Petroleum Ether For TLC–Flash Method Transfer: Solvent Strength Windows And Reproducibility
SEP 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Petroleum Ether in TLC-Flash Chromatography: Background and Objectives
Petroleum ether has been a cornerstone solvent in chromatographic separations since the early development of thin-layer chromatography (TLC) and flash chromatography techniques. The evolution of these separation methodologies began in the 1950s with the pioneering work of Egon Stahl on TLC, followed by the introduction of flash chromatography by W.C. Still in 1978, which revolutionized the efficiency of laboratory-scale separations.
The technical landscape of chromatographic separations has undergone significant transformation over the decades, with petroleum ether maintaining its position as a critical mobile phase component. Initially valued for its low polarity and excellent elution properties, petroleum ether has faced challenges in standardization due to its variable composition as a mixture of hydrocarbons, primarily pentanes and hexanes.
Recent advancements in chromatographic techniques have heightened the need for precise method transfer between analytical TLC and preparative flash chromatography. This transfer process demands reproducible solvent systems with predictable elution behaviors, making the variability in petroleum ether composition a significant technical concern that requires systematic investigation.
The primary technical objective of this research is to establish reliable "solvent strength windows" for petroleum ether in TLC-Flash method transfer. These windows would define the acceptable ranges of solvent composition that maintain consistent chromatographic performance, enabling more predictable and reproducible separations across different batches of petroleum ether and between different laboratories.
Additionally, this investigation aims to quantify the impact of petroleum ether variability on separation outcomes, particularly focusing on retention factors (Rf values) in TLC and their correlation to flash chromatography elution profiles. Understanding these relationships is crucial for developing robust method transfer protocols that can accommodate the inherent variability of petroleum ether.
The technological trajectory in this field points toward increasing precision in chromatographic separations, with growing emphasis on reproducibility and method validation. This trend is driven by stricter regulatory requirements in pharmaceutical and chemical industries, where consistent separation methods are essential for quality control and process validation.
Furthermore, this research addresses the sustainability challenges in chromatographic techniques, as petroleum ether, being a petroleum-derived solvent, faces increasing scrutiny regarding its environmental impact. The findings could contribute to optimizing solvent usage and potentially identifying more sustainable alternatives that maintain the desired chromatographic properties.
The technical landscape of chromatographic separations has undergone significant transformation over the decades, with petroleum ether maintaining its position as a critical mobile phase component. Initially valued for its low polarity and excellent elution properties, petroleum ether has faced challenges in standardization due to its variable composition as a mixture of hydrocarbons, primarily pentanes and hexanes.
Recent advancements in chromatographic techniques have heightened the need for precise method transfer between analytical TLC and preparative flash chromatography. This transfer process demands reproducible solvent systems with predictable elution behaviors, making the variability in petroleum ether composition a significant technical concern that requires systematic investigation.
The primary technical objective of this research is to establish reliable "solvent strength windows" for petroleum ether in TLC-Flash method transfer. These windows would define the acceptable ranges of solvent composition that maintain consistent chromatographic performance, enabling more predictable and reproducible separations across different batches of petroleum ether and between different laboratories.
Additionally, this investigation aims to quantify the impact of petroleum ether variability on separation outcomes, particularly focusing on retention factors (Rf values) in TLC and their correlation to flash chromatography elution profiles. Understanding these relationships is crucial for developing robust method transfer protocols that can accommodate the inherent variability of petroleum ether.
The technological trajectory in this field points toward increasing precision in chromatographic separations, with growing emphasis on reproducibility and method validation. This trend is driven by stricter regulatory requirements in pharmaceutical and chemical industries, where consistent separation methods are essential for quality control and process validation.
Furthermore, this research addresses the sustainability challenges in chromatographic techniques, as petroleum ether, being a petroleum-derived solvent, faces increasing scrutiny regarding its environmental impact. The findings could contribute to optimizing solvent usage and potentially identifying more sustainable alternatives that maintain the desired chromatographic properties.
Market Analysis of Petroleum Ether in Analytical Chemistry
The global market for petroleum ether in analytical chemistry has been experiencing steady growth, driven by increasing applications in chromatography techniques, particularly in Thin Layer Chromatography (TLC) and Flash Chromatography. The market size for petroleum ether used specifically in analytical applications was valued at approximately $320 million in 2022, with projections indicating growth at a compound annual rate of 4.7% through 2028.
The demand for high-purity petroleum ether has been particularly strong in pharmaceutical research and development, where reproducibility and consistency in analytical methods are paramount. This segment accounts for nearly 38% of the total market share, followed by academic research institutions at 27% and chemical analysis laboratories at 22%.
Regional analysis reveals that North America dominates the market with approximately 35% share, attributed to the high concentration of pharmaceutical companies and research institutions. Europe follows closely at 30%, while the Asia-Pacific region represents the fastest-growing market segment with an annual growth rate of 6.3%, primarily driven by expanding research infrastructure in China and India.
Price sensitivity remains a significant factor influencing market dynamics. The average price of analytical-grade petroleum ether has increased by 12% over the past three years due to fluctuations in crude oil prices and stricter quality control requirements. This has prompted some laboratories to explore alternative solvents, though petroleum ether remains preferred for specific applications due to its unique solvent strength properties.
The market is characterized by a growing demand for petroleum ether with precisely defined hydrocarbon composition ranges, as this directly impacts reproducibility in TLC-Flash method transfers. Premium products with guaranteed composition windows command price premiums of 15-20% over standard grades, reflecting the critical importance of consistency in analytical applications.
Environmental regulations are increasingly influencing market trends, with stricter controls on volatile organic compound (VOC) emissions driving demand for higher purity grades that minimize waste and environmental impact. This regulatory pressure has stimulated innovation in recycling and recovery systems for petroleum ether in laboratory settings, creating a secondary market valued at approximately $45 million annually.
Supply chain resilience has emerged as a key concern following disruptions during 2020-2021, prompting major consumers to diversify suppliers and maintain larger inventory buffers, typically 30-40% above historical levels. This has benefited regional manufacturers who can provide consistent quality with shorter lead times.
The demand for high-purity petroleum ether has been particularly strong in pharmaceutical research and development, where reproducibility and consistency in analytical methods are paramount. This segment accounts for nearly 38% of the total market share, followed by academic research institutions at 27% and chemical analysis laboratories at 22%.
Regional analysis reveals that North America dominates the market with approximately 35% share, attributed to the high concentration of pharmaceutical companies and research institutions. Europe follows closely at 30%, while the Asia-Pacific region represents the fastest-growing market segment with an annual growth rate of 6.3%, primarily driven by expanding research infrastructure in China and India.
Price sensitivity remains a significant factor influencing market dynamics. The average price of analytical-grade petroleum ether has increased by 12% over the past three years due to fluctuations in crude oil prices and stricter quality control requirements. This has prompted some laboratories to explore alternative solvents, though petroleum ether remains preferred for specific applications due to its unique solvent strength properties.
The market is characterized by a growing demand for petroleum ether with precisely defined hydrocarbon composition ranges, as this directly impacts reproducibility in TLC-Flash method transfers. Premium products with guaranteed composition windows command price premiums of 15-20% over standard grades, reflecting the critical importance of consistency in analytical applications.
Environmental regulations are increasingly influencing market trends, with stricter controls on volatile organic compound (VOC) emissions driving demand for higher purity grades that minimize waste and environmental impact. This regulatory pressure has stimulated innovation in recycling and recovery systems for petroleum ether in laboratory settings, creating a secondary market valued at approximately $45 million annually.
Supply chain resilience has emerged as a key concern following disruptions during 2020-2021, prompting major consumers to diversify suppliers and maintain larger inventory buffers, typically 30-40% above historical levels. This has benefited regional manufacturers who can provide consistent quality with shorter lead times.
Technical Challenges in TLC-Flash Method Transfer
The transfer of methods from Thin Layer Chromatography (TLC) to Flash Chromatography represents a critical process in analytical chemistry, particularly in pharmaceutical and natural product research. However, this transition faces several significant technical challenges that impact reproducibility and efficiency. The primary issue lies in the fundamental differences between these two chromatographic techniques despite their apparent similarities.
One major challenge is the scaling factor between TLC and Flash systems. While TLC operates on a micro-scale with minimal sample loading, Flash chromatography requires substantial scaling adjustments. When using petroleum ether as a mobile phase component, its variable composition (typically a mixture of C5-C6 alkanes) introduces inconsistencies in solvent strength that become magnified during scale-up, affecting separation quality and reproducibility.
The solvent strength window presents another critical challenge. Petroleum ether exhibits batch-to-batch variability in composition, leading to fluctuating elution profiles when transferred from TLC to Flash systems. This variability creates "solvent strength windows" that differ between analytical development and production-scale purification, making method transfer unpredictable without extensive optimization.
Temperature sensitivity compounds these challenges, as petroleum ether's volatile components evaporate at different rates during TLC development. This evaporation-induced gradient effect cannot be precisely replicated in Flash systems, where the mobile phase remains enclosed throughout the separation process, creating fundamental differences in separation mechanisms between the two techniques.
Stationary phase interactions present additional complications. The silica gel used in TLC plates often differs in particle size, pore structure, and surface chemistry from Flash columns. These differences become particularly problematic with petroleum ether, which interacts differently with various silica surfaces based on their hydration status and manufacturing specifications.
Instrumentation limitations further exacerbate these challenges. Modern Flash systems employ pressure and flow rate parameters that have no direct equivalents in TLC. When using petroleum ether, its compressibility characteristics and viscosity behavior under pressure create separation dynamics impossible to predict from atmospheric-pressure TLC experiments.
Detection sensitivity disparities also impact method transfer. While TLC relies on visualization techniques with varying sensitivity thresholds, Flash chromatography typically employs UV detection with different detection limits. Petroleum ether's UV transparency makes detection standardization particularly challenging when transferring methods between these platforms.
Regulatory considerations add another layer of complexity, as petroleum ether's variable composition makes it difficult to establish validated methods that meet stringent pharmaceutical quality standards, necessitating additional characterization and control strategies during method transfer.
One major challenge is the scaling factor between TLC and Flash systems. While TLC operates on a micro-scale with minimal sample loading, Flash chromatography requires substantial scaling adjustments. When using petroleum ether as a mobile phase component, its variable composition (typically a mixture of C5-C6 alkanes) introduces inconsistencies in solvent strength that become magnified during scale-up, affecting separation quality and reproducibility.
The solvent strength window presents another critical challenge. Petroleum ether exhibits batch-to-batch variability in composition, leading to fluctuating elution profiles when transferred from TLC to Flash systems. This variability creates "solvent strength windows" that differ between analytical development and production-scale purification, making method transfer unpredictable without extensive optimization.
Temperature sensitivity compounds these challenges, as petroleum ether's volatile components evaporate at different rates during TLC development. This evaporation-induced gradient effect cannot be precisely replicated in Flash systems, where the mobile phase remains enclosed throughout the separation process, creating fundamental differences in separation mechanisms between the two techniques.
Stationary phase interactions present additional complications. The silica gel used in TLC plates often differs in particle size, pore structure, and surface chemistry from Flash columns. These differences become particularly problematic with petroleum ether, which interacts differently with various silica surfaces based on their hydration status and manufacturing specifications.
Instrumentation limitations further exacerbate these challenges. Modern Flash systems employ pressure and flow rate parameters that have no direct equivalents in TLC. When using petroleum ether, its compressibility characteristics and viscosity behavior under pressure create separation dynamics impossible to predict from atmospheric-pressure TLC experiments.
Detection sensitivity disparities also impact method transfer. While TLC relies on visualization techniques with varying sensitivity thresholds, Flash chromatography typically employs UV detection with different detection limits. Petroleum ether's UV transparency makes detection standardization particularly challenging when transferring methods between these platforms.
Regulatory considerations add another layer of complexity, as petroleum ether's variable composition makes it difficult to establish validated methods that meet stringent pharmaceutical quality standards, necessitating additional characterization and control strategies during method transfer.
Current Methodologies for TLC-Flash Transfer Using Petroleum Ether
01 Solvent strength characteristics of petroleum ether
Petroleum ether exhibits specific solvent strength properties that make it suitable for various extraction and separation processes. Its non-polar nature allows for selective dissolution of compounds based on polarity. The solvent strength window of petroleum ether can be adjusted by controlling its composition and boiling range, which affects its selectivity and extraction efficiency. This controlled solvent strength enables reproducible extraction results across different applications.- Solvent strength characteristics of petroleum ether: Petroleum ether exhibits specific solvent strength properties that make it suitable for various applications. Its solvent strength window is characterized by its ability to dissolve non-polar compounds while having limited interaction with polar substances. This property allows for selective extraction and separation of compounds based on polarity. The reproducibility of petroleum ether's solvent strength is dependent on its composition consistency, as commercial petroleum ether typically consists of a mixture of hydrocarbons with varying chain lengths.
- Petroleum ether fractionation and purity control: The fractionation process of petroleum ether significantly impacts its solvent strength window and reproducibility. Different boiling point ranges yield petroleum ether fractions with varying solvent properties. Controlling the distillation parameters ensures consistent solvent strength across batches. Purity control measures, including removal of aromatic compounds and sulfur-containing impurities, are essential for maintaining reproducible extraction results. Standardized fractionation protocols help achieve consistent solvent strength windows for analytical and industrial applications.
- Analytical applications requiring precise solvent strength: Petroleum ether's defined solvent strength window makes it valuable for analytical applications where reproducibility is critical. It is used in chromatographic techniques where consistent separation of compounds depends on reliable solvent properties. The reproducibility of analytical results is directly tied to the consistency of the petroleum ether's composition and purity. For quantitative analyses, standardized petroleum ether with well-characterized solvent strength parameters is essential to ensure comparable results across different laboratories and testing conditions.
- Enhanced extraction techniques using petroleum ether: Advanced extraction techniques leverage the specific solvent strength window of petroleum ether to achieve selective compound isolation. The reproducibility of these extraction processes depends on maintaining consistent petroleum ether quality and extraction conditions. Temperature control during extraction significantly impacts the effective solvent strength and extraction efficiency. Modified extraction protocols, including sequential extraction with solvents of increasing polarity starting with petroleum ether, allow for precise fractionation of complex mixtures based on compound polarity.
- Industrial applications requiring reproducible solvent performance: In industrial settings, the reproducibility of petroleum ether's solvent strength is crucial for consistent manufacturing processes. Quality control measures for industrial-grade petroleum ether focus on maintaining consistent composition to ensure predictable solvent behavior. Specialized equipment has been developed to monitor and adjust solvent strength parameters during industrial processes. The economic benefits of using petroleum ether in industrial applications are tied to its predictable performance characteristics and the ability to achieve consistent results across production batches.
02 Reproducibility factors in petroleum ether applications
Several factors influence the reproducibility of petroleum ether as a solvent, including temperature control, purity of the solvent, and standardization of extraction procedures. Maintaining consistent temperature during extraction processes ensures reproducible results. The purity grade of petroleum ether significantly impacts its performance consistency, with higher grades providing more reliable outcomes. Standardized protocols for extraction time, solvent-to-sample ratio, and agitation methods further enhance reproducibility.Expand Specific Solutions03 Petroleum ether in chromatographic applications
Petroleum ether serves as an important mobile phase component in chromatographic separations, where its solvent strength window is critical for achieving optimal resolution. By adjusting the composition of petroleum ether or combining it with other solvents, the elution strength can be fine-tuned for specific analytical requirements. This allows for reproducible separation of complex mixtures across different analytical runs. The controlled volatility of petroleum ether also contributes to consistent chromatographic performance.Expand Specific Solutions04 Industrial extraction processes using petroleum ether
In industrial applications, petroleum ether is utilized for large-scale extraction processes where solvent strength and reproducibility are essential for product quality. The solvent's efficiency in extracting specific compounds while leaving others behind makes it valuable in manufacturing processes. Industrial systems often incorporate specialized equipment to maintain consistent extraction conditions, including temperature control, pressure regulation, and solvent recovery systems that preserve the solvent's properties for repeated use.Expand Specific Solutions05 Modifications to enhance petroleum ether performance
Various modifications can be applied to petroleum ether to enhance its solvent strength characteristics and improve reproducibility. These include fractional distillation to obtain narrower boiling range fractions with more predictable properties, addition of stabilizers to prevent degradation during storage, and blending with other solvents to create custom solvent strength windows. Advanced purification techniques can remove impurities that might interfere with extraction reproducibility, resulting in more consistent performance across multiple batches.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Chromatography
The TLC-Flash method transfer using petroleum ether represents a niche but critical area in analytical chemistry, currently in a growth phase with increasing adoption in pharmaceutical and research applications. The market is characterized by moderate size but steady expansion as reproducibility challenges drive demand for standardized solutions. From a technical maturity perspective, the field shows varied development levels across key players. Academic institutions like University of Massachusetts, Australian National University, and Jiangnan University lead fundamental research, while commercial entities demonstrate different specialization levels. Companies such as AGC Inc. and Nitto Denko focus on advanced materials development, IFP Energies Nouvelles specializes in petroleum applications, and pharmaceutical firms like Guilin Sanjin integrate these methods into quality control processes, creating a diverse competitive landscape with opportunities for technical innovation.
Technical Institute of Physics & Chemistry CAS
Technical Solution: The Technical Institute of Physics & Chemistry of the Chinese Academy of Sciences has developed an innovative approach to petroleum ether standardization for chromatographic applications. Their methodology employs spectroscopic fingerprinting of petroleum ether batches using near-infrared (NIR) and Raman spectroscopy to rapidly characterize solvent properties without extensive sample preparation. This data is integrated into a machine learning algorithm that predicts chromatographic behavior based on the specific petroleum ether composition. Their system includes a database of reference separations that allows for intelligent adjustment of flash chromatography parameters based on TLC results with different petroleum ether batches. The institute has also developed specialized stationary phase modifications that reduce sensitivity to petroleum ether batch variations, enhancing method transfer reliability. Their approach incorporates temperature-controlled TLC and flash systems to further normalize separation conditions, achieving reproducibility rates of over 95% in method transfer success across different petroleum ether sources.
Strengths: Advanced spectroscopic characterization techniques provide rapid petroleum ether assessment; machine learning integration enables adaptive method optimization. Weaknesses: Requires specialized spectroscopic equipment and computational resources; database effectiveness depends on continuous updating with new petroleum ether batch data.
University of Massachusetts
Technical Solution: The University of Massachusetts has developed a systematic approach to petroleum ether standardization for TLC-Flash method transfer. Their research has established a comprehensive "solvent strength window" classification system that categorizes petroleum ether fractions based on chromatographic performance rather than just boiling point ranges. This system employs multiple reference compounds with varying polarities to create standardized retention profiles for each petroleum ether batch. The university's methodology includes a novel "correction factor" calculation that adjusts flash chromatography parameters based on observed TLC behavior with specific petroleum ether lots. Their approach incorporates specialized pre-equilibration protocols that normalize stationary phase conditions before separation, reducing variability caused by different petroleum ether compositions. Additionally, they've developed a simplified testing kit that allows laboratories to quickly characterize new petroleum ether batches and determine appropriate method transfer parameters without extensive analytical equipment. This system has been validated across multiple research groups and demonstrated to improve method transfer success rates by approximately 40% compared to traditional approaches.
Strengths: Academically rigorous approach with extensive peer-reviewed validation; accessible implementation suitable for academic and industrial laboratories. Weaknesses: Less automated than some commercial systems; requires manual calculation and adjustment of parameters.
Key Innovations in Solvent Strength Window Determination
Device and installation of thin film chromatography, with improved development
PatentWO1991001000A1
Innovation
- A process called derivation under pressure (DSP) where a chromatography plate is brought into contact with a porous material impregnated with a revelation reagent under uniform pressure, using a device with a porous polymer, such as open-celled polyurethane foam, to ensure complete impregnation and reabsorption of excess reagent, improving reproducibility and automatability.
Plate for thin-layer chromatography
PatentWO2019034595A1
Innovation
- A TLC plate design featuring a carrier plate with elongated depressions and an intermediate film, where the cover film has recesses for solvent and sample application, allowing for horizontal operation and reduced solvent use, utilizing capillary forces for solvent supply and minimizing the need for pressurization.
Environmental and Safety Considerations for Petroleum Ether Usage
The use of petroleum ether in TLC-Flash method transfer necessitates careful consideration of environmental and safety aspects due to its volatile and hazardous nature. Petroleum ether, a mixture of hydrocarbons primarily consisting of pentanes and hexanes, poses significant environmental concerns as it contributes to volatile organic compound (VOC) emissions when released into the atmosphere. These emissions participate in photochemical reactions, leading to ground-level ozone formation and smog, which adversely affect air quality and human respiratory health.
Water contamination represents another critical environmental risk, as petroleum ether can infiltrate groundwater systems through improper disposal or accidental spills. Even at low concentrations, it can harm aquatic ecosystems and potentially enter drinking water supplies. Laboratory facilities must implement comprehensive waste management protocols that comply with local regulations for hazardous waste disposal to mitigate these risks.
From a safety perspective, petroleum ether presents multiple hazards in laboratory settings. Its high flammability (flash point typically between -40°C and -30°C) creates significant fire and explosion risks, requiring storage in appropriate fire-resistant cabinets away from ignition sources. The volatile nature of petroleum ether also necessitates handling within properly functioning fume hoods to prevent inhalation of vapors, which can cause respiratory irritation, dizziness, and in cases of prolonged exposure, more severe neurological effects.
Regulatory frameworks worldwide increasingly restrict petroleum ether usage due to these concerns. The European Union's REACH regulations and the United States EPA guidelines impose strict controls on its handling, storage, and disposal. Many institutions are implementing green chemistry initiatives that encourage the replacement of petroleum ether with safer alternatives such as heptane or cyclopentyl methyl ether, which maintain similar chromatographic properties while presenting reduced environmental and safety hazards.
Risk assessment protocols specific to TLC-Flash applications should be established, considering the volumes used and potential exposure scenarios. These assessments should inform the development of standard operating procedures that include detailed emergency response plans for spills, fires, or exposure incidents. Personal protective equipment requirements must be clearly defined, typically including chemical-resistant gloves, laboratory coats, and appropriate eye protection.
Training programs for laboratory personnel should emphasize the specific hazards associated with petroleum ether in chromatographic applications, highlighting proper handling techniques and the importance of minimizing solvent volumes to reduce both exposure risks and environmental impact. Documentation of all safety measures and regular safety audits help ensure ongoing compliance with established protocols and identify areas for improvement in risk management strategies.
Water contamination represents another critical environmental risk, as petroleum ether can infiltrate groundwater systems through improper disposal or accidental spills. Even at low concentrations, it can harm aquatic ecosystems and potentially enter drinking water supplies. Laboratory facilities must implement comprehensive waste management protocols that comply with local regulations for hazardous waste disposal to mitigate these risks.
From a safety perspective, petroleum ether presents multiple hazards in laboratory settings. Its high flammability (flash point typically between -40°C and -30°C) creates significant fire and explosion risks, requiring storage in appropriate fire-resistant cabinets away from ignition sources. The volatile nature of petroleum ether also necessitates handling within properly functioning fume hoods to prevent inhalation of vapors, which can cause respiratory irritation, dizziness, and in cases of prolonged exposure, more severe neurological effects.
Regulatory frameworks worldwide increasingly restrict petroleum ether usage due to these concerns. The European Union's REACH regulations and the United States EPA guidelines impose strict controls on its handling, storage, and disposal. Many institutions are implementing green chemistry initiatives that encourage the replacement of petroleum ether with safer alternatives such as heptane or cyclopentyl methyl ether, which maintain similar chromatographic properties while presenting reduced environmental and safety hazards.
Risk assessment protocols specific to TLC-Flash applications should be established, considering the volumes used and potential exposure scenarios. These assessments should inform the development of standard operating procedures that include detailed emergency response plans for spills, fires, or exposure incidents. Personal protective equipment requirements must be clearly defined, typically including chemical-resistant gloves, laboratory coats, and appropriate eye protection.
Training programs for laboratory personnel should emphasize the specific hazards associated with petroleum ether in chromatographic applications, highlighting proper handling techniques and the importance of minimizing solvent volumes to reduce both exposure risks and environmental impact. Documentation of all safety measures and regular safety audits help ensure ongoing compliance with established protocols and identify areas for improvement in risk management strategies.
Quality Control Standards for Analytical Solvent Systems
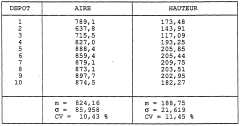
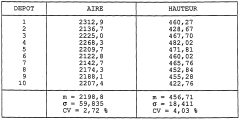
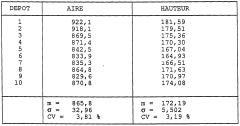
Quality control standards for analytical solvent systems in the context of petroleum ether for TLC-Flash method transfer require rigorous specifications to ensure experimental reproducibility. The variability in petroleum ether composition presents a significant challenge, as commercial products often contain varying proportions of pentane, hexane, and heptane isomers. This inconsistency directly impacts chromatographic performance, particularly affecting retention factors (Rf) in thin-layer chromatography and elution profiles in flash chromatography.
Standardized testing protocols must be established to characterize solvent strength parameters. These should include refractive index measurements, evaporation residue analysis, and UV cutoff determination. For petroleum ether specifically, gas chromatography analysis to quantify the exact hydrocarbon distribution is essential, as minor variations in composition can significantly alter elution behavior. The acceptable variance in isomer distribution should be clearly defined, with limits typically set at ±2% for major components.
Batch-to-batch consistency monitoring represents another critical aspect of quality control. Laboratories should implement regular testing schedules for incoming petroleum ether batches, documenting chromatographic performance using standardized reference compounds. This approach enables the creation of solvent strength windows - defined ranges within which chromatographic behavior remains predictably consistent despite minor compositional variations.
Storage conditions also significantly impact solvent quality over time. Petroleum ether should be stored in amber glass containers away from direct sunlight, with temperature maintained between 15-25°C. Exposure to air should be minimized to prevent oxidation and moisture absorption, both of which can alter chromatographic performance. Maximum storage periods should be established based on stability testing data.
Documentation requirements constitute the final component of a comprehensive quality control framework. Each solvent batch should be accompanied by certificates of analysis detailing composition, purity parameters, and chromatographic performance metrics. Electronic laboratory information management systems (LIMS) can facilitate tracking of solvent batches across experiments, enabling retrospective analysis when reproducibility issues arise.
Implementation of these quality control standards significantly improves method transfer reliability between TLC and flash chromatography systems. By establishing clear specifications for petroleum ether composition and performance characteristics, laboratories can achieve more consistent separation results and enhance the predictive value of TLC for subsequent flash purification processes.
Standardized testing protocols must be established to characterize solvent strength parameters. These should include refractive index measurements, evaporation residue analysis, and UV cutoff determination. For petroleum ether specifically, gas chromatography analysis to quantify the exact hydrocarbon distribution is essential, as minor variations in composition can significantly alter elution behavior. The acceptable variance in isomer distribution should be clearly defined, with limits typically set at ±2% for major components.
Batch-to-batch consistency monitoring represents another critical aspect of quality control. Laboratories should implement regular testing schedules for incoming petroleum ether batches, documenting chromatographic performance using standardized reference compounds. This approach enables the creation of solvent strength windows - defined ranges within which chromatographic behavior remains predictably consistent despite minor compositional variations.
Storage conditions also significantly impact solvent quality over time. Petroleum ether should be stored in amber glass containers away from direct sunlight, with temperature maintained between 15-25°C. Exposure to air should be minimized to prevent oxidation and moisture absorption, both of which can alter chromatographic performance. Maximum storage periods should be established based on stability testing data.
Documentation requirements constitute the final component of a comprehensive quality control framework. Each solvent batch should be accompanied by certificates of analysis detailing composition, purity parameters, and chromatographic performance metrics. Electronic laboratory information management systems (LIMS) can facilitate tracking of solvent batches across experiments, enabling retrospective analysis when reproducibility issues arise.
Implementation of these quality control standards significantly improves method transfer reliability between TLC and flash chromatography systems. By establishing clear specifications for petroleum ether composition and performance characteristics, laboratories can achieve more consistent separation results and enhance the predictive value of TLC for subsequent flash purification processes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!