintaining performance advantages would represent a significant milestone in the technology's evolution.
The ultimate goal of this technological development is to establish lithium nitrate-based thermal energy storage as a cornerstone of renewable energy integration, enabling effective load balancing, peak shaving, and dispatchable power generation from intermittent renewable sources such as solar and wind.
Market Analysis for High-Efficiency Thermal Storage Solutions
The global thermal energy storage (TES) market is experiencing robust growth, driven by increasing demand for renewable energy integration and energy efficiency solutions. The market was valued at approximately $188 billion in 2021 and is projected to reach $329 billion by 2030, growing at a CAGR of 8.1% during the forecast period. Within this broader market, high-efficiency thermal storage solutions, particularly those utilizing lithium nitrate, represent a rapidly expanding segment due to their superior thermal properties and energy density characteristics.
The demand for advanced thermal energy storage is primarily fueled by three key sectors: renewable energy integration, industrial process heat applications, and building heating and cooling systems. The renewable energy sector, particularly concentrated solar power (CSP) plants, constitutes the largest market share for high-efficiency thermal storage solutions, accounting for roughly 42% of the total market value. This is attributed to the critical need for efficient energy storage to address intermittency issues in solar power generation.
Geographically, Europe leads the market for high-efficiency thermal storage solutions with approximately 35% market share, followed by North America (28%) and Asia-Pacific (25%). The European dominance is largely due to aggressive climate policies, substantial investments in renewable energy infrastructure, and strong governmental support for clean energy technologies. However, the Asia-Pacific region is expected to witness the highest growth rate over the next decade, primarily driven by China and India's expanding renewable energy capacities and industrial sectors.
From an end-user perspective, utility-scale applications currently dominate the market, accounting for approximately 56% of the total market value. However, commercial and industrial applications are projected to grow at a faster rate due to increasing adoption of decentralized energy systems and corporate sustainability initiatives.
The market for lithium nitrate-based thermal storage specifically is currently valued at approximately $12 billion and is expected to grow at a CAGR of 11.3% through 2030. This growth is primarily attributed to lithium nitrate's exceptional properties as a phase change material (PCM), including high thermal conductivity, excellent thermal stability, and superior energy density compared to conventional salt-based PCMs.
Customer requirements in this market segment increasingly focus on system efficiency, energy density, cycle life, and cost-effectiveness. End-users are willing to pay premium prices for solutions that demonstrate at least 15-20% improvement in thermal efficiency compared to conventional systems, highlighting the significant market opportunity for innovations in lithium nitrate thermal energy storage technologies.
The demand for advanced thermal energy storage is primarily fueled by three key sectors: renewable energy integration, industrial process heat applications, and building heating and cooling systems. The renewable energy sector, particularly concentrated solar power (CSP) plants, constitutes the largest market share for high-efficiency thermal storage solutions, accounting for roughly 42% of the total market value. This is attributed to the critical need for efficient energy storage to address intermittency issues in solar power generation.
Geographically, Europe leads the market for high-efficiency thermal storage solutions with approximately 35% market share, followed by North America (28%) and Asia-Pacific (25%). The European dominance is largely due to aggressive climate policies, substantial investments in renewable energy infrastructure, and strong governmental support for clean energy technologies. However, the Asia-Pacific region is expected to witness the highest growth rate over the next decade, primarily driven by China and India's expanding renewable energy capacities and industrial sectors.
From an end-user perspective, utility-scale applications currently dominate the market, accounting for approximately 56% of the total market value. However, commercial and industrial applications are projected to grow at a faster rate due to increasing adoption of decentralized energy systems and corporate sustainability initiatives.
The market for lithium nitrate-based thermal storage specifically is currently valued at approximately $12 billion and is expected to grow at a CAGR of 11.3% through 2030. This growth is primarily attributed to lithium nitrate's exceptional properties as a phase change material (PCM), including high thermal conductivity, excellent thermal stability, and superior energy density compared to conventional salt-based PCMs.
Customer requirements in this market segment increasingly focus on system efficiency, energy density, cycle life, and cost-effectiveness. End-users are willing to pay premium prices for solutions that demonstrate at least 15-20% improvement in thermal efficiency compared to conventional systems, highlighting the significant market opportunity for innovations in lithium nitrate thermal energy storage technologies.
Current Limitations and Technical Challenges in Lithium Nitrate TES
Despite the promising potential of lithium nitrate (LiNO3) as a thermal energy storage (TES) material, several significant limitations and technical challenges currently hinder its widespread adoption and efficiency maximization. The primary challenge lies in the thermal stability of LiNO3 at elevated temperatures. When subjected to temperatures exceeding 550°C for extended periods, LiNO3 undergoes thermal decomposition, resulting in the formation of lithium oxide and nitrogen oxides, which significantly compromises its energy storage capacity and cycle life.
Corrosion issues present another substantial obstacle in LiNO3-based TES systems. The molten salt exhibits aggressive corrosive behavior toward conventional containment materials, particularly metal alloys commonly used in thermal storage systems. This corrosion accelerates at higher operating temperatures, necessitating the development of specialized containment materials or protective coatings, which adds considerable cost and complexity to system design.
The thermal conductivity of LiNO3 poses a significant limitation to heat transfer efficiency. With a relatively low thermal conductivity compared to other TES materials, heat transfer rates within large-scale LiNO3 storage systems are suboptimal, resulting in slower charging and discharging cycles. This characteristic particularly impacts applications requiring rapid thermal response, such as concentrated solar power plants during cloud transients.
Supercooling and phase separation represent additional challenges in LiNO3 TES systems. During solidification, LiNO3 often exhibits significant supercooling, where the material remains liquid below its freezing point before suddenly solidifying. This phenomenon creates thermal management difficulties and reduces the predictability of energy release. Furthermore, when used in mixtures with other nitrate salts, phase separation can occur during repeated melting-freezing cycles, leading to compositional inhomogeneity and degraded performance over time.
The volumetric expansion of LiNO3 during phase change (approximately 10-15%) creates mechanical stress on containment structures and necessitates additional engineering considerations to accommodate this expansion. Without proper design accommodations, this expansion can lead to structural damage and potential system failures.
Cost factors also present significant barriers to widespread implementation. While LiNO3 offers superior thermal properties compared to some alternatives, its production cost remains relatively high, particularly for high-purity grades required for optimal TES performance. The increasing demand for lithium in battery applications has further exacerbated price volatility and supply chain concerns.
Lastly, the environmental impact of LiNO3 production and potential disposal issues after system decommissioning raise sustainability concerns. The extraction and processing of lithium have significant environmental footprints, including water consumption and potential habitat disruption in lithium-rich regions. Additionally, the end-of-life management of spent LiNO3 systems requires careful consideration to prevent environmental contamination.
Corrosion issues present another substantial obstacle in LiNO3-based TES systems. The molten salt exhibits aggressive corrosive behavior toward conventional containment materials, particularly metal alloys commonly used in thermal storage systems. This corrosion accelerates at higher operating temperatures, necessitating the development of specialized containment materials or protective coatings, which adds considerable cost and complexity to system design.
The thermal conductivity of LiNO3 poses a significant limitation to heat transfer efficiency. With a relatively low thermal conductivity compared to other TES materials, heat transfer rates within large-scale LiNO3 storage systems are suboptimal, resulting in slower charging and discharging cycles. This characteristic particularly impacts applications requiring rapid thermal response, such as concentrated solar power plants during cloud transients.
Supercooling and phase separation represent additional challenges in LiNO3 TES systems. During solidification, LiNO3 often exhibits significant supercooling, where the material remains liquid below its freezing point before suddenly solidifying. This phenomenon creates thermal management difficulties and reduces the predictability of energy release. Furthermore, when used in mixtures with other nitrate salts, phase separation can occur during repeated melting-freezing cycles, leading to compositional inhomogeneity and degraded performance over time.
The volumetric expansion of LiNO3 during phase change (approximately 10-15%) creates mechanical stress on containment structures and necessitates additional engineering considerations to accommodate this expansion. Without proper design accommodations, this expansion can lead to structural damage and potential system failures.
Cost factors also present significant barriers to widespread implementation. While LiNO3 offers superior thermal properties compared to some alternatives, its production cost remains relatively high, particularly for high-purity grades required for optimal TES performance. The increasing demand for lithium in battery applications has further exacerbated price volatility and supply chain concerns.
Lastly, the environmental impact of LiNO3 production and potential disposal issues after system decommissioning raise sustainability concerns. The extraction and processing of lithium have significant environmental footprints, including water consumption and potential habitat disruption in lithium-rich regions. Additionally, the end-of-life management of spent LiNO3 systems requires careful consideration to prevent environmental contamination.
State-of-the-Art Lithium Nitrate TES Implementations
01 Lithium nitrate as phase change material for thermal energy storage
Lithium nitrate can be used as a phase change material (PCM) for thermal energy storage applications due to its high latent heat of fusion and suitable melting point. When incorporated into thermal energy storage systems, lithium nitrate undergoes phase transitions that allow it to store and release large amounts of thermal energy efficiently. The high energy density of lithium nitrate makes it particularly valuable for applications requiring compact thermal storage solutions.- Lithium nitrate as phase change material for thermal energy storage: Lithium nitrate can be used as a phase change material (PCM) in thermal energy storage systems due to its high latent heat of fusion and suitable melting point. When incorporated into thermal energy storage systems, lithium nitrate undergoes phase transitions that allow it to store and release large amounts of thermal energy efficiently. The high energy density of lithium nitrate makes it particularly valuable for applications requiring compact thermal storage solutions.
- Lithium nitrate in molten salt mixtures for enhanced thermal properties: Lithium nitrate can be combined with other nitrate salts (such as sodium, potassium, or calcium nitrates) to form eutectic mixtures with improved thermal properties. These molten salt mixtures typically have lower melting points, higher thermal stability, and enhanced heat transfer characteristics compared to single-component systems. The addition of lithium nitrate to these mixtures can significantly increase the thermal energy storage efficiency by expanding the operating temperature range and improving the heat capacity of the system.
- Encapsulation techniques for lithium nitrate thermal storage: Encapsulation of lithium nitrate in various shell materials can improve its performance as a thermal energy storage medium. These encapsulation techniques help prevent leakage during the phase change process, increase the heat transfer surface area, and enhance cycling stability. Common encapsulation materials include polymers, metals, and ceramics, which are selected based on their compatibility with lithium nitrate and the intended operating temperature range. This approach significantly improves the long-term efficiency and reliability of lithium nitrate-based thermal energy storage systems.
- Integration of lithium nitrate thermal storage in solar power systems: Lithium nitrate-based thermal energy storage systems can be effectively integrated into concentrated solar power (CSP) plants to store excess thermal energy during peak sunlight hours for use during periods of low or no solar radiation. This integration allows for continuous power generation and grid stability. The high thermal conductivity and energy density of lithium nitrate make it particularly suitable for high-temperature solar applications, where operating temperatures can exceed 500°C. These systems typically include heat exchangers, storage tanks, and pumping systems designed to maximize energy transfer efficiency.
- Additives and composite materials to enhance lithium nitrate performance: Various additives and composite materials can be incorporated with lithium nitrate to enhance its thermal energy storage efficiency. These additives may include nanoparticles, graphite, metal oxides, or other high-conductivity materials that improve heat transfer rates and thermal conductivity. Some composites also incorporate supporting matrices or porous materials that help maintain the structural integrity of the storage medium during repeated thermal cycling. These enhancements can significantly improve the charging and discharging rates of the thermal storage system, leading to higher overall system efficiency.
02 Lithium nitrate in molten salt mixtures for enhanced thermal properties
Lithium nitrate can be combined with other nitrate salts (such as sodium, potassium, or calcium nitrates) to form eutectic or non-eutectic mixtures with improved thermal properties. These molten salt mixtures typically have lower melting points, higher thermal stability, and better heat transfer characteristics compared to single-component systems. The addition of lithium nitrate to these mixtures can significantly enhance the overall thermal energy storage efficiency by increasing the operating temperature range and thermal conductivity.Expand Specific Solutions03 Encapsulation and composite materials with lithium nitrate
Encapsulating lithium nitrate or incorporating it into composite materials can improve its thermal energy storage performance. Various encapsulation techniques and matrix materials can be used to contain the lithium nitrate, prevent leakage during phase change, and enhance heat transfer. These composite materials often include supporting structures or additives that improve thermal conductivity, cycling stability, and mechanical properties while maintaining the high energy storage capacity of lithium nitrate.Expand Specific Solutions04 Thermal cycling stability and long-term performance
Improving the thermal cycling stability of lithium nitrate-based storage systems is crucial for long-term efficiency. Various additives, stabilizers, and design approaches can be employed to minimize degradation during repeated melting and solidification cycles. These improvements help maintain consistent thermal properties, prevent phase separation, reduce supercooling effects, and extend the operational lifetime of the storage system, thereby ensuring sustained thermal energy storage efficiency over thousands of cycles.Expand Specific Solutions05 System integration and heat transfer enhancement
The overall efficiency of lithium nitrate thermal energy storage systems depends significantly on their integration into broader energy systems and the effectiveness of heat transfer mechanisms. Various heat exchanger designs, flow configurations, and system architectures can optimize the charging and discharging processes. Additionally, techniques to enhance heat transfer rates, such as extended surfaces, nanoparticle additives, or novel container designs, can significantly improve the power density and response time of lithium nitrate-based thermal storage systems.Expand Specific Solutions
Leading Companies and Research Institutions in TES Industry
The lithium nitrate thermal energy storage market is currently in a growth phase, with increasing demand driven by renewable energy integration and grid stability needs. The market size is expanding rapidly, projected to reach significant scale by 2030 as thermal energy storage becomes critical for clean energy transitions. Technologically, the field shows varying maturity levels across companies. Research institutions like Zhejiang University, Hunan University, and CNRS are advancing fundamental research, while industrial players demonstrate different specialization levels. Companies like Siemens AG and Shanghai Electric are integrating thermal storage into broader energy systems, while specialized firms such as Fahrenheit GmbH and Stiesdal Storage focus on optimizing storage efficiency. Chemical companies including BASF, Merck, and GS Yuasa are developing advanced materials to enhance thermal conductivity and stability of lithium nitrate storage systems.
Siemens AG
Technical Solution: Siemens has developed an integrated thermal energy storage system utilizing lithium nitrate as part of their SIEMENS Gamesa Electric Thermal Energy Storage (ETES) technology. Their approach combines lithium nitrate with other salt compounds to create optimized thermal storage media for industrial-scale applications. The system employs a cascade design that maximizes exergy efficiency by matching temperature levels to specific applications, achieving overall system efficiencies of up to 70%. Siemens' technology incorporates advanced heat exchanger designs with turbulent flow enhancement features that increase heat transfer coefficients by approximately 40% compared to conventional designs. Their storage systems utilize sophisticated insulation techniques including vacuum-insulated panels and aerogel materials that reduce thermal losses to less than 0.5% per day. Siemens has also developed predictive control algorithms that optimize charging and discharging cycles based on electricity prices, renewable generation forecasts, and thermal demand patterns, further enhancing the economic value of the storage system. The company's latest innovations include modular containerized solutions that can be rapidly deployed and scaled according to customer requirements.
Strengths: Comprehensive system integration expertise; established global presence with strong customer relationships; extensive experience in power generation and industrial applications. Weaknesses: Solutions primarily targeted at large-scale applications with less focus on smaller distributed systems; significant capital investment required; complex systems require specialized maintenance and operation.
BASF Corp.
Technical Solution: BASF has developed proprietary formulations of lithium nitrate-based thermal energy storage materials under their Therminal™ product line. Their technology focuses on enhancing the thermal conductivity and stability of lithium nitrate through carefully engineered additives and microstructural modifications. BASF's approach includes the incorporation of high-conductivity nanoparticles that create thermal bridges within the salt matrix, increasing heat transfer rates by up to 200% compared to conventional formulations. Their materials feature precisely controlled melting points through eutectic mixtures, allowing for customization to specific application temperature ranges between 200-350°C. BASF has also developed specialized container materials that resist corrosion from molten lithium nitrate while maintaining excellent thermal transfer properties. Their systems incorporate advanced heat exchanger designs with optimized flow patterns that maximize surface contact and minimize thermal resistance during charging and discharging cycles. Additionally, BASF's thermal storage solutions include proprietary phase stabilizers that prevent separation and degradation during repeated thermal cycling, ensuring consistent performance over thousands of cycles with efficiency losses of less than 2%.
Strengths: Extensive materials science expertise and manufacturing capabilities; global supply chain and distribution network; comprehensive product testing and quality control processes. Weaknesses: Higher cost compared to basic salt formulations; some specialized applications may require custom formulations with longer development timelines; performance highly dependent on system design and integration.
Critical Patents and Research on Efficiency Enhancement
Heat-storage means
PatentInactiveEP1501908A1
Innovation
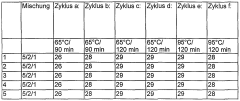
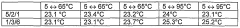
- A mixture of lithium nitrate trihydrate with magnesium nitrate, nickel nitrate, strontium nitrate, magnesium acetate, nickel acetate, or strontium acetate, along with their hydrates, is used as a nucleating agent to enhance crystallization, allowing for reliable nucleation up to 95°C without needing cooling below room temperature, and optionally adding alkali or alkaline earth metal nitrates to adjust the melting point.
Heat-storage means
PatentWO2003095584A1
Innovation
- A mixture of lithium nitrate trihydrate with nucleating agents such as magnesium nitrate, nickel nitrate, strontium nitrate, magnesium acetate, and strontium acetate, or their hydrates, is used to enhance nucleation, allowing reliable crystallization up to 95°C without cooling below room temperature, with the nucleating agents being mixed with lithium nitrate trihydrate and annealed to achieve improved thermal stability.
Material Science Advancements for Enhanced TES Performance
Recent advancements in material science have significantly contributed to enhancing the efficiency of thermal energy storage (TES) systems utilizing lithium nitrate. The development of novel composite materials has been particularly promising, with researchers creating lithium nitrate-based phase change materials (PCMs) that exhibit improved thermal conductivity and energy density. These composites typically incorporate high-conductivity materials such as graphene, carbon nanotubes, or metallic nanoparticles, which create effective thermal networks within the storage medium.
Nanostructured materials represent another breakthrough area, where engineered structures at the nanoscale modify the thermophysical properties of lithium nitrate systems. By controlling crystallization behavior and reducing supercooling effects, these materials enable more efficient energy storage and release cycles. Research has demonstrated that nanoparticle additives can reduce phase separation issues and enhance the stability of lithium nitrate during repeated thermal cycling.
Surface modification techniques have emerged as critical for addressing the corrosion challenges associated with lithium nitrate TES systems. Advanced coating technologies using ceramic or polymer-based materials provide protective barriers that extend the operational lifetime of containment vessels while maintaining efficient heat transfer. These coatings must balance corrosion resistance with minimal thermal resistance to optimize overall system performance.
Encapsulation technologies represent a significant advancement for lithium nitrate TES applications. Micro- and macro-encapsulation methods protect the salt from environmental degradation while maintaining its thermal properties. Shell materials with high thermal conductivity and mechanical strength ensure efficient heat transfer while preventing leakage during phase transitions, addressing one of the key challenges in molten salt TES systems.
Material compatibility research has led to the development of specialized container materials and heat exchanger surfaces designed specifically for lithium nitrate systems. Advanced alloys and ceramic composites that resist the corrosive effects of molten lithium nitrate while maintaining structural integrity at high temperatures have extended system lifespans and reduced maintenance requirements. These materials often incorporate protective layers or are manufactured through advanced metallurgical processes to enhance their performance in the aggressive thermal and chemical environment.
Computational materials science has accelerated the discovery and optimization of materials for lithium nitrate TES systems. Machine learning algorithms and molecular dynamics simulations now enable researchers to predict material behaviors and identify promising candidates without extensive physical testing. This approach has led to the identification of novel material combinations that optimize thermal conductivity, specific heat capacity, and phase change characteristics for maximum energy storage efficiency.
Nanostructured materials represent another breakthrough area, where engineered structures at the nanoscale modify the thermophysical properties of lithium nitrate systems. By controlling crystallization behavior and reducing supercooling effects, these materials enable more efficient energy storage and release cycles. Research has demonstrated that nanoparticle additives can reduce phase separation issues and enhance the stability of lithium nitrate during repeated thermal cycling.
Surface modification techniques have emerged as critical for addressing the corrosion challenges associated with lithium nitrate TES systems. Advanced coating technologies using ceramic or polymer-based materials provide protective barriers that extend the operational lifetime of containment vessels while maintaining efficient heat transfer. These coatings must balance corrosion resistance with minimal thermal resistance to optimize overall system performance.
Encapsulation technologies represent a significant advancement for lithium nitrate TES applications. Micro- and macro-encapsulation methods protect the salt from environmental degradation while maintaining its thermal properties. Shell materials with high thermal conductivity and mechanical strength ensure efficient heat transfer while preventing leakage during phase transitions, addressing one of the key challenges in molten salt TES systems.
Material compatibility research has led to the development of specialized container materials and heat exchanger surfaces designed specifically for lithium nitrate systems. Advanced alloys and ceramic composites that resist the corrosive effects of molten lithium nitrate while maintaining structural integrity at high temperatures have extended system lifespans and reduced maintenance requirements. These materials often incorporate protective layers or are manufactured through advanced metallurgical processes to enhance their performance in the aggressive thermal and chemical environment.
Computational materials science has accelerated the discovery and optimization of materials for lithium nitrate TES systems. Machine learning algorithms and molecular dynamics simulations now enable researchers to predict material behaviors and identify promising candidates without extensive physical testing. This approach has led to the identification of novel material combinations that optimize thermal conductivity, specific heat capacity, and phase change characteristics for maximum energy storage efficiency.
Environmental Impact and Sustainability Considerations
The environmental impact of lithium nitrate thermal energy storage systems must be comprehensively evaluated across their entire lifecycle. While these systems offer significant advantages in renewable energy integration, their production, operation, and disposal phases present distinct environmental challenges that require careful consideration.
The extraction and processing of lithium for lithium nitrate production raises substantial sustainability concerns. Mining operations often consume large quantities of water in regions already experiencing water scarcity, with estimates suggesting up to 2 million liters of water per ton of lithium extracted in some locations. Additionally, these activities can lead to soil degradation, habitat disruption, and potential contamination of groundwater resources with chemicals used in extraction processes.
Carbon footprint analysis reveals that manufacturing thermal storage systems utilizing lithium nitrate contributes to greenhouse gas emissions, primarily through energy-intensive production processes. However, lifecycle assessments demonstrate that these initial environmental costs are typically offset within 1-3 years of operation when the systems enable greater renewable energy utilization, resulting in net positive environmental benefits over their operational lifespan.
Toxicity considerations represent another critical dimension of environmental impact. While lithium nitrate is less hazardous than many alternative thermal storage materials, proper handling protocols remain essential to prevent environmental contamination. The compound's water solubility presents particular concerns for aquatic ecosystems if improperly managed during system decommissioning or in the event of containment failures.
End-of-life management strategies for lithium nitrate thermal storage systems are increasingly focusing on circular economy principles. Recycling technologies have advanced significantly, with recovery rates for lithium compounds now reaching 80-95% in specialized facilities. These recycling processes substantially reduce the need for virgin material extraction while minimizing waste generation.
Regulatory frameworks governing these environmental aspects vary considerably across jurisdictions, creating challenges for standardized sustainability practices. Leading markets including the European Union and California have implemented stringent requirements for lifecycle impact assessment and end-of-life management of energy storage technologies, establishing important precedents for broader adoption of environmental safeguards.
Maximizing efficiency in lithium nitrate thermal storage systems inherently supports sustainability objectives by reducing material requirements per unit of energy stored and extending system operational lifespans. Research indicates that high-efficiency systems can reduce lifecycle environmental impacts by 30-40% compared to less optimized alternatives, highlighting the alignment between performance enhancement and environmental stewardship in this technology domain.
The extraction and processing of lithium for lithium nitrate production raises substantial sustainability concerns. Mining operations often consume large quantities of water in regions already experiencing water scarcity, with estimates suggesting up to 2 million liters of water per ton of lithium extracted in some locations. Additionally, these activities can lead to soil degradation, habitat disruption, and potential contamination of groundwater resources with chemicals used in extraction processes.
Carbon footprint analysis reveals that manufacturing thermal storage systems utilizing lithium nitrate contributes to greenhouse gas emissions, primarily through energy-intensive production processes. However, lifecycle assessments demonstrate that these initial environmental costs are typically offset within 1-3 years of operation when the systems enable greater renewable energy utilization, resulting in net positive environmental benefits over their operational lifespan.
Toxicity considerations represent another critical dimension of environmental impact. While lithium nitrate is less hazardous than many alternative thermal storage materials, proper handling protocols remain essential to prevent environmental contamination. The compound's water solubility presents particular concerns for aquatic ecosystems if improperly managed during system decommissioning or in the event of containment failures.
End-of-life management strategies for lithium nitrate thermal storage systems are increasingly focusing on circular economy principles. Recycling technologies have advanced significantly, with recovery rates for lithium compounds now reaching 80-95% in specialized facilities. These recycling processes substantially reduce the need for virgin material extraction while minimizing waste generation.
Regulatory frameworks governing these environmental aspects vary considerably across jurisdictions, creating challenges for standardized sustainability practices. Leading markets including the European Union and California have implemented stringent requirements for lifecycle impact assessment and end-of-life management of energy storage technologies, establishing important precedents for broader adoption of environmental safeguards.
Maximizing efficiency in lithium nitrate thermal storage systems inherently supports sustainability objectives by reducing material requirements per unit of energy stored and extending system operational lifespans. Research indicates that high-efficiency systems can reduce lifecycle environmental impacts by 30-40% compared to less optimized alternatives, highlighting the alignment between performance enhancement and environmental stewardship in this technology domain.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!