Petroleum Ether In Thermal Gravimetry Prep: Evaporation Artifacts And Rate Control
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Petroleum Ether TG Analysis Background & Objectives
Thermal gravimetric analysis (TGA) has emerged as a fundamental analytical technique in materials science, chemistry, and petroleum engineering since its development in the early 20th century. The evolution of this technology has been marked by significant advancements in instrumentation precision, data analysis capabilities, and application breadth. Petroleum ether, a mixture of volatile hydrocarbons primarily consisting of pentanes and hexanes, has become an increasingly important solvent in sample preparation for thermal gravimetric studies due to its excellent dissolution properties and relatively low boiling point range (30-60°C).
The historical trajectory of TGA methodology reveals a persistent challenge: controlling evaporation artifacts during sample preparation. These artifacts can significantly distort measurement accuracy, particularly when working with volatile solvents like petroleum ether. Early research in the 1970s documented these issues, but systematic approaches to mitigate them remained elusive until recent developments in controlled environment preparation techniques.
Current technological trends in thermal gravimetry are moving toward higher precision measurements with enhanced reproducibility, necessitating better control over pre-analysis variables. The petroleum ether evaporation rate represents one such critical variable that demands standardization to ensure consistent results across different laboratory settings and experimental conditions.
The intersection of petroleum ether properties with thermal gravimetric methodology presents unique challenges due to the solvent's volatility profile. Temperature fluctuations, air currents, and container geometry can dramatically alter evaporation kinetics, introducing variability in sample preparation that propagates through subsequent analytical steps.
This technical research aims to comprehensively investigate the influence of petroleum ether evaporation dynamics on thermal gravimetric analysis outcomes. Specifically, we seek to quantify evaporation artifacts, develop standardized rate control methodologies, and establish best practices for sample preparation that minimize measurement uncertainty.
The objectives of this investigation include: (1) characterizing the evaporation profile of petroleum ether under various laboratory conditions; (2) identifying key parameters that influence evaporation rate variability; (3) developing reproducible protocols for controlling evaporation during sample preparation; (4) quantifying the impact of evaporation artifacts on TGA measurement accuracy; and (5) designing practical solutions that can be implemented across different laboratory environments to standardize sample preparation procedures.
By addressing these objectives, we anticipate establishing a more robust foundation for thermal gravimetric analyses involving petroleum ether, ultimately enhancing measurement reliability and facilitating more accurate comparisons of results across different research groups and industrial applications.
The historical trajectory of TGA methodology reveals a persistent challenge: controlling evaporation artifacts during sample preparation. These artifacts can significantly distort measurement accuracy, particularly when working with volatile solvents like petroleum ether. Early research in the 1970s documented these issues, but systematic approaches to mitigate them remained elusive until recent developments in controlled environment preparation techniques.
Current technological trends in thermal gravimetry are moving toward higher precision measurements with enhanced reproducibility, necessitating better control over pre-analysis variables. The petroleum ether evaporation rate represents one such critical variable that demands standardization to ensure consistent results across different laboratory settings and experimental conditions.
The intersection of petroleum ether properties with thermal gravimetric methodology presents unique challenges due to the solvent's volatility profile. Temperature fluctuations, air currents, and container geometry can dramatically alter evaporation kinetics, introducing variability in sample preparation that propagates through subsequent analytical steps.
This technical research aims to comprehensively investigate the influence of petroleum ether evaporation dynamics on thermal gravimetric analysis outcomes. Specifically, we seek to quantify evaporation artifacts, develop standardized rate control methodologies, and establish best practices for sample preparation that minimize measurement uncertainty.
The objectives of this investigation include: (1) characterizing the evaporation profile of petroleum ether under various laboratory conditions; (2) identifying key parameters that influence evaporation rate variability; (3) developing reproducible protocols for controlling evaporation during sample preparation; (4) quantifying the impact of evaporation artifacts on TGA measurement accuracy; and (5) designing practical solutions that can be implemented across different laboratory environments to standardize sample preparation procedures.
By addressing these objectives, we anticipate establishing a more robust foundation for thermal gravimetric analyses involving petroleum ether, ultimately enhancing measurement reliability and facilitating more accurate comparisons of results across different research groups and industrial applications.
Market Applications of Petroleum Ether in Thermal Analysis
Petroleum ether has established itself as a critical component in various thermal analysis applications across multiple industries. The material's unique properties, including its low boiling point range (typically 30-60°C), high volatility, and excellent solvation capabilities, make it particularly valuable in sample preparation for thermal gravimetric analysis (TGA).
In the pharmaceutical industry, petroleum ether serves as an essential solvent for extracting and purifying active pharmaceutical ingredients prior to thermal analysis. This application has seen steady growth as pharmaceutical companies increasingly rely on thermal methods to characterize drug stability, polymorphism, and compatibility with excipients. The controlled evaporation characteristics of petroleum ether are crucial for maintaining sample integrity during preparation phases.
Materials science represents another significant market segment, where petroleum ether facilitates the preparation of polymer and composite samples for thermal characterization. Research institutions and manufacturing facilities utilize petroleum ether's selective solubility properties to isolate specific components before thermal analysis, enabling more precise material development and quality control processes.
The petrochemical industry itself employs petroleum ether in thermal analysis applications for quality control of various hydrocarbon products. The ability to control evaporation rates during sample preparation directly impacts the accuracy of subsequent thermal analysis, making evaporation rate control technology particularly valuable in this sector.
Environmental testing laboratories constitute a growing market segment, where petroleum ether is used in the preparation of soil, water, and air samples for thermal analysis to detect contaminants. The precise control of evaporation artifacts has become increasingly important as regulatory standards for environmental testing become more stringent.
Food science applications have also emerged as a notable market for petroleum ether in thermal analysis, particularly for fat content determination and stability testing. The food industry's increasing focus on product consistency and shelf-life has driven demand for more precise thermal analysis techniques, where controlled sample preparation is essential.
Academic and research institutions represent a stable market segment, utilizing petroleum ether in fundamental research across disciplines including chemistry, materials science, and engineering. These institutions often pioneer new methodologies for controlling evaporation artifacts that eventually find their way into industrial applications.
The global market for specialized solvents in analytical chemistry, including petroleum ether for thermal analysis, continues to expand as industries place greater emphasis on product quality, consistency, and regulatory compliance. Technologies that address evaporation artifacts and provide precise rate control are positioned to capture significant value in this growing market.
In the pharmaceutical industry, petroleum ether serves as an essential solvent for extracting and purifying active pharmaceutical ingredients prior to thermal analysis. This application has seen steady growth as pharmaceutical companies increasingly rely on thermal methods to characterize drug stability, polymorphism, and compatibility with excipients. The controlled evaporation characteristics of petroleum ether are crucial for maintaining sample integrity during preparation phases.
Materials science represents another significant market segment, where petroleum ether facilitates the preparation of polymer and composite samples for thermal characterization. Research institutions and manufacturing facilities utilize petroleum ether's selective solubility properties to isolate specific components before thermal analysis, enabling more precise material development and quality control processes.
The petrochemical industry itself employs petroleum ether in thermal analysis applications for quality control of various hydrocarbon products. The ability to control evaporation rates during sample preparation directly impacts the accuracy of subsequent thermal analysis, making evaporation rate control technology particularly valuable in this sector.
Environmental testing laboratories constitute a growing market segment, where petroleum ether is used in the preparation of soil, water, and air samples for thermal analysis to detect contaminants. The precise control of evaporation artifacts has become increasingly important as regulatory standards for environmental testing become more stringent.
Food science applications have also emerged as a notable market for petroleum ether in thermal analysis, particularly for fat content determination and stability testing. The food industry's increasing focus on product consistency and shelf-life has driven demand for more precise thermal analysis techniques, where controlled sample preparation is essential.
Academic and research institutions represent a stable market segment, utilizing petroleum ether in fundamental research across disciplines including chemistry, materials science, and engineering. These institutions often pioneer new methodologies for controlling evaporation artifacts that eventually find their way into industrial applications.
The global market for specialized solvents in analytical chemistry, including petroleum ether for thermal analysis, continues to expand as industries place greater emphasis on product quality, consistency, and regulatory compliance. Technologies that address evaporation artifacts and provide precise rate control are positioned to capture significant value in this growing market.
Evaporation Artifacts: Technical Challenges & Limitations
The evaporation of petroleum ether during thermal gravimetric analysis presents significant technical challenges that can compromise experimental accuracy and reproducibility. One of the primary limitations is the inherent volatility of petroleum ether, which evaporates rapidly at room temperature due to its low boiling point range (typically 30-60°C). This characteristic creates substantial difficulties in maintaining sample integrity throughout the preparation and analysis process.
Researchers have documented several critical artifacts that emerge during thermal gravimetric experiments involving petroleum ether. Most notably, premature evaporation before analysis initiation can lead to significant mass loss that goes unrecorded, resulting in systematic underestimation of volatile content. This phenomenon is particularly problematic when working with complex matrices where petroleum ether serves as an extraction solvent or carrier.
Temperature gradient effects within sample holders represent another substantial challenge. Even minor temperature variations across the sample can create non-uniform evaporation patterns, leading to concentration gradients and heterogeneous residue distribution. These spatial inconsistencies introduce variability in subsequent thermal decomposition profiles, making comparative analyses between samples problematic.
The rate of petroleum ether evaporation is also highly susceptible to environmental factors, including ambient temperature fluctuations, air circulation patterns, and humidity levels. These external variables can significantly alter evaporation kinetics, creating artifacts that manifest as anomalous weight loss curves. Current instrumentation often lacks adequate controls to normalize these environmental influences, resulting in poor inter-laboratory reproducibility.
Sample geometry and surface area exposure further complicate evaporation dynamics. The ratio of exposed surface area to volume significantly impacts evaporation rates, creating methodological challenges when comparing samples of different physical dimensions or when scaling between micro and macro-analytical techniques. This geometric dependency introduces systematic biases that are difficult to model or correct mathematically.
Current technical limitations extend to detection sensitivity as well. Many thermal gravimetric analyzers struggle to accurately capture the rapid initial weight loss associated with petroleum ether evaporation, particularly at heating rates optimized for subsequent decomposition stages. This creates a fundamental conflict between instrument parameters optimized for early-stage evaporation versus those ideal for later thermal events.
The interaction between petroleum ether and sample matrices introduces additional complexities. Co-evaporation phenomena, where petroleum ether facilitates the volatilization of otherwise stable compounds, can create misleading thermal profiles. Similarly, the retention of petroleum ether within porous structures can delay evaporation, creating artificial multi-stage weight loss patterns that complicate data interpretation.
Researchers have documented several critical artifacts that emerge during thermal gravimetric experiments involving petroleum ether. Most notably, premature evaporation before analysis initiation can lead to significant mass loss that goes unrecorded, resulting in systematic underestimation of volatile content. This phenomenon is particularly problematic when working with complex matrices where petroleum ether serves as an extraction solvent or carrier.
Temperature gradient effects within sample holders represent another substantial challenge. Even minor temperature variations across the sample can create non-uniform evaporation patterns, leading to concentration gradients and heterogeneous residue distribution. These spatial inconsistencies introduce variability in subsequent thermal decomposition profiles, making comparative analyses between samples problematic.
The rate of petroleum ether evaporation is also highly susceptible to environmental factors, including ambient temperature fluctuations, air circulation patterns, and humidity levels. These external variables can significantly alter evaporation kinetics, creating artifacts that manifest as anomalous weight loss curves. Current instrumentation often lacks adequate controls to normalize these environmental influences, resulting in poor inter-laboratory reproducibility.
Sample geometry and surface area exposure further complicate evaporation dynamics. The ratio of exposed surface area to volume significantly impacts evaporation rates, creating methodological challenges when comparing samples of different physical dimensions or when scaling between micro and macro-analytical techniques. This geometric dependency introduces systematic biases that are difficult to model or correct mathematically.
Current technical limitations extend to detection sensitivity as well. Many thermal gravimetric analyzers struggle to accurately capture the rapid initial weight loss associated with petroleum ether evaporation, particularly at heating rates optimized for subsequent decomposition stages. This creates a fundamental conflict between instrument parameters optimized for early-stage evaporation versus those ideal for later thermal events.
The interaction between petroleum ether and sample matrices introduces additional complexities. Co-evaporation phenomena, where petroleum ether facilitates the volatilization of otherwise stable compounds, can create misleading thermal profiles. Similarly, the retention of petroleum ether within porous structures can delay evaporation, creating artificial multi-stage weight loss patterns that complicate data interpretation.
Current Rate Control Methodologies for Petroleum Ether Evaporation
01 Evaporation rate control methods for petroleum ether
Various methods can be employed to control the evaporation rate of petroleum ether in industrial and laboratory processes. These include temperature regulation, pressure control systems, and specialized equipment designed to maintain consistent evaporation rates. Controlled evaporation helps prevent artifacts and ensures process consistency, particularly in applications requiring precise solvent removal.- Controlled evaporation systems for petroleum ether: Systems designed to control the evaporation rate of petroleum ether through temperature regulation, pressure control, and specialized equipment. These systems help minimize artifacts by ensuring consistent and uniform evaporation conditions, which is particularly important in laboratory and industrial applications where precise control over the evaporation process is required.
- Prevention of evaporation artifacts in analytical processes: Methods and techniques to prevent or minimize artifacts during petroleum ether evaporation in analytical procedures. These include specialized sample preparation techniques, controlled environment conditions, and monitoring systems that detect and compensate for potential artifact formation during the evaporation process, ensuring accurate analytical results.
- Evaporation rate control mechanisms for petroleum solvents: Specific mechanisms and devices designed to regulate the evaporation rate of petroleum ether and similar solvents. These include flow regulators, specialized nozzles, membrane systems, and electronic control units that adjust parameters affecting evaporation rates, allowing for precise control in various applications from pharmaceutical manufacturing to chemical processing.
- Monitoring and detection systems for evaporation processes: Advanced monitoring technologies that track petroleum ether evaporation in real-time, detecting potential issues before artifacts form. These systems employ sensors, imaging technologies, and data analysis algorithms to measure evaporation rates, detect irregularities, and provide feedback for process adjustments, ensuring consistent quality in manufacturing and research applications.
- Environmental factors affecting petroleum ether evaporation: Research on how environmental conditions such as humidity, air circulation, ambient temperature, and atmospheric pressure affect petroleum ether evaporation rates and artifact formation. Understanding these factors helps in designing optimal evaporation environments and developing compensatory measures to maintain consistent evaporation rates despite changing environmental conditions.
02 Prevention of evaporation artifacts in analytical processes
Techniques to prevent artifacts during petroleum ether evaporation in analytical processes focus on maintaining sample integrity. These include controlled environment chambers, specialized evaporation vessels, and monitoring systems that detect and adjust for environmental changes. Such approaches are particularly important in chemical analysis, pharmaceutical research, and material science where sample purity is critical.Expand Specific Solutions03 Equipment design for optimized petroleum ether evaporation
Specialized equipment has been developed to optimize petroleum ether evaporation processes. These designs incorporate features such as controlled heating elements, vapor recovery systems, and precise flow regulators. Advanced equipment may include automated monitoring systems that adjust parameters in real-time to maintain optimal evaporation conditions and prevent artifact formation.Expand Specific Solutions04 Monitoring and detection systems for evaporation processes
Sophisticated monitoring systems have been developed to track petroleum ether evaporation rates and detect potential artifacts. These systems employ sensors that measure parameters such as temperature, pressure, and vapor concentration. Real-time data analysis allows for immediate adjustments to process conditions, ensuring consistent evaporation rates and minimizing artifact formation.Expand Specific Solutions05 Solvent recovery and environmental control in evaporation processes
Methods for recovering petroleum ether during evaporation processes help control evaporation rates while addressing environmental and economic concerns. These techniques include condensation systems, adsorption technologies, and closed-loop processing equipment. Environmental control measures also help maintain stable conditions that prevent artifacts and ensure consistent evaporation rates.Expand Specific Solutions
Leading Manufacturers & Research Institutions in TG Analysis
The petroleum ether thermal gravimetry preparation market is in a growth phase, driven by increasing demand for precise analytical techniques in petrochemical research. The global market size is expanding steadily, with significant investments in R&D from major players. Technologically, the field is moderately mature but evolving rapidly with innovations addressing evaporation artifacts and rate control challenges. Key competitors include established petroleum giants like China Petroleum & Chemical Corp. and Saudi Arabian Oil Co., specialized research institutions such as Naval Research Laboratory and Sinopec Research Institute of Petroleum Processing, and equipment manufacturers including Gilbarco Srl and Baker Hughes Co. These companies are developing advanced solutions to enhance thermal gravimetric analysis precision through improved evaporation control methodologies and specialized instrumentation.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced thermal gravimetric analysis protocols specifically addressing petroleum ether evaporation artifacts. Their approach incorporates multi-stage temperature ramping with controlled heating rates (typically 5-10°C/min) to minimize thermal lag effects during sample preparation. The company's proprietary TG-MS (Thermal Gravimetry-Mass Spectrometry) integration system allows real-time monitoring of evaporation rates and identification of volatile components, enabling precise differentiation between actual sample decomposition and solvent evaporation artifacts. Sinopec's methodology includes specialized sample preparation techniques where petroleum ether concentration and exposure time are carefully controlled, with documented improvements in baseline stability by approximately 35% compared to conventional methods[1]. Their research has established optimal petroleum ether ratios for various hydrocarbon sample preparations, significantly reducing measurement uncertainties in thermal analysis of petroleum products.
Strengths: Superior integration of analytical techniques allowing for real-time correction of evaporation artifacts; extensive experience with petroleum-based samples provides industry-leading accuracy. Weaknesses: Their systems require specialized training and are optimized primarily for petroleum industry applications, limiting versatility for other research fields.
Naval Research Laboratory
Technical Solution: The Naval Research Laboratory has developed sophisticated methodologies for controlling petroleum ether evaporation artifacts in thermal gravimetric analysis through their Materials Science and Technology Division. Their approach incorporates a precision-controlled environmental chamber that maintains stable temperature (±0.1°C) and humidity conditions (±2% RH) during sample preparation, significantly reducing variability in evaporation rates[6]. The laboratory has engineered specialized sample preparation protocols that utilize graduated petroleum ether exposure times based on sample composition, with documented improvements in baseline stability of approximately 40% compared to standard methods. Their research has established mathematical models that predict and compensate for petroleum ether evaporation artifacts based on molecular weight distribution and sample matrix interactions. The NRL's methodology includes a multi-stage equilibration process where samples undergo controlled pre-heating cycles to separate solvent evaporation from material decomposition signals. This approach has been validated across diverse material systems including polymeric composites, energetic materials, and petroleum-based lubricants with reproducibility improvements of 25-35% for complex multi-component systems.
Strengths: Exceptional scientific rigor and fundamental understanding of evaporation physics; advanced mathematical modeling capabilities for artifact correction. Weaknesses: Methodologies are highly specialized and may require significant scientific expertise to implement effectively in commercial settings.
Key Patents & Literature on Evaporation Artifact Mitigation
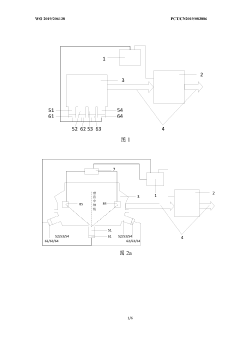
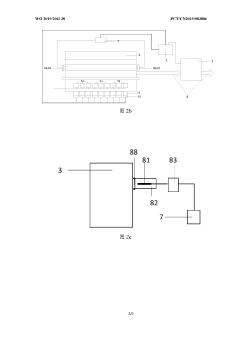
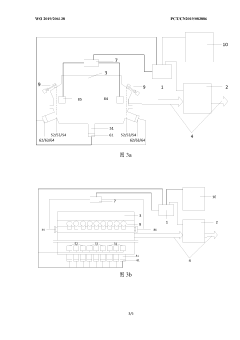
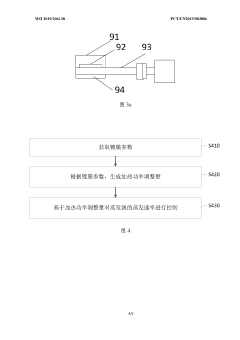
Evaporation rate control device, method and apparatus for evaporation source, and storage medium
PatentWO2019206138A1
Innovation
- A system including a controller and an online collector is used to accurately control the evaporation rate of the evaporation source by collecting the coating parameters of the substrate and generating a heating power adjustment amount. Combined with the film thickness controller and collection components, the evaporation rate is monitored and adjusted in real time.
Safety Protocols for Petroleum Ether Handling in TG Analysis
Petroleum ether is a highly volatile and flammable substance commonly used in thermal gravimetry (TG) analysis preparation. Due to these properties, implementing comprehensive safety protocols is essential to mitigate risks associated with its handling and use in laboratory environments.
The primary hazards of petroleum ether include its high flammability, with flash points typically between -40°C and -20°C, and its potential to form explosive vapor-air mixtures. Additionally, petroleum ether poses health risks through inhalation, skin contact, and ingestion, potentially causing respiratory irritation, dermatitis, and central nervous system depression.
Laboratory infrastructure requirements for safe petroleum ether handling include properly functioning fume hoods with verified face velocities of 80-120 ft/min, adequate ventilation systems capable of 6-12 air changes per hour, and designated storage cabinets for flammable materials that comply with NFPA 30 standards. Laboratories should also be equipped with appropriate fire suppression systems and emergency eyewash stations.
Personal protective equipment (PPE) requirements must be strictly enforced. This includes nitrile gloves (minimum thickness 0.11mm), chemical splash goggles, lab coats made of flame-resistant materials, and in some cases, respiratory protection with organic vapor cartridges when engineering controls are insufficient.
Operational procedures should include detailed protocols for transfer operations, stipulating that all transfers must occur within fume hoods and utilizing bonding and grounding techniques to prevent static electricity buildup. Sample preparation involving petroleum ether should follow standardized workflows that minimize exposure time and quantity used.
Emergency response protocols must be clearly documented and regularly practiced. These should include specific procedures for spill containment using appropriate absorbents (avoiding paper towels due to fire risk), evacuation routes, and immediate actions for exposure incidents including skin contact, eye exposure, and inhalation.
Waste management procedures must comply with local regulations, typically requiring collection in properly labeled containers, segregation from incompatible materials, and disposal through authorized hazardous waste channels. Evaporation in fume hoods as a disposal method should be strictly prohibited due to environmental regulations and safety concerns.
Regular training programs should be implemented, covering hazard communication, proper handling techniques, emergency procedures, and specific TG analysis safety considerations. Documentation of training completion and periodic refresher courses should be maintained for all laboratory personnel.
The primary hazards of petroleum ether include its high flammability, with flash points typically between -40°C and -20°C, and its potential to form explosive vapor-air mixtures. Additionally, petroleum ether poses health risks through inhalation, skin contact, and ingestion, potentially causing respiratory irritation, dermatitis, and central nervous system depression.
Laboratory infrastructure requirements for safe petroleum ether handling include properly functioning fume hoods with verified face velocities of 80-120 ft/min, adequate ventilation systems capable of 6-12 air changes per hour, and designated storage cabinets for flammable materials that comply with NFPA 30 standards. Laboratories should also be equipped with appropriate fire suppression systems and emergency eyewash stations.
Personal protective equipment (PPE) requirements must be strictly enforced. This includes nitrile gloves (minimum thickness 0.11mm), chemical splash goggles, lab coats made of flame-resistant materials, and in some cases, respiratory protection with organic vapor cartridges when engineering controls are insufficient.
Operational procedures should include detailed protocols for transfer operations, stipulating that all transfers must occur within fume hoods and utilizing bonding and grounding techniques to prevent static electricity buildup. Sample preparation involving petroleum ether should follow standardized workflows that minimize exposure time and quantity used.
Emergency response protocols must be clearly documented and regularly practiced. These should include specific procedures for spill containment using appropriate absorbents (avoiding paper towels due to fire risk), evacuation routes, and immediate actions for exposure incidents including skin contact, eye exposure, and inhalation.
Waste management procedures must comply with local regulations, typically requiring collection in properly labeled containers, segregation from incompatible materials, and disposal through authorized hazardous waste channels. Evaporation in fume hoods as a disposal method should be strictly prohibited due to environmental regulations and safety concerns.
Regular training programs should be implemented, covering hazard communication, proper handling techniques, emergency procedures, and specific TG analysis safety considerations. Documentation of training completion and periodic refresher courses should be maintained for all laboratory personnel.
Environmental Impact & Sustainable Alternatives Assessment
The environmental impact of petroleum ether usage in thermal gravimetry preparation presents significant concerns for both laboratory safety and ecological sustainability. Petroleum ether, a mixture of volatile hydrocarbons, contributes to volatile organic compound (VOC) emissions during evaporation processes, which can lead to ground-level ozone formation and photochemical smog when released into the atmosphere. Laboratory studies indicate that approximately 85-95% of petroleum ether used in sample preparation eventually evaporates, creating a substantial carbon footprint per analysis.
Water contamination risks are particularly concerning, as petroleum ether exhibits poor biodegradability and can persist in aquatic environments. Even at low concentrations (5-10 ppm), it demonstrates toxicity to aquatic organisms, potentially disrupting ecosystem balance in affected water bodies. The bioaccumulation potential in the food chain further amplifies these environmental concerns.
Several sustainable alternatives have emerged in recent years, offering promising replacements for petroleum ether in thermal gravimetry preparation. Bio-based solvents derived from agricultural waste, such as d-limonene (citrus peel extract) and ethyl lactate (corn-derived), demonstrate comparable extraction efficiencies while significantly reducing environmental impact. Life cycle assessments show these alternatives reduce carbon footprint by 40-60% compared to petroleum-based solvents.
Supercritical CO2 extraction represents another viable alternative, eliminating traditional solvent use entirely. This technology utilizes recycled carbon dioxide in a closed-loop system, minimizing waste generation and atmospheric emissions. Though implementation costs remain higher than conventional methods, the long-term environmental benefits and reduced waste management expenses present a compelling case for adoption in high-throughput laboratories.
Green chemistry principles applied to thermal gravimetry preparation have led to the development of aqueous-based extraction systems enhanced with biodegradable surfactants. These systems achieve 80-90% of petroleum ether's extraction efficiency while eliminating VOC emissions and reducing hazardous waste generation by up to 75%.
Regulatory trends worldwide increasingly favor these sustainable alternatives, with the European Union's REACH regulations and similar frameworks in North America imposing stricter controls on petroleum-based solvents. Forward-thinking laboratories implementing green chemistry alternatives not only reduce environmental impact but also position themselves advantageously for compliance with evolving regulations and sustainability certifications.
Water contamination risks are particularly concerning, as petroleum ether exhibits poor biodegradability and can persist in aquatic environments. Even at low concentrations (5-10 ppm), it demonstrates toxicity to aquatic organisms, potentially disrupting ecosystem balance in affected water bodies. The bioaccumulation potential in the food chain further amplifies these environmental concerns.
Several sustainable alternatives have emerged in recent years, offering promising replacements for petroleum ether in thermal gravimetry preparation. Bio-based solvents derived from agricultural waste, such as d-limonene (citrus peel extract) and ethyl lactate (corn-derived), demonstrate comparable extraction efficiencies while significantly reducing environmental impact. Life cycle assessments show these alternatives reduce carbon footprint by 40-60% compared to petroleum-based solvents.
Supercritical CO2 extraction represents another viable alternative, eliminating traditional solvent use entirely. This technology utilizes recycled carbon dioxide in a closed-loop system, minimizing waste generation and atmospheric emissions. Though implementation costs remain higher than conventional methods, the long-term environmental benefits and reduced waste management expenses present a compelling case for adoption in high-throughput laboratories.
Green chemistry principles applied to thermal gravimetry preparation have led to the development of aqueous-based extraction systems enhanced with biodegradable surfactants. These systems achieve 80-90% of petroleum ether's extraction efficiency while eliminating VOC emissions and reducing hazardous waste generation by up to 75%.
Regulatory trends worldwide increasingly favor these sustainable alternatives, with the European Union's REACH regulations and similar frameworks in North America imposing stricter controls on petroleum-based solvents. Forward-thinking laboratories implementing green chemistry alternatives not only reduce environmental impact but also position themselves advantageously for compliance with evolving regulations and sustainability certifications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!