How to Achieve High-Purity Lithium Nitrate via Solvent Recrystallization
OCT 9, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Nitrate Purification Background and Objectives
Lithium nitrate (LiNO3) has emerged as a critical compound in various high-tech applications, particularly in energy storage systems, thermal energy storage, and advanced materials manufacturing. The historical development of lithium nitrate purification techniques dates back to the early 20th century, with significant advancements occurring in the post-World War II era as demand for high-purity lithium compounds increased for nuclear and later electronic applications.
The evolution of purification technologies has progressed from basic precipitation methods to more sophisticated approaches including ion exchange, membrane filtration, and various crystallization techniques. Among these, solvent recrystallization has shown particular promise due to its scalability and effectiveness in achieving high purity levels without introducing additional contaminants.
Current industry standards typically require lithium nitrate purity levels of 99.5% for general applications, while specialized sectors such as advanced battery technologies and pharmaceutical applications demand ultra-high purity exceeding 99.9%. These escalating purity requirements are driving innovation in purification methodologies, with solvent recrystallization emerging as a leading approach.
The technical objectives for high-purity lithium nitrate via solvent recrystallization encompass several key parameters: achieving consistent purity levels above 99.9%, minimizing process-induced contamination, optimizing solvent selection for maximum yield and purity, developing energy-efficient crystallization protocols, and establishing scalable processes suitable for industrial implementation.
Recent technological trends indicate a growing focus on green chemistry principles in purification processes, with emphasis on environmentally benign solvents, reduced energy consumption, and minimization of waste streams. Additionally, there is increasing interest in developing continuous flow processes for recrystallization to replace traditional batch methods, potentially offering advantages in consistency, throughput, and quality control.
The global push toward electrification and renewable energy storage has significantly accelerated research in this field, with particular attention to the role of high-purity lithium compounds in next-generation battery technologies. This trend is expected to continue, with projections suggesting a compound annual growth rate of 18-22% for high-purity lithium compounds over the next decade.
The technical evolution path forward likely involves integration of advanced analytical techniques for real-time purity monitoring, development of tailored solvent systems for specific impurity profiles, and implementation of machine learning approaches to optimize crystallization parameters based on feedstock variations and desired output specifications.
The evolution of purification technologies has progressed from basic precipitation methods to more sophisticated approaches including ion exchange, membrane filtration, and various crystallization techniques. Among these, solvent recrystallization has shown particular promise due to its scalability and effectiveness in achieving high purity levels without introducing additional contaminants.
Current industry standards typically require lithium nitrate purity levels of 99.5% for general applications, while specialized sectors such as advanced battery technologies and pharmaceutical applications demand ultra-high purity exceeding 99.9%. These escalating purity requirements are driving innovation in purification methodologies, with solvent recrystallization emerging as a leading approach.
The technical objectives for high-purity lithium nitrate via solvent recrystallization encompass several key parameters: achieving consistent purity levels above 99.9%, minimizing process-induced contamination, optimizing solvent selection for maximum yield and purity, developing energy-efficient crystallization protocols, and establishing scalable processes suitable for industrial implementation.
Recent technological trends indicate a growing focus on green chemistry principles in purification processes, with emphasis on environmentally benign solvents, reduced energy consumption, and minimization of waste streams. Additionally, there is increasing interest in developing continuous flow processes for recrystallization to replace traditional batch methods, potentially offering advantages in consistency, throughput, and quality control.
The global push toward electrification and renewable energy storage has significantly accelerated research in this field, with particular attention to the role of high-purity lithium compounds in next-generation battery technologies. This trend is expected to continue, with projections suggesting a compound annual growth rate of 18-22% for high-purity lithium compounds over the next decade.
The technical evolution path forward likely involves integration of advanced analytical techniques for real-time purity monitoring, development of tailored solvent systems for specific impurity profiles, and implementation of machine learning approaches to optimize crystallization parameters based on feedstock variations and desired output specifications.
Market Demand Analysis for High-Purity Lithium Nitrate
The global market for high-purity lithium nitrate has experienced significant growth in recent years, primarily driven by its expanding applications in energy storage systems, particularly molten salt thermal energy storage for concentrated solar power (CSP) plants. The demand for high-purity lithium nitrate (99.9% and above) has been increasing at a compound annual growth rate of approximately 8-10% since 2018, with the market value reaching several hundred million dollars globally.
The renewable energy sector represents the largest consumer of high-purity lithium nitrate, accounting for over 60% of total demand. Within this sector, CSP plants utilize lithium nitrate as a critical component in heat transfer fluids and thermal storage media due to its excellent thermal properties and stability at high temperatures. The global installed capacity of CSP plants has been growing steadily, creating sustained demand for high-purity lithium compounds.
Secondary markets for high-purity lithium nitrate include the pharmaceutical industry, where it serves as a precursor in drug synthesis, and the electronics sector, where it is used in specialized applications requiring high-purity lithium compounds. These sectors collectively represent about 25% of the total market demand but are growing at faster rates than the energy storage segment.
Geographically, the demand is concentrated in regions with significant investments in renewable energy infrastructure. Spain, the United States, China, and Middle Eastern countries lead in CSP installations and consequently in lithium nitrate consumption. China has emerged as both a major consumer and producer, with domestic demand growing at over 12% annually due to aggressive renewable energy targets.
Market analysis indicates that price sensitivity varies significantly across application segments. The energy storage sector demonstrates moderate price elasticity, as lithium nitrate represents a small but critical component of overall project costs. In contrast, pharmaceutical and electronic applications show lower price sensitivity due to the emphasis on purity and performance over cost considerations.
Supply chain dynamics reveal potential vulnerabilities, as lithium raw material availability continues to face constraints. The market currently experiences periodic supply-demand imbalances, which has led to price volatility. This situation underscores the importance of developing more efficient purification methods like advanced solvent recrystallization techniques to optimize resource utilization and ensure consistent supply of high-purity material.
Future market projections suggest continued strong growth through 2030, with emerging applications in next-generation batteries and advanced ceramics potentially creating new demand streams. The development of more cost-effective purification technologies could significantly impact market dynamics by lowering production costs and expanding the accessible market size.
The renewable energy sector represents the largest consumer of high-purity lithium nitrate, accounting for over 60% of total demand. Within this sector, CSP plants utilize lithium nitrate as a critical component in heat transfer fluids and thermal storage media due to its excellent thermal properties and stability at high temperatures. The global installed capacity of CSP plants has been growing steadily, creating sustained demand for high-purity lithium compounds.
Secondary markets for high-purity lithium nitrate include the pharmaceutical industry, where it serves as a precursor in drug synthesis, and the electronics sector, where it is used in specialized applications requiring high-purity lithium compounds. These sectors collectively represent about 25% of the total market demand but are growing at faster rates than the energy storage segment.
Geographically, the demand is concentrated in regions with significant investments in renewable energy infrastructure. Spain, the United States, China, and Middle Eastern countries lead in CSP installations and consequently in lithium nitrate consumption. China has emerged as both a major consumer and producer, with domestic demand growing at over 12% annually due to aggressive renewable energy targets.
Market analysis indicates that price sensitivity varies significantly across application segments. The energy storage sector demonstrates moderate price elasticity, as lithium nitrate represents a small but critical component of overall project costs. In contrast, pharmaceutical and electronic applications show lower price sensitivity due to the emphasis on purity and performance over cost considerations.
Supply chain dynamics reveal potential vulnerabilities, as lithium raw material availability continues to face constraints. The market currently experiences periodic supply-demand imbalances, which has led to price volatility. This situation underscores the importance of developing more efficient purification methods like advanced solvent recrystallization techniques to optimize resource utilization and ensure consistent supply of high-purity material.
Future market projections suggest continued strong growth through 2030, with emerging applications in next-generation batteries and advanced ceramics potentially creating new demand streams. The development of more cost-effective purification technologies could significantly impact market dynamics by lowering production costs and expanding the accessible market size.
Current Challenges in Solvent Recrystallization Techniques
Solvent recrystallization represents a critical purification technique for obtaining high-purity lithium nitrate, yet several significant challenges impede optimal implementation. The primary obstacle lies in solvent selection, as the ideal solvent must exhibit appropriate solubility differentials between lithium nitrate and impurities across temperature ranges. Many conventional solvents fail to provide sufficient separation efficiency, particularly when dealing with chemically similar impurities such as sodium or potassium nitrates.
Temperature control during crystallization presents another formidable challenge. The crystallization kinetics of lithium nitrate are highly sensitive to cooling rates and temperature gradients. Rapid cooling often leads to impurity entrapment within crystal structures, while excessively slow cooling reduces production efficiency. Industry reports indicate that temperature fluctuations as small as 2°C can significantly impact final product purity.
Impurity co-crystallization remains particularly problematic in lithium nitrate purification. Common contaminants including calcium, magnesium, and iron compounds demonstrate similar crystallization behaviors, making their complete separation challenging through conventional recrystallization alone. These impurities can form inclusion complexes or adhere to crystal surfaces, requiring multiple recrystallization cycles that diminish overall yield.
Scale-up difficulties present substantial barriers to industrial implementation. Laboratory-scale successes often fail to translate directly to production environments due to heat transfer limitations, mixing inefficiencies, and altered crystallization dynamics in larger vessels. The economic viability of multi-stage recrystallization processes is frequently compromised by yield losses exceeding 15-20% per cycle.
Filtration and washing procedures introduce additional complexities. The morphology of lithium nitrate crystals—often forming fine, needle-like structures—creates filtration challenges and increases the risk of product loss. Washing steps must be precisely calibrated to remove mother liquor without redissolving significant amounts of product.
Environmental and safety concerns further complicate solvent selection. Many effective organic solvents pose health and environmental hazards, while more benign alternatives often deliver inferior separation performance. Regulatory pressures increasingly restrict the use of certain solvents, necessitating compromises between purification efficiency and sustainability considerations.
Recent research has highlighted the challenge of controlling crystal habit and size distribution. Inconsistent crystal morphology affects not only filtration efficiency but also the propensity for impurity inclusion. Advanced techniques such as seeding, ultrasonic assistance, and anti-solvent addition show promise but introduce additional process variables requiring precise control.
Temperature control during crystallization presents another formidable challenge. The crystallization kinetics of lithium nitrate are highly sensitive to cooling rates and temperature gradients. Rapid cooling often leads to impurity entrapment within crystal structures, while excessively slow cooling reduces production efficiency. Industry reports indicate that temperature fluctuations as small as 2°C can significantly impact final product purity.
Impurity co-crystallization remains particularly problematic in lithium nitrate purification. Common contaminants including calcium, magnesium, and iron compounds demonstrate similar crystallization behaviors, making their complete separation challenging through conventional recrystallization alone. These impurities can form inclusion complexes or adhere to crystal surfaces, requiring multiple recrystallization cycles that diminish overall yield.
Scale-up difficulties present substantial barriers to industrial implementation. Laboratory-scale successes often fail to translate directly to production environments due to heat transfer limitations, mixing inefficiencies, and altered crystallization dynamics in larger vessels. The economic viability of multi-stage recrystallization processes is frequently compromised by yield losses exceeding 15-20% per cycle.
Filtration and washing procedures introduce additional complexities. The morphology of lithium nitrate crystals—often forming fine, needle-like structures—creates filtration challenges and increases the risk of product loss. Washing steps must be precisely calibrated to remove mother liquor without redissolving significant amounts of product.
Environmental and safety concerns further complicate solvent selection. Many effective organic solvents pose health and environmental hazards, while more benign alternatives often deliver inferior separation performance. Regulatory pressures increasingly restrict the use of certain solvents, necessitating compromises between purification efficiency and sustainability considerations.
Recent research has highlighted the challenge of controlling crystal habit and size distribution. Inconsistent crystal morphology affects not only filtration efficiency but also the propensity for impurity inclusion. Advanced techniques such as seeding, ultrasonic assistance, and anti-solvent addition show promise but introduce additional process variables requiring precise control.
Current Solvent Recrystallization Methodologies
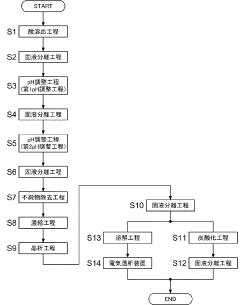
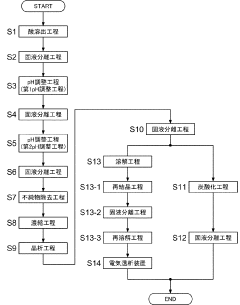
01 Purification methods for lithium nitrate
Various methods are employed to purify lithium nitrate to achieve high purity levels. These methods include recrystallization, solvent extraction, and ion exchange techniques. The purification processes remove impurities such as other metal ions, sulfates, and chlorides that can affect the performance of lithium nitrate in its applications. Advanced purification techniques can achieve purity levels exceeding 99.9%, which is crucial for specialized applications.- Purification methods for lithium nitrate: Various methods are employed to purify lithium nitrate to achieve high purity levels. These include recrystallization techniques, solvent extraction, and chemical precipitation processes. Advanced purification methods can remove impurities such as sodium, potassium, calcium, and magnesium ions, resulting in lithium nitrate with purity levels exceeding 99.9%. These purification processes are critical for applications requiring high-purity lithium nitrate.
- High-purity lithium nitrate for battery applications: High-purity lithium nitrate is essential for advanced battery technologies, particularly for lithium-ion and solid-state batteries. The presence of impurities can significantly impact battery performance, cycle life, and safety. Battery-grade lithium nitrate typically requires purity levels of 99.95% or higher, with strict limits on metallic impurities and moisture content. Purification processes specifically designed for battery applications focus on removing contaminants that could interfere with electrochemical reactions.
- Analytical methods for determining lithium nitrate purity: Various analytical techniques are used to determine the purity of lithium nitrate and identify impurities. These include inductively coupled plasma mass spectrometry (ICP-MS), atomic absorption spectroscopy, ion chromatography, and thermal analysis methods. These techniques can detect impurities at parts-per-million or even parts-per-billion levels, allowing for precise quality control of lithium nitrate. Standard testing protocols have been developed to ensure consistent purity assessment across different manufacturing batches.
- Industrial production of high-purity lithium nitrate: Industrial-scale production of high-purity lithium nitrate involves specialized equipment and controlled processes to maintain quality. Manufacturing methods include the reaction of lithium carbonate or hydroxide with nitric acid, followed by multiple purification steps. Advanced production techniques incorporate continuous monitoring systems, automated process controls, and clean room environments to prevent contamination. These industrial processes can consistently produce lithium nitrate with purity levels suitable for demanding applications in electronics, pharmaceuticals, and energy storage.
- Applications requiring specific lithium nitrate purity levels: Different applications require specific purity levels of lithium nitrate. For thermal energy storage systems, lithium nitrate with moderate purity (99-99.5%) may be sufficient, while pharmaceutical and electronic applications often require ultra-high purity (>99.99%). In catalytic applications, certain trace elements may be tolerated or even beneficial, while others must be strictly limited. The relationship between lithium nitrate purity and performance characteristics in various applications has been extensively studied, allowing for optimization of purity specifications based on end-use requirements.
02 High-purity lithium nitrate for battery applications
High-purity lithium nitrate is essential for lithium-ion battery applications, particularly as an electrolyte additive. The presence of impurities can significantly impact battery performance, safety, and longevity. Battery-grade lithium nitrate typically requires purity levels of 99.5% or higher, with strict limits on specific impurities such as sodium, potassium, calcium, and heavy metals. Purification processes for battery applications focus on removing these critical impurities to enhance the solid electrolyte interphase formation and improve battery cycling stability.Expand Specific Solutions03 Analytical methods for determining lithium nitrate purity
Various analytical techniques are used to determine the purity of lithium nitrate and identify impurities. These methods include inductively coupled plasma mass spectrometry (ICP-MS), atomic absorption spectroscopy, ion chromatography, and thermal analysis. These techniques can detect impurities at parts per million or even parts per billion levels, allowing for precise quality control of lithium nitrate products. Standard testing protocols have been developed to ensure consistent purity assessment across different batches and manufacturers.Expand Specific Solutions04 Industrial production of high-purity lithium nitrate
Industrial processes for producing high-purity lithium nitrate involve multiple stages of purification and quality control. Starting materials such as lithium carbonate or lithium hydroxide are reacted with nitric acid, followed by concentration, crystallization, and drying steps. Advanced manufacturing techniques incorporate continuous monitoring and process controls to maintain consistent purity levels. Some processes include specialized steps to remove specific impurities that are problematic for particular applications, such as transition metals for battery applications or calcium for heat transfer fluids.Expand Specific Solutions05 Applications requiring specific lithium nitrate purity levels
Different applications of lithium nitrate require specific purity levels. For thermal energy storage systems, lithium nitrate with purity above 99% is typically required to ensure optimal heat transfer properties and prevent corrosion. In pharmaceutical and food applications, ultra-high purity lithium nitrate meeting USP or food-grade standards is necessary. For catalytic applications, specific impurity profiles may be tolerated or even beneficial, while maintaining overall high purity. The relationship between purity specifications and performance characteristics is well-documented for various industrial applications.Expand Specific Solutions
Key Industry Players in High-Purity Chemical Production
The high-purity lithium nitrate via solvent recrystallization market is in a growth phase, driven by increasing demand for advanced battery technologies and energy storage solutions. The global market size is expanding rapidly, projected to reach significant volumes as lithium-based technologies become more prevalent in renewable energy applications. Technologically, the field shows moderate maturity with established processes, but innovation continues for higher purity and efficiency. Key players demonstrate varying levels of technological advancement: Albemarle and Ganfeng Lithium lead with comprehensive lithium processing capabilities; SQM brings extensive experience in lithium extraction; while companies like AGC and BASF contribute advanced chemical processing expertise. Research institutions such as Qinghai Institute of Salt Lakes and Purdue Research Foundation are driving next-generation purification techniques, indicating ongoing evolution in this specialized chemical processing domain.
Albemarle Corp.
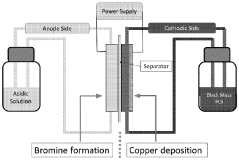
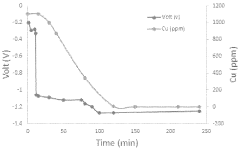
Technical Solution: Albemarle has developed an advanced solvent recrystallization process for high-purity lithium nitrate production that achieves 99.9% purity levels. Their proprietary method involves a multi-stage crystallization process using carefully selected organic solvents with specific polarity characteristics. The process begins with crude lithium nitrate dissolved in a primary solvent at elevated temperatures (60-80°C), followed by controlled cooling crystallization with precise temperature ramping rates (0.5-1°C/min). Albemarle employs counter-current washing techniques with secondary solvents to remove metal impurities, particularly sodium, calcium, and magnesium ions. Their system incorporates in-line monitoring with spectroscopic analysis to ensure consistent crystal formation and purity levels. The final product undergoes vacuum drying at moderate temperatures (80-100°C) to prevent thermal decomposition while ensuring complete solvent removal.
Strengths: Superior impurity removal capability, particularly for alkali metal contaminants; highly scalable process suitable for industrial production; consistent batch-to-batch quality with >99.9% purity. Weaknesses: Higher production costs due to solvent recovery systems and energy requirements; process requires precise temperature control that can be challenging to maintain at scale.
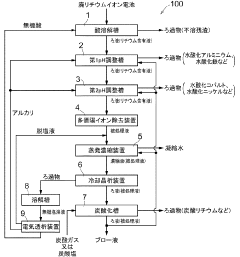
Qinghai Institute of Salt Lakes, Chinese Academy of Sciences
Technical Solution: The Qinghai Institute has developed an innovative solvent recrystallization process specifically designed for salt lake-derived lithium resources. Their approach utilizes a dual-solvent system with water as the primary solvent and carefully selected organic co-solvents that enhance selectivity for lithium nitrate crystallization. The process begins with a pre-concentration step using solar evaporation, followed by selective precipitation of magnesium and calcium impurities. The recrystallization occurs in a temperature-controlled environment (55-75°C) with precise cooling rates tailored to the specific impurity profile of Qinghai salt lake brines. Their technology incorporates a novel impurity complexation step where specific organic agents bind to metal contaminants, preventing their co-crystallization with lithium nitrate. The institute has also developed specialized washing protocols using temperature-gradient techniques that minimize product loss while maximizing impurity removal. Their process achieves lithium nitrate with >99.5% purity while maintaining high yields (>90%) from salt lake resources that traditionally present purification challenges.
Strengths: Specifically optimized for salt lake resources with high magnesium content; lower production costs through integration with existing salt lake operations; environmentally sustainable approach with reduced water consumption. Weaknesses: Process efficiency varies with seasonal changes in brine composition; requires specialized knowledge of salt lake chemistry; limited scalability compared to some commercial processes.
Critical Patents and Literature on Recrystallization Processes
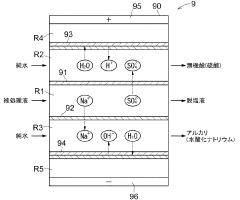
Lithium recovery method
PatentActiveJP2020132952A
Innovation
- A method involving concentration, solid-liquid separation, carbonation, and electrodialysis steps to separate and recover high-purity lithium carbonate, including impurity removal and recycling of inorganic salts and acids.
Precursor preparation from recycled rechargeable batteries
PatentWO2024057307A1
Innovation
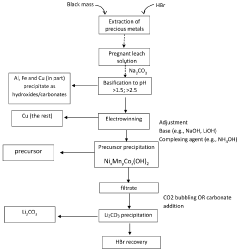
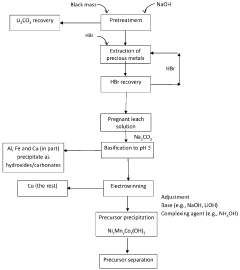
- A process involving acidic leaching of lithium-ion battery electrodes to create a pregnant leach solution, followed by post-leaching purification steps to remove aluminum, iron, and copper, and subsequent coprecipitation of nickel, manganese, and cobalt in a controlled pH environment to produce high-purity mixed metal oxides or hydroxides/carbonates with a predetermined molar ratio, suitable for cathode material production.
Environmental Impact Assessment of Purification Processes
The environmental impact of lithium nitrate purification processes, particularly solvent recrystallization, requires comprehensive assessment to ensure sustainable production practices. Traditional purification methods often involve significant water consumption, energy usage, and chemical waste generation that can adversely affect ecosystems and contribute to resource depletion.
Solvent recrystallization techniques for lithium nitrate purification present both environmental challenges and opportunities. The primary environmental concerns include volatile organic compound (VOC) emissions from solvents, wastewater contamination with dissolved lithium compounds, and energy-intensive heating and cooling cycles. Studies indicate that conventional recrystallization processes can generate approximately 10-15 liters of wastewater per kilogram of high-purity lithium nitrate produced.
Recent advancements in green chemistry approaches have demonstrated potential for reducing these impacts. Closed-loop solvent recovery systems can achieve 85-95% solvent recycling rates, significantly reducing fresh solvent requirements and waste generation. Implementation of such systems in industrial settings has shown reduction in VOC emissions by up to 70% compared to traditional open systems.
Energy consumption represents another significant environmental factor. Temperature-controlled crystallization processes typically require 2.5-4.0 kWh of energy per kilogram of purified product. Integration of heat recovery systems and optimization of crystallization parameters can reduce this energy footprint by 30-40%, with corresponding reductions in greenhouse gas emissions.
Water usage efficiency has improved through implementation of counter-current washing techniques and advanced filtration systems. These innovations have demonstrated potential to reduce freshwater consumption by up to 60% while maintaining product purity specifications above 99.5%.
Life cycle assessment (LCA) studies comparing various purification routes indicate that solvent selection plays a critical role in overall environmental impact. Bio-derived solvents show promise in reducing carbon footprint by 25-35% compared to petroleum-based alternatives, though challenges remain regarding their production scale and economic viability.
Waste management strategies for spent solutions containing lithium compounds have evolved toward recovery and valorization approaches. Advanced membrane separation and selective precipitation techniques enable recovery of 85-95% of lithium from waste streams, transforming what was previously considered waste into valuable secondary raw materials.
Regulatory frameworks increasingly emphasize environmental performance metrics for chemical manufacturing processes. Companies implementing best available techniques (BAT) for lithium nitrate purification can achieve compliance while simultaneously improving operational efficiency and reducing long-term environmental liabilities.
Solvent recrystallization techniques for lithium nitrate purification present both environmental challenges and opportunities. The primary environmental concerns include volatile organic compound (VOC) emissions from solvents, wastewater contamination with dissolved lithium compounds, and energy-intensive heating and cooling cycles. Studies indicate that conventional recrystallization processes can generate approximately 10-15 liters of wastewater per kilogram of high-purity lithium nitrate produced.
Recent advancements in green chemistry approaches have demonstrated potential for reducing these impacts. Closed-loop solvent recovery systems can achieve 85-95% solvent recycling rates, significantly reducing fresh solvent requirements and waste generation. Implementation of such systems in industrial settings has shown reduction in VOC emissions by up to 70% compared to traditional open systems.
Energy consumption represents another significant environmental factor. Temperature-controlled crystallization processes typically require 2.5-4.0 kWh of energy per kilogram of purified product. Integration of heat recovery systems and optimization of crystallization parameters can reduce this energy footprint by 30-40%, with corresponding reductions in greenhouse gas emissions.
Water usage efficiency has improved through implementation of counter-current washing techniques and advanced filtration systems. These innovations have demonstrated potential to reduce freshwater consumption by up to 60% while maintaining product purity specifications above 99.5%.
Life cycle assessment (LCA) studies comparing various purification routes indicate that solvent selection plays a critical role in overall environmental impact. Bio-derived solvents show promise in reducing carbon footprint by 25-35% compared to petroleum-based alternatives, though challenges remain regarding their production scale and economic viability.
Waste management strategies for spent solutions containing lithium compounds have evolved toward recovery and valorization approaches. Advanced membrane separation and selective precipitation techniques enable recovery of 85-95% of lithium from waste streams, transforming what was previously considered waste into valuable secondary raw materials.
Regulatory frameworks increasingly emphasize environmental performance metrics for chemical manufacturing processes. Companies implementing best available techniques (BAT) for lithium nitrate purification can achieve compliance while simultaneously improving operational efficiency and reducing long-term environmental liabilities.
Quality Control and Analytical Methods for Purity Verification
Quality control and analytical methods are critical components in the production of high-purity lithium nitrate via solvent recrystallization. The verification of product purity requires a comprehensive suite of analytical techniques that can detect impurities at trace levels and confirm the chemical composition of the final product.
X-ray diffraction (XRD) serves as a primary method for crystalline phase identification, enabling researchers to confirm the crystal structure of lithium nitrate and detect any crystalline impurities. This technique provides valuable information about the lattice parameters and phase purity, which directly correlates with the overall quality of the recrystallized product.
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Atomic Absorption Spectroscopy (AAS) are essential for quantifying metallic impurities at parts-per-million (ppm) or parts-per-billion (ppb) levels. These techniques can detect trace amounts of sodium, potassium, calcium, and other metal contaminants that might compromise the performance of lithium nitrate in battery applications or other industrial uses.
Ion chromatography plays a crucial role in analyzing anionic impurities, particularly sulfates, chlorides, and carbonates that may remain after the recrystallization process. The presence of these impurities can significantly affect the electrochemical properties of lithium nitrate when used in battery applications.
Thermal analysis techniques, including Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA), provide insights into the thermal stability and water content of the lithium nitrate product. These parameters are particularly important for applications requiring high thermal stability or anhydrous conditions.
Spectroscopic methods such as Fourier Transform Infrared Spectroscopy (FTIR) and Raman spectroscopy offer complementary information about molecular structure and potential organic impurities introduced during the recrystallization process. These techniques can detect solvent residues that might remain trapped within the crystal structure.
Statistical quality control methods, including Six Sigma and Statistical Process Control (SPC), should be implemented to monitor the consistency of the recrystallization process. These approaches involve establishing control charts for critical parameters such as crystal size distribution, impurity levels, and yield, enabling early detection of process deviations.
In-process testing during various stages of recrystallization helps identify potential issues before they affect the final product quality. This includes monitoring solution concentration, temperature profiles, cooling rates, and filtration efficiency, all of which can impact the purity of the final lithium nitrate crystals.
X-ray diffraction (XRD) serves as a primary method for crystalline phase identification, enabling researchers to confirm the crystal structure of lithium nitrate and detect any crystalline impurities. This technique provides valuable information about the lattice parameters and phase purity, which directly correlates with the overall quality of the recrystallized product.
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Atomic Absorption Spectroscopy (AAS) are essential for quantifying metallic impurities at parts-per-million (ppm) or parts-per-billion (ppb) levels. These techniques can detect trace amounts of sodium, potassium, calcium, and other metal contaminants that might compromise the performance of lithium nitrate in battery applications or other industrial uses.
Ion chromatography plays a crucial role in analyzing anionic impurities, particularly sulfates, chlorides, and carbonates that may remain after the recrystallization process. The presence of these impurities can significantly affect the electrochemical properties of lithium nitrate when used in battery applications.
Thermal analysis techniques, including Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA), provide insights into the thermal stability and water content of the lithium nitrate product. These parameters are particularly important for applications requiring high thermal stability or anhydrous conditions.
Spectroscopic methods such as Fourier Transform Infrared Spectroscopy (FTIR) and Raman spectroscopy offer complementary information about molecular structure and potential organic impurities introduced during the recrystallization process. These techniques can detect solvent residues that might remain trapped within the crystal structure.
Statistical quality control methods, including Six Sigma and Statistical Process Control (SPC), should be implemented to monitor the consistency of the recrystallization process. These approaches involve establishing control charts for critical parameters such as crystal size distribution, impurity levels, and yield, enabling early detection of process deviations.
In-process testing during various stages of recrystallization helps identify potential issues before they affect the final product quality. This includes monitoring solution concentration, temperature profiles, cooling rates, and filtration efficiency, all of which can impact the purity of the final lithium nitrate crystals.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!