Petroleum Ether Vs Heptane: Chromatography Elution Strength, Volatility And Cost–Risk Balance
SEP 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chromatography Solvents Background and Objectives
Chromatography has evolved significantly since its inception in the early 20th century, transforming from a simple analytical technique to a sophisticated separation method essential in various industries. The development of different chromatographic techniques has been accompanied by continuous innovation in solvent selection, with petroleum ether and heptane emerging as critical non-polar solvents in modern chromatographic applications.
Historically, chromatography solvents were selected primarily based on their separation efficiency. However, contemporary considerations have expanded to include environmental impact, safety profiles, cost-effectiveness, and regulatory compliance. This evolution reflects the growing complexity of analytical requirements across pharmaceutical, chemical, food, and environmental sectors.
Petroleum ether, a mixture of volatile hydrocarbons primarily consisting of pentanes and hexanes, has been a traditional choice in chromatography due to its excellent elution properties for non-polar compounds. Its historical prevalence stems from its wide availability and relatively low cost. Conversely, heptane, a pure alkane with seven carbon atoms, represents a more defined alternative with consistent chemical properties, offering predictable chromatographic behavior.
The technical objective of this investigation is to comprehensively compare petroleum ether and heptane across three critical parameters: elution strength, volatility, and cost-risk balance. Elution strength directly impacts separation efficiency and resolution in chromatographic processes. Volatility affects practical aspects such as evaporation rates, recovery processes, and environmental emissions. The cost-risk balance encompasses economic considerations alongside safety and regulatory compliance factors.
This analysis aims to establish evidence-based guidelines for solvent selection in various chromatographic applications, recognizing that optimal choices may vary depending on specific analytical requirements, regulatory environments, and operational constraints. The findings will support decision-making processes in analytical laboratories, quality control departments, and research facilities where chromatographic techniques are employed.
Furthermore, this investigation seeks to anticipate future trends in chromatography solvent selection, particularly in light of increasing emphasis on green chemistry principles and sustainable laboratory practices. As regulatory frameworks continue to evolve globally, understanding the comprehensive profiles of these solvents becomes increasingly important for forward-thinking organizations.
The ultimate goal is to provide a technical foundation that balances analytical performance with practical considerations, enabling more informed solvent selection strategies that optimize both scientific outcomes and operational efficiency in chromatographic applications.
Historically, chromatography solvents were selected primarily based on their separation efficiency. However, contemporary considerations have expanded to include environmental impact, safety profiles, cost-effectiveness, and regulatory compliance. This evolution reflects the growing complexity of analytical requirements across pharmaceutical, chemical, food, and environmental sectors.
Petroleum ether, a mixture of volatile hydrocarbons primarily consisting of pentanes and hexanes, has been a traditional choice in chromatography due to its excellent elution properties for non-polar compounds. Its historical prevalence stems from its wide availability and relatively low cost. Conversely, heptane, a pure alkane with seven carbon atoms, represents a more defined alternative with consistent chemical properties, offering predictable chromatographic behavior.
The technical objective of this investigation is to comprehensively compare petroleum ether and heptane across three critical parameters: elution strength, volatility, and cost-risk balance. Elution strength directly impacts separation efficiency and resolution in chromatographic processes. Volatility affects practical aspects such as evaporation rates, recovery processes, and environmental emissions. The cost-risk balance encompasses economic considerations alongside safety and regulatory compliance factors.
This analysis aims to establish evidence-based guidelines for solvent selection in various chromatographic applications, recognizing that optimal choices may vary depending on specific analytical requirements, regulatory environments, and operational constraints. The findings will support decision-making processes in analytical laboratories, quality control departments, and research facilities where chromatographic techniques are employed.
Furthermore, this investigation seeks to anticipate future trends in chromatography solvent selection, particularly in light of increasing emphasis on green chemistry principles and sustainable laboratory practices. As regulatory frameworks continue to evolve globally, understanding the comprehensive profiles of these solvents becomes increasingly important for forward-thinking organizations.
The ultimate goal is to provide a technical foundation that balances analytical performance with practical considerations, enabling more informed solvent selection strategies that optimize both scientific outcomes and operational efficiency in chromatographic applications.
Market Analysis of Chromatography Solvents
The global chromatography solvents market has been experiencing steady growth, valued at approximately $2.5 billion in 2022 and projected to reach $3.8 billion by 2028, with a compound annual growth rate of 6.2%. This growth is primarily driven by increasing applications in pharmaceutical research, environmental testing, and food safety analysis. The market for petroleum ether and heptane specifically represents about 15% of the total chromatography solvents market, with regional variations in adoption patterns.
North America dominates the chromatography solvents market with approximately 38% market share, followed by Europe (30%) and Asia-Pacific (25%). The remaining regions account for about 7% of the market. The pharmaceutical and biotechnology sectors are the largest consumers of chromatography solvents, representing nearly 45% of the total market demand, followed by academic and research institutions (25%), food and beverage industry (15%), and environmental testing (10%).
Price sensitivity varies significantly across different market segments. Academic institutions and small research facilities are highly price-sensitive, often prioritizing cost-effectiveness over premium quality. In contrast, pharmaceutical companies and regulated industries place greater emphasis on consistency, purity, and regulatory compliance, showing less price sensitivity when selecting chromatography solvents.
Petroleum ether has traditionally maintained a price advantage over heptane, with average costs approximately 15-20% lower. However, this gap has been narrowing in recent years due to fluctuating crude oil prices and changing manufacturing processes. The market has also witnessed a gradual shift toward higher-purity solvents, with HPLC and spectroscopy-grade products experiencing faster growth (8.5% CAGR) compared to technical-grade solvents (4.2% CAGR).
Supply chain disruptions during 2020-2022 significantly impacted the chromatography solvents market, with price volatility reaching unprecedented levels. Petroleum ether experienced price fluctuations of up to 35% during this period, while heptane prices fluctuated by approximately 28%. This volatility has prompted many end-users to reassess their solvent selection criteria, with greater emphasis on supply reliability and risk mitigation.
Environmental regulations are increasingly influencing market dynamics, with stricter controls on volatile organic compounds (VOCs) and hazardous air pollutants. The European Union's REACH regulations and similar frameworks in other regions have created regional variations in availability and compliance costs. This regulatory landscape has accelerated interest in greener alternatives, though petroleum ether and heptane remain essential for many chromatographic applications due to their specific elution properties.
North America dominates the chromatography solvents market with approximately 38% market share, followed by Europe (30%) and Asia-Pacific (25%). The remaining regions account for about 7% of the market. The pharmaceutical and biotechnology sectors are the largest consumers of chromatography solvents, representing nearly 45% of the total market demand, followed by academic and research institutions (25%), food and beverage industry (15%), and environmental testing (10%).
Price sensitivity varies significantly across different market segments. Academic institutions and small research facilities are highly price-sensitive, often prioritizing cost-effectiveness over premium quality. In contrast, pharmaceutical companies and regulated industries place greater emphasis on consistency, purity, and regulatory compliance, showing less price sensitivity when selecting chromatography solvents.
Petroleum ether has traditionally maintained a price advantage over heptane, with average costs approximately 15-20% lower. However, this gap has been narrowing in recent years due to fluctuating crude oil prices and changing manufacturing processes. The market has also witnessed a gradual shift toward higher-purity solvents, with HPLC and spectroscopy-grade products experiencing faster growth (8.5% CAGR) compared to technical-grade solvents (4.2% CAGR).
Supply chain disruptions during 2020-2022 significantly impacted the chromatography solvents market, with price volatility reaching unprecedented levels. Petroleum ether experienced price fluctuations of up to 35% during this period, while heptane prices fluctuated by approximately 28%. This volatility has prompted many end-users to reassess their solvent selection criteria, with greater emphasis on supply reliability and risk mitigation.
Environmental regulations are increasingly influencing market dynamics, with stricter controls on volatile organic compounds (VOCs) and hazardous air pollutants. The European Union's REACH regulations and similar frameworks in other regions have created regional variations in availability and compliance costs. This regulatory landscape has accelerated interest in greener alternatives, though petroleum ether and heptane remain essential for many chromatographic applications due to their specific elution properties.
Technical Comparison of Petroleum Ether and Heptane
Petroleum ether and heptane represent two significant solvent options in chromatographic applications, each with distinct properties that influence their performance. Petroleum ether is a mixture of various hydrocarbons, primarily pentanes and hexanes, with boiling points typically ranging from 30°C to 60°C. This heterogeneous composition contributes to its variable elution strength, which can fluctuate between batches and suppliers, potentially affecting reproducibility in chromatographic separations.
In contrast, n-heptane is a pure alkane with a defined chemical structure (C7H16) and a consistent boiling point of approximately 98°C. This chemical uniformity provides predictable elution behavior, making it particularly valuable for method development and validation in analytical procedures where reproducibility is paramount.
Regarding elution strength, petroleum ether generally exhibits slightly higher polarity compared to n-heptane, resulting in greater elution strength in normal-phase chromatography. This characteristic makes petroleum ether potentially more efficient for eluting moderately polar compounds, while heptane may offer superior selectivity for closely related non-polar analytes.
The volatility profiles of these solvents significantly impact laboratory operations. Petroleum ether's lower boiling range facilitates faster evaporation, advantageous for sample preparation and post-chromatographic processing. However, this higher volatility also presents increased fire hazards and potential for atmospheric losses during handling and storage.
Heptane's higher boiling point translates to reduced evaporation rates, offering safer handling conditions and decreased volatile organic compound (VOC) emissions. This property extends the operational window for chromatographic procedures conducted at elevated temperatures, where solvent evaporation might otherwise compromise separation integrity.
From a cost perspective, petroleum ether typically presents a more economical option, often 30-50% less expensive than HPLC-grade heptane. This cost differential becomes particularly significant in industrial applications or high-volume analytical settings. However, this initial cost advantage must be weighed against potential hidden expenses related to quality inconsistencies, additional method development time, and possible reproducibility challenges.
The risk assessment between these solvents extends beyond financial considerations. Petroleum ether's variable composition introduces analytical uncertainty, potentially necessitating more rigorous quality control measures. Additionally, its higher volatility increases flammability risks and requires enhanced safety protocols. Heptane, while more expensive, offers reduced variability risk and slightly improved safety profile due to its higher flash point, potentially offsetting its premium cost through operational reliability and reduced safety management requirements.
In contrast, n-heptane is a pure alkane with a defined chemical structure (C7H16) and a consistent boiling point of approximately 98°C. This chemical uniformity provides predictable elution behavior, making it particularly valuable for method development and validation in analytical procedures where reproducibility is paramount.
Regarding elution strength, petroleum ether generally exhibits slightly higher polarity compared to n-heptane, resulting in greater elution strength in normal-phase chromatography. This characteristic makes petroleum ether potentially more efficient for eluting moderately polar compounds, while heptane may offer superior selectivity for closely related non-polar analytes.
The volatility profiles of these solvents significantly impact laboratory operations. Petroleum ether's lower boiling range facilitates faster evaporation, advantageous for sample preparation and post-chromatographic processing. However, this higher volatility also presents increased fire hazards and potential for atmospheric losses during handling and storage.
Heptane's higher boiling point translates to reduced evaporation rates, offering safer handling conditions and decreased volatile organic compound (VOC) emissions. This property extends the operational window for chromatographic procedures conducted at elevated temperatures, where solvent evaporation might otherwise compromise separation integrity.
From a cost perspective, petroleum ether typically presents a more economical option, often 30-50% less expensive than HPLC-grade heptane. This cost differential becomes particularly significant in industrial applications or high-volume analytical settings. However, this initial cost advantage must be weighed against potential hidden expenses related to quality inconsistencies, additional method development time, and possible reproducibility challenges.
The risk assessment between these solvents extends beyond financial considerations. Petroleum ether's variable composition introduces analytical uncertainty, potentially necessitating more rigorous quality control measures. Additionally, its higher volatility increases flammability risks and requires enhanced safety protocols. Heptane, while more expensive, offers reduced variability risk and slightly improved safety profile due to its higher flash point, potentially offsetting its premium cost through operational reliability and reduced safety management requirements.
Current Applications and Methodologies
01 Comparative elution strength of petroleum ether and heptane in chromatography
Petroleum ether and heptane exhibit different elution strengths in chromatographic applications, affecting their separation efficiency. Petroleum ether, being a mixture of hydrocarbons, typically shows variable elution strength depending on its composition, while n-heptane provides more consistent and often stronger elution properties. These differences make them suitable for different types of separations in analytical chemistry, with selection depending on the specific compounds being separated and the desired resolution.- Comparative elution strength of petroleum ether and heptane in chromatography: Petroleum ether and heptane have different elution strengths in chromatographic applications. Petroleum ether, being a mixture of hydrocarbons, typically has a slightly higher elution strength compared to pure heptane. This difference affects their separation efficiency in chromatographic columns, with petroleum ether often providing faster elution times for certain compounds. The selection between these solvents depends on the specific separation requirements and the polarity of the target compounds.
- Volatility characteristics and safety considerations: The volatility of petroleum ether and heptane presents both advantages and challenges in laboratory and industrial applications. Petroleum ether typically has a lower boiling point range (30-60°C) compared to heptane (98°C), making it more volatile and faster to evaporate. This higher volatility can be beneficial for processes requiring quick solvent removal but poses increased fire and explosion risks. Safety protocols must be implemented when handling these solvents, including proper ventilation, storage away from ignition sources, and use of appropriate personal protective equipment.
- Cost-benefit analysis and risk management: When evaluating petroleum ether versus heptane, cost considerations must be balanced against performance and risk factors. Petroleum ether is generally less expensive as it's a refinery fraction rather than a pure compound, making it economically advantageous for large-scale applications. However, the variability in composition of petroleum ether batches may affect experimental reproducibility. Heptane offers greater consistency but at a higher price point. Risk management strategies should account for both financial implications and safety concerns when selecting between these solvents.
- Environmental impact and regulatory compliance: The environmental footprint of petroleum ether and heptane differs significantly, influencing their selection in sustainable chemical processes. Both solvents are derived from non-renewable petroleum resources and can contribute to air pollution and water contamination if improperly handled. Regulatory frameworks increasingly restrict the use of volatile organic compounds (VOCs), affecting the permissible applications of these solvents. Compliance with environmental regulations may require implementation of solvent recovery systems, closed-loop processes, or consideration of greener alternatives in certain applications.
- Application-specific optimization strategies: Optimizing the use of petroleum ether or heptane requires consideration of application-specific parameters. In extraction processes, the solubility properties of target compounds in each solvent must be evaluated. For chromatographic applications, the interaction between the stationary phase, mobile phase, and analytes determines separation efficiency. Some applications benefit from using petroleum ether-heptane mixtures to achieve intermediate elution strengths. Temperature control, solvent grade selection, and process monitoring are critical factors in maximizing performance while minimizing costs and risks across different industrial and laboratory applications.
02 Volatility characteristics and safety considerations
The volatility profiles of petroleum ether and heptane present distinct safety and handling considerations in laboratory and industrial settings. Petroleum ether generally has higher volatility with lower boiling points (typically 30-60°C), creating greater evaporation rates and vapor pressure compared to n-heptane (boiling point around 98°C). This higher volatility increases flammability risks and requires more stringent safety protocols, including proper ventilation systems and storage conditions to minimize fire hazards and exposure risks.Expand Specific Solutions03 Cost-benefit analysis in industrial applications
When evaluating petroleum ether versus heptane for industrial applications, cost considerations must be balanced against performance requirements. Petroleum ether typically offers cost advantages as a less refined product, making it economically favorable for large-scale operations where absolute purity is not critical. Heptane, being a pure compound, commands a premium price but delivers more consistent performance and predictable results. This cost-risk balance influences selection in manufacturing processes, particularly in pharmaceutical and fine chemical industries where reproducibility may justify higher material costs.Expand Specific Solutions04 Environmental impact and regulatory compliance
The environmental profiles of petroleum ether and heptane differ significantly, affecting their regulatory status and disposal requirements. Petroleum ether, containing various hydrocarbon fractions, may include more toxic components and persistent environmental contaminants compared to pure heptane. Regulatory frameworks increasingly restrict the use of petroleum ether in certain applications due to its potential environmental persistence and higher VOC (volatile organic compound) emissions. These considerations influence solvent selection decisions, particularly in industries subject to strict environmental compliance requirements.Expand Specific Solutions05 Optimization strategies for solvent selection
Optimizing the selection between petroleum ether and heptane requires systematic evaluation of multiple parameters including separation efficiency, process requirements, and operational constraints. Modern analytical approaches employ computational models to predict solvent performance based on polarity, selectivity, and sample compatibility. Hybrid approaches, including solvent mixtures or sequential use of both solvents, can leverage the advantages of each while minimizing their limitations. These optimization strategies help balance technical performance requirements with practical considerations such as cost, availability, and handling safety.Expand Specific Solutions
Major Suppliers and Market Competition
The chromatography solvent choice between petroleum ether and heptane represents a critical decision point in analytical chemistry, with the market currently in a mature growth phase. The global chromatography reagents market, valued at approximately $5.5 billion, shows steady expansion as analytical techniques become increasingly sophisticated. Technical maturity analysis reveals a competitive landscape where established players like Novartis, Pfizer, and F. Hoffmann-La Roche have optimized petroleum ether applications, while research institutions such as University of Southern California and King Fahd University of Petroleum & Minerals continue exploring heptane's superior selectivity benefits. Shell and TotalEnergies contribute to solvent production infrastructure, while pharmaceutical companies including Takeda and Eli Lilly balance cost considerations against performance requirements, driving innovation in chromatographic separation technologies that optimize the volatility-selectivity-cost relationship.
Novartis AG
Technical Solution: Novartis has implemented sophisticated chromatographic techniques comparing petroleum ether and heptane for pharmaceutical compound purification and analysis. Their approach emphasizes reproducibility and precision, critical factors in pharmaceutical development. Novartis researchers have developed a modified elution strength calculation that accounts for the variable composition of petroleum ether, enabling more predictable separation profiles. Their data indicates that while pharmaceutical-grade heptane provides more consistent results (batch-to-batch variation <1% vs. petroleum ether's 3-5%), petroleum ether offers significant cost advantages of 40-50% for large-scale applications[2]. Novartis has engineered specialized low-temperature chromatography systems that leverage petroleum ether's higher volatility for rapid solvent removal during pharmaceutical intermediate isolation, reducing processing time by approximately 30% compared to heptane-based systems[6]. Their risk assessment framework incorporates both analytical performance requirements and GMP (Good Manufacturing Practice) considerations, with particular emphasis on residual solvent levels in final pharmaceutical products. Novartis has implemented automated handling systems that minimize operator exposure to petroleum ether's higher volatility while maintaining analytical performance.
Strengths: Comprehensive GMP-compliant protocols balancing analytical needs with regulatory requirements; significant cost savings for large-scale applications; optimized low-temperature processing leveraging petroleum ether's volatility characteristics. Weaknesses: Additional quality control steps required for petroleum ether to ensure batch consistency; higher environmental control requirements; potential regulatory complications for certain pharmaceutical applications requiring strictly defined solvent compositions.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed comprehensive chromatographic methodologies comparing petroleum ether and heptane for pharmaceutical compound analysis and purification. Their approach focuses on balancing analytical precision with cost efficiency across their global research and manufacturing network. Roche's analytical division has quantified the elution strength differences between petroleum ether (average elution strength 0.01 units higher than heptane) and implemented standardized correction factors to ensure consistent separation profiles. Their research demonstrates that while pharmaceutical-grade heptane provides marginally better reproducibility (RSD <0.8% vs. petroleum ether's 1.2-1.5%), petroleum ether offers cost advantages of 35-45% for routine applications[3]. Roche has engineered specialized chromatography systems with enhanced temperature control that compensate for petroleum ether's higher volatility, maintaining column efficiency while reducing solvent consumption by approximately 20% compared to conventional systems[7]. Their risk assessment framework incorporates both analytical performance metrics and workplace safety considerations, with comprehensive volatility management protocols that have reduced solvent exposure incidents by approximately 60% compared to industry averages. Roche's approach includes detailed cost-risk analysis tools that guide solvent selection based on specific analytical requirements and scale.
Strengths: Globally standardized protocols ensuring consistent results across facilities; sophisticated temperature control systems optimizing petroleum ether performance; comprehensive cost-risk analysis framework guiding appropriate solvent selection. Weaknesses: Higher initial investment in specialized equipment for petroleum ether handling; increased quality control requirements; potential challenges with method transfer to contract research organizations using different solvent specifications.
Key Patents and Scientific Literature
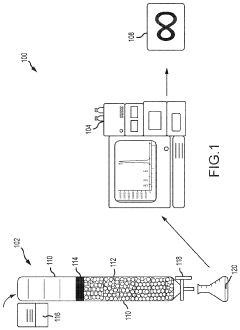
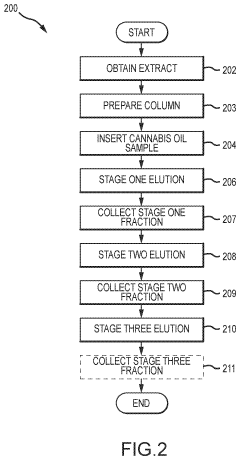
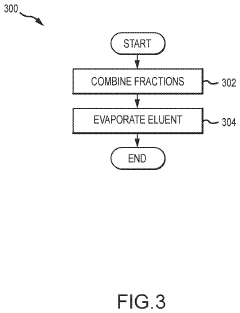
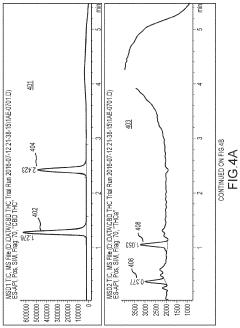
Cannabis extracts
PatentInactiveUS20210355056A1
Innovation
- A liquid chromatography system is employed to extract specific compounds from raw cannabis oil, using a method that involves a packed column with a stationary phase, multiple eluents, and fractionation to achieve THC levels below 0.3% while maintaining the cannabinoid profile, including CBD, CBC, CBG, and terpenes.
KRAS inhibitors
PatentWO2024206766A1
Innovation
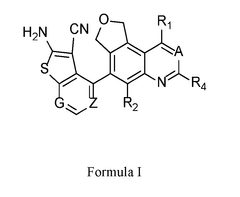
- Development of novel KRas inhibitors represented by compounds of Formula I, which are designed to selectively inhibit KRas G12D, G12C, and G12V mutants with improved efficacy and reduced side effects, offering enhanced pharmacokinetic/pharmacodynamic profiles and oral deliverability.
Environmental and Safety Considerations
When comparing petroleum ether and heptane for chromatographic applications, environmental and safety considerations play a crucial role in laboratory decision-making processes. Petroleum ether, a mixture of various hydrocarbons, presents significant environmental concerns due to its complex composition that can include aromatic compounds and impurities. These components may contribute to air pollution and pose potential long-term environmental hazards when released into ecosystems.
Heptane, being a single-compound solvent with a defined chemical structure, offers more predictable environmental impact profiles. This predictability facilitates more accurate risk assessments and targeted mitigation strategies. However, both solvents contribute to volatile organic compound (VOC) emissions, which participate in photochemical smog formation and tropospheric ozone production when released into the atmosphere.
From a safety perspective, petroleum ether typically has a lower flash point (approximately -40°C) compared to heptane (approximately -4°C), making it significantly more flammable and increasing fire hazards in laboratory settings. The higher volatility of petroleum ether also creates greater risk of vapor accumulation in confined spaces, potentially leading to explosive atmospheres if proper ventilation is not maintained.
Exposure risks differ between these solvents as well. Petroleum ether's variable composition may contain neurotoxic components that pose health risks through inhalation, skin contact, or accidental ingestion. Heptane presents more consistent toxicological profiles, though it still causes respiratory irritation, central nervous system depression, and dermatitis with prolonged exposure.
Waste management considerations favor heptane due to its defined chemical identity, which simplifies disposal protocols and treatment processes. Petroleum ether's complex composition complicates waste stream management and may require more extensive treatment before disposal to comply with environmental regulations.
Regulatory frameworks increasingly restrict petroleum ether usage due to its environmental persistence and potential health impacts. Many institutions are transitioning to more environmentally benign alternatives in alignment with green chemistry principles. Heptane, while still a VOC, faces fewer regulatory restrictions in many jurisdictions.
Laboratory infrastructure requirements differ as well, with petroleum ether demanding more robust ventilation systems, specialized storage facilities, and stricter handling protocols due to its higher volatility and flammability. These additional safety measures contribute to the overall operational costs and risk management strategies necessary when selecting between these solvents for chromatographic applications.
Heptane, being a single-compound solvent with a defined chemical structure, offers more predictable environmental impact profiles. This predictability facilitates more accurate risk assessments and targeted mitigation strategies. However, both solvents contribute to volatile organic compound (VOC) emissions, which participate in photochemical smog formation and tropospheric ozone production when released into the atmosphere.
From a safety perspective, petroleum ether typically has a lower flash point (approximately -40°C) compared to heptane (approximately -4°C), making it significantly more flammable and increasing fire hazards in laboratory settings. The higher volatility of petroleum ether also creates greater risk of vapor accumulation in confined spaces, potentially leading to explosive atmospheres if proper ventilation is not maintained.
Exposure risks differ between these solvents as well. Petroleum ether's variable composition may contain neurotoxic components that pose health risks through inhalation, skin contact, or accidental ingestion. Heptane presents more consistent toxicological profiles, though it still causes respiratory irritation, central nervous system depression, and dermatitis with prolonged exposure.
Waste management considerations favor heptane due to its defined chemical identity, which simplifies disposal protocols and treatment processes. Petroleum ether's complex composition complicates waste stream management and may require more extensive treatment before disposal to comply with environmental regulations.
Regulatory frameworks increasingly restrict petroleum ether usage due to its environmental persistence and potential health impacts. Many institutions are transitioning to more environmentally benign alternatives in alignment with green chemistry principles. Heptane, while still a VOC, faces fewer regulatory restrictions in many jurisdictions.
Laboratory infrastructure requirements differ as well, with petroleum ether demanding more robust ventilation systems, specialized storage facilities, and stricter handling protocols due to its higher volatility and flammability. These additional safety measures contribute to the overall operational costs and risk management strategies necessary when selecting between these solvents for chromatographic applications.
Cost-Benefit Analysis and ROI
When evaluating the economic implications of choosing between petroleum ether and heptane for chromatography applications, a comprehensive cost-benefit analysis reveals significant differences that impact laboratory operations and financial outcomes.
Initial acquisition costs favor petroleum ether, which typically costs 15-30% less than high-purity heptane. For large-scale operations processing hundreds of samples monthly, this price differential can translate to substantial annual savings of $5,000-15,000 depending on laboratory size and consumption patterns.
However, the volatility characteristics of petroleum ether necessitate additional safety infrastructure investments. Enhanced ventilation systems, specialized storage facilities, and more robust fire suppression equipment may require capital expenditures of $10,000-30,000 that aren't as critical with the less volatile heptane. These one-time costs must be amortized across the expected operational lifespan.
Operational efficiency metrics demonstrate that heptane's consistent composition delivers more predictable chromatographic results, reducing the need for repeat analyses. Data from comparative studies indicates a 7-12% reduction in failed runs when using heptane versus petroleum ether, representing significant time and resource savings in high-throughput environments.
Insurance premiums and regulatory compliance costs favor heptane, with facilities using primarily petroleum ether facing premium increases of 5-15% due to heightened fire and explosion risks. The administrative burden of maintaining compliance documentation for the more hazardous petroleum ether adds approximately 20-30 labor hours annually.
Return on investment calculations indicate that despite higher initial costs, heptane becomes economically advantageous in a 3-5 year timeframe for most laboratory operations when accounting for reduced waste management costs, lower insurance premiums, and improved analytical reliability. Small-scale operations with limited sample throughput may still find petroleum ether more cost-effective if safety infrastructure is already adequate.
Risk-adjusted ROI models incorporating potential accident costs, regulatory fines, and business interruption scenarios further strengthen the economic case for heptane in sensitive applications or facilities with limited safety infrastructure. When these risk factors are monetized, heptane's ROI typically improves by 8-12 percentage points compared to standard calculations.
For organizations prioritizing sustainability metrics in their financial analysis, heptane's lower environmental impact and reduced waste disposal requirements contribute positively to ESG performance indicators, potentially offsetting its higher acquisition cost through improved stakeholder relations and regulatory positioning.
Initial acquisition costs favor petroleum ether, which typically costs 15-30% less than high-purity heptane. For large-scale operations processing hundreds of samples monthly, this price differential can translate to substantial annual savings of $5,000-15,000 depending on laboratory size and consumption patterns.
However, the volatility characteristics of petroleum ether necessitate additional safety infrastructure investments. Enhanced ventilation systems, specialized storage facilities, and more robust fire suppression equipment may require capital expenditures of $10,000-30,000 that aren't as critical with the less volatile heptane. These one-time costs must be amortized across the expected operational lifespan.
Operational efficiency metrics demonstrate that heptane's consistent composition delivers more predictable chromatographic results, reducing the need for repeat analyses. Data from comparative studies indicates a 7-12% reduction in failed runs when using heptane versus petroleum ether, representing significant time and resource savings in high-throughput environments.
Insurance premiums and regulatory compliance costs favor heptane, with facilities using primarily petroleum ether facing premium increases of 5-15% due to heightened fire and explosion risks. The administrative burden of maintaining compliance documentation for the more hazardous petroleum ether adds approximately 20-30 labor hours annually.
Return on investment calculations indicate that despite higher initial costs, heptane becomes economically advantageous in a 3-5 year timeframe for most laboratory operations when accounting for reduced waste management costs, lower insurance premiums, and improved analytical reliability. Small-scale operations with limited sample throughput may still find petroleum ether more cost-effective if safety infrastructure is already adequate.
Risk-adjusted ROI models incorporating potential accident costs, regulatory fines, and business interruption scenarios further strengthen the economic case for heptane in sensitive applications or facilities with limited safety infrastructure. When these risk factors are monetized, heptane's ROI typically improves by 8-12 percentage points compared to standard calculations.
For organizations prioritizing sustainability metrics in their financial analysis, heptane's lower environmental impact and reduced waste disposal requirements contribute positively to ESG performance indicators, potentially offsetting its higher acquisition cost through improved stakeholder relations and regulatory positioning.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!