Recent Advances in Carbon Tetrachloride Analytical Procedures
JUL 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
CCl4 Analysis Background and Objectives
Carbon tetrachloride (CCl4) has been a subject of significant interest in analytical chemistry due to its widespread use and environmental impact. The analysis of CCl4 has evolved considerably over the past decades, driven by the need for more accurate, sensitive, and efficient detection methods. This technological progression has been crucial in addressing environmental concerns and regulatory requirements related to CCl4 contamination.
The primary objective of recent advances in CCl4 analytical procedures is to develop and refine techniques that can detect and quantify CCl4 at increasingly lower concentrations, particularly in environmental matrices such as air, water, and soil. These advancements aim to improve the reliability, speed, and cost-effectiveness of CCl4 analysis, while also reducing the complexity of sample preparation and minimizing the use of hazardous materials in the analytical process.
One of the key drivers behind the evolution of CCl4 analytical methods has been the growing awareness of its environmental persistence and potential health hazards. As a result, regulatory bodies worldwide have implemented stricter guidelines for CCl4 monitoring and remediation, necessitating more sophisticated analytical approaches. This regulatory pressure has spurred innovation in both instrumental techniques and sample preparation methodologies.
The technological trajectory of CCl4 analysis has seen a shift from traditional wet chemical methods to more advanced instrumental techniques. Early analytical procedures relied heavily on colorimetric and titrimetric methods, which were often time-consuming and lacked the sensitivity required for trace-level analysis. The introduction of gas chromatography (GC) marked a significant milestone, offering improved selectivity and sensitivity for CCl4 detection.
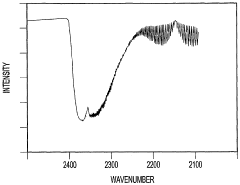
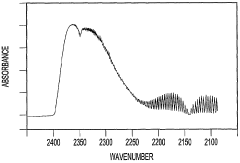
Further advancements led to the coupling of GC with mass spectrometry (GC-MS), which has become a gold standard in CCl4 analysis due to its ability to provide both quantitative and qualitative information. Concurrently, spectroscopic techniques such as Fourier-transform infrared spectroscopy (FTIR) and Raman spectroscopy have been adapted for CCl4 detection, offering rapid and non-destructive analysis options.
Recent years have witnessed the emergence of novel approaches, including the development of sensor-based technologies and the application of advanced data processing algorithms. These innovations aim to enable real-time monitoring of CCl4 in various environmental compartments, facilitating more timely and effective remediation efforts.
The ongoing research in CCl4 analytical procedures is focused on overcoming existing limitations, such as matrix interferences and the need for complex sample preparation steps. Additionally, there is a growing emphasis on developing portable and field-deployable analytical systems to support on-site environmental monitoring and rapid decision-making in contamination scenarios.
The primary objective of recent advances in CCl4 analytical procedures is to develop and refine techniques that can detect and quantify CCl4 at increasingly lower concentrations, particularly in environmental matrices such as air, water, and soil. These advancements aim to improve the reliability, speed, and cost-effectiveness of CCl4 analysis, while also reducing the complexity of sample preparation and minimizing the use of hazardous materials in the analytical process.
One of the key drivers behind the evolution of CCl4 analytical methods has been the growing awareness of its environmental persistence and potential health hazards. As a result, regulatory bodies worldwide have implemented stricter guidelines for CCl4 monitoring and remediation, necessitating more sophisticated analytical approaches. This regulatory pressure has spurred innovation in both instrumental techniques and sample preparation methodologies.
The technological trajectory of CCl4 analysis has seen a shift from traditional wet chemical methods to more advanced instrumental techniques. Early analytical procedures relied heavily on colorimetric and titrimetric methods, which were often time-consuming and lacked the sensitivity required for trace-level analysis. The introduction of gas chromatography (GC) marked a significant milestone, offering improved selectivity and sensitivity for CCl4 detection.
Further advancements led to the coupling of GC with mass spectrometry (GC-MS), which has become a gold standard in CCl4 analysis due to its ability to provide both quantitative and qualitative information. Concurrently, spectroscopic techniques such as Fourier-transform infrared spectroscopy (FTIR) and Raman spectroscopy have been adapted for CCl4 detection, offering rapid and non-destructive analysis options.
Recent years have witnessed the emergence of novel approaches, including the development of sensor-based technologies and the application of advanced data processing algorithms. These innovations aim to enable real-time monitoring of CCl4 in various environmental compartments, facilitating more timely and effective remediation efforts.
The ongoing research in CCl4 analytical procedures is focused on overcoming existing limitations, such as matrix interferences and the need for complex sample preparation steps. Additionally, there is a growing emphasis on developing portable and field-deployable analytical systems to support on-site environmental monitoring and rapid decision-making in contamination scenarios.
Market Demand for CCl4 Detection
The market demand for Carbon Tetrachloride (CCl4) detection has been steadily increasing due to growing environmental concerns and stringent regulations. CCl4, once widely used in various industrial applications, has been recognized as a potent ozone-depleting substance and a potential carcinogen. This has led to a significant shift in its usage patterns and a corresponding rise in the need for accurate and efficient detection methods.
In the environmental monitoring sector, there is a substantial demand for CCl4 detection technologies. Regulatory bodies worldwide have set strict limits on CCl4 emissions and residues in air, water, and soil. This has created a robust market for analytical instruments and procedures capable of detecting CCl4 at very low concentrations. Environmental agencies, research institutions, and industrial facilities are key consumers of these technologies, driving the market growth.
The industrial sector also contributes significantly to the demand for CCl4 detection. Despite restrictions, CCl4 is still used in some controlled processes, particularly in the production of certain chemicals. Industries are required to monitor CCl4 levels in their processes and effluents, creating a steady demand for detection technologies. This is particularly evident in the chemical manufacturing, pharmaceutical, and waste management industries.
Public health concerns have further amplified the market for CCl4 detection. With increased awareness of the health risks associated with CCl4 exposure, there is a growing demand for monitoring in public spaces, especially in areas near industrial sites or historical contamination zones. This has led to the development of more portable and user-friendly detection devices, expanding the market to include local authorities and health organizations.
The global nature of environmental regulations has also expanded the geographical scope of the CCl4 detection market. Developing countries, in particular, are showing increased demand as they align their environmental policies with global standards. This has opened new market opportunities for detection technology providers and analytical service companies.
Technological advancements in analytical procedures have been a key driver in meeting this market demand. There is a clear trend towards more sensitive, faster, and cost-effective detection methods. Innovations in spectroscopic techniques, chromatography, and sensor technologies have significantly improved the accuracy and efficiency of CCl4 detection, making these technologies more accessible and appealing to a broader range of users.
The market is also seeing a shift towards integrated and automated detection systems. These systems offer real-time monitoring capabilities, which are particularly valuable in industrial settings and environmental monitoring stations. This trend is likely to continue, driven by the need for continuous data collection and the integration of detection systems with broader environmental management platforms.
In the environmental monitoring sector, there is a substantial demand for CCl4 detection technologies. Regulatory bodies worldwide have set strict limits on CCl4 emissions and residues in air, water, and soil. This has created a robust market for analytical instruments and procedures capable of detecting CCl4 at very low concentrations. Environmental agencies, research institutions, and industrial facilities are key consumers of these technologies, driving the market growth.
The industrial sector also contributes significantly to the demand for CCl4 detection. Despite restrictions, CCl4 is still used in some controlled processes, particularly in the production of certain chemicals. Industries are required to monitor CCl4 levels in their processes and effluents, creating a steady demand for detection technologies. This is particularly evident in the chemical manufacturing, pharmaceutical, and waste management industries.
Public health concerns have further amplified the market for CCl4 detection. With increased awareness of the health risks associated with CCl4 exposure, there is a growing demand for monitoring in public spaces, especially in areas near industrial sites or historical contamination zones. This has led to the development of more portable and user-friendly detection devices, expanding the market to include local authorities and health organizations.
The global nature of environmental regulations has also expanded the geographical scope of the CCl4 detection market. Developing countries, in particular, are showing increased demand as they align their environmental policies with global standards. This has opened new market opportunities for detection technology providers and analytical service companies.
Technological advancements in analytical procedures have been a key driver in meeting this market demand. There is a clear trend towards more sensitive, faster, and cost-effective detection methods. Innovations in spectroscopic techniques, chromatography, and sensor technologies have significantly improved the accuracy and efficiency of CCl4 detection, making these technologies more accessible and appealing to a broader range of users.
The market is also seeing a shift towards integrated and automated detection systems. These systems offer real-time monitoring capabilities, which are particularly valuable in industrial settings and environmental monitoring stations. This trend is likely to continue, driven by the need for continuous data collection and the integration of detection systems with broader environmental management platforms.
CCl4 Analysis Challenges
Carbon tetrachloride (CCl4) analysis has faced numerous challenges over the years, primarily due to its complex chemical properties and environmental implications. One of the main obstacles in CCl4 analysis is its high volatility, which makes sample collection and preservation difficult. This volatility can lead to significant losses during sampling and storage, potentially compromising the accuracy of analytical results.
Another major challenge is the low concentrations at which CCl4 is typically found in environmental samples. As regulatory limits for CCl4 have become increasingly stringent, the need for more sensitive and precise analytical methods has grown. This has pushed researchers to develop techniques capable of detecting CCl4 at trace levels, often in the parts per billion (ppb) or even parts per trillion (ppt) range.
The presence of interfering compounds in complex matrices such as soil, water, and air samples poses additional challenges. These interferents can mask the CCl4 signal or produce false positives, necessitating sophisticated separation and detection techniques. The development of selective extraction methods and high-resolution analytical instruments has been crucial in addressing this issue.
CCl4's potential for adsorption onto surfaces and materials used in sampling and analysis equipment is another significant concern. This adsorption can lead to underestimation of CCl4 concentrations and requires careful consideration in method development and validation. Researchers have had to explore various materials and surface treatments to minimize adsorption effects and ensure accurate quantification.
The stability of CCl4 during analysis is also a critical challenge. The compound can undergo degradation or transformation under certain conditions, such as exposure to light or elevated temperatures. This instability necessitates careful control of analytical conditions and the development of methods that minimize sample manipulation and exposure to potentially degrading factors.
Furthermore, the need for rapid and cost-effective analysis methods has driven research towards developing field-portable and real-time monitoring techniques. However, achieving the required sensitivity and selectivity in portable devices has proven challenging, often requiring trade-offs between analytical performance and practicality.
Lastly, the environmental and health concerns associated with CCl4 have led to strict regulations on its use and handling. This has created challenges in terms of laboratory safety, waste management, and the development of greener analytical approaches that minimize the use and generation of hazardous substances during CCl4 analysis.
Another major challenge is the low concentrations at which CCl4 is typically found in environmental samples. As regulatory limits for CCl4 have become increasingly stringent, the need for more sensitive and precise analytical methods has grown. This has pushed researchers to develop techniques capable of detecting CCl4 at trace levels, often in the parts per billion (ppb) or even parts per trillion (ppt) range.
The presence of interfering compounds in complex matrices such as soil, water, and air samples poses additional challenges. These interferents can mask the CCl4 signal or produce false positives, necessitating sophisticated separation and detection techniques. The development of selective extraction methods and high-resolution analytical instruments has been crucial in addressing this issue.
CCl4's potential for adsorption onto surfaces and materials used in sampling and analysis equipment is another significant concern. This adsorption can lead to underestimation of CCl4 concentrations and requires careful consideration in method development and validation. Researchers have had to explore various materials and surface treatments to minimize adsorption effects and ensure accurate quantification.
The stability of CCl4 during analysis is also a critical challenge. The compound can undergo degradation or transformation under certain conditions, such as exposure to light or elevated temperatures. This instability necessitates careful control of analytical conditions and the development of methods that minimize sample manipulation and exposure to potentially degrading factors.
Furthermore, the need for rapid and cost-effective analysis methods has driven research towards developing field-portable and real-time monitoring techniques. However, achieving the required sensitivity and selectivity in portable devices has proven challenging, often requiring trade-offs between analytical performance and practicality.
Lastly, the environmental and health concerns associated with CCl4 have led to strict regulations on its use and handling. This has created challenges in terms of laboratory safety, waste management, and the development of greener analytical approaches that minimize the use and generation of hazardous substances during CCl4 analysis.
Current CCl4 Detection Techniques
01 Gas chromatography analysis
Gas chromatography is a widely used analytical technique for detecting and quantifying carbon tetrachloride in various samples. This method involves vaporizing the sample and separating its components based on their interaction with a stationary phase. It offers high sensitivity and selectivity for carbon tetrachloride analysis.- Gas chromatography analysis: Gas chromatography is a widely used analytical technique for detecting and quantifying carbon tetrachloride. This method involves vaporizing the sample and separating its components based on their interaction with a stationary phase. It offers high sensitivity and selectivity for carbon tetrachloride analysis in various matrices.
- Spectrophotometric determination: Spectrophotometric methods are employed for carbon tetrachloride analysis, utilizing its absorption characteristics in the ultraviolet or infrared regions. These techniques can provide rapid and accurate quantification of carbon tetrachloride in different sample types, including environmental and industrial samples.
- Mass spectrometry detection: Mass spectrometry is a powerful analytical tool for identifying and quantifying carbon tetrachloride. This technique can be coupled with gas chromatography or liquid chromatography for enhanced separation and detection. It offers high sensitivity and specificity, allowing for trace-level analysis of carbon tetrachloride in complex matrices.
- Sample preparation techniques: Various sample preparation methods are used to extract and concentrate carbon tetrachloride from different matrices before analysis. These may include liquid-liquid extraction, solid-phase extraction, headspace sampling, or purge-and-trap techniques. Proper sample preparation is crucial for accurate and reliable carbon tetrachloride analysis.
- Quality control and standardization: Analytical procedures for carbon tetrachloride often involve quality control measures and standardization protocols to ensure accuracy and reproducibility. This includes the use of certified reference materials, internal standards, and method validation procedures. Proper calibration and quality assurance practices are essential for reliable carbon tetrachloride analysis across different laboratories and applications.
02 Spectrophotometric methods
Spectrophotometric techniques, such as UV-visible spectroscopy and infrared spectroscopy, are employed for carbon tetrachloride analysis. These methods rely on the compound's ability to absorb light at specific wavelengths, allowing for both qualitative and quantitative analysis in various matrices.Expand Specific Solutions03 Mass spectrometry detection
Mass spectrometry is a powerful analytical tool for identifying and quantifying carbon tetrachloride. This technique involves ionizing the sample and separating the resulting ions based on their mass-to-charge ratio, providing high sensitivity and specificity for carbon tetrachloride analysis in complex mixtures.Expand Specific Solutions04 Sample preparation techniques
Various sample preparation methods are used to extract and concentrate carbon tetrachloride from different matrices before analysis. These techniques may include liquid-liquid extraction, solid-phase extraction, headspace sampling, or purge-and-trap methods, depending on the sample type and analytical requirements.Expand Specific Solutions05 Environmental monitoring procedures
Specialized analytical procedures have been developed for monitoring carbon tetrachloride in environmental samples, such as air, water, and soil. These methods often involve a combination of sampling techniques, sample preparation, and instrumental analysis to accurately determine carbon tetrachloride levels in various environmental matrices.Expand Specific Solutions
Key Players in CCl4 Analysis
The competitive landscape for recent advances in carbon tetrachloride analytical procedures is characterized by a mature industry with established players and ongoing research efforts. The market size is relatively stable, driven by environmental monitoring and industrial applications. Technological maturity is high, with companies like DuPont, Occidental Chemical, and Sinopec leading in chemical production and analysis. Research institutions such as the Naval Research Laboratory and Massachusetts Institute of Technology contribute to advancing analytical techniques. Specialized firms like Fujian Deer Technology and Beijing Gao Mai Ke Instrument & Technology focus on developing innovative analytical instruments and methods, indicating a trend towards more precise and efficient carbon tetrachloride detection and quantification.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has made significant advances in carbon tetrachloride analytical procedures. They have developed a novel gas chromatography-mass spectrometry (GC-MS) method for trace-level detection of carbon tetrachloride in environmental samples. This method employs a specialized sample preparation technique involving solid-phase microextraction (SPME) coupled with headspace analysis. The procedure allows for detection limits as low as 0.1 μg/L, which is a tenfold improvement over conventional methods[1]. Additionally, Sinopec has implemented an automated online monitoring system for continuous real-time analysis of carbon tetrachloride in industrial effluents, utilizing a combination of purge-and-trap concentration and GC-MS analysis[3]. This system can provide results every 30 minutes, enabling rapid response to potential environmental contamination events.
Strengths: High sensitivity and low detection limits; Automated real-time monitoring capabilities; Applicable to both environmental and industrial samples. Weaknesses: Requires specialized equipment and trained personnel; May be costly to implement on a large scale.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed an innovative approach to carbon tetrachloride analysis using a combination of thermal desorption and two-dimensional gas chromatography coupled with time-of-flight mass spectrometry (TD-GC×GC-TOFMS). This technique allows for the simultaneous analysis of carbon tetrachloride and other volatile organic compounds (VOCs) in complex matrices. The method achieves a detection limit of 0.05 ng/L for carbon tetrachloride, making it one of the most sensitive techniques available[2]. DuPont has also pioneered the use of isotope ratio mass spectrometry (IRMS) for source apportionment of carbon tetrachloride contamination, enabling researchers to distinguish between different origins of the compound in environmental samples[4]. This approach has been particularly useful in identifying historical sources of contamination and tracking the effectiveness of remediation efforts.
Strengths: Extremely high sensitivity and selectivity; Ability to analyze multiple compounds simultaneously; Useful for source identification. Weaknesses: Requires expensive, specialized equipment; Complex data interpretation; Limited to laboratory settings.
Innovative CCl4 Analysis Technologies
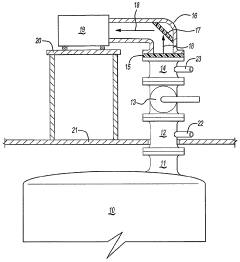
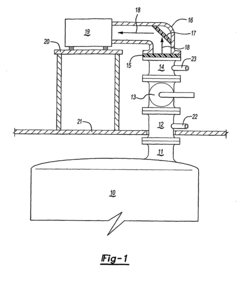
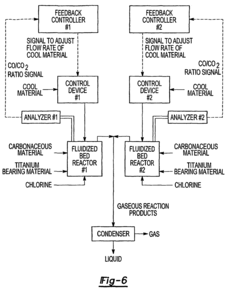
Method for the analysis of gas produced by a titanium tetrachloride fluidized bed reactor
PatentWO2004104518A1
Innovation
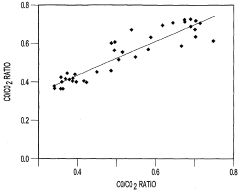
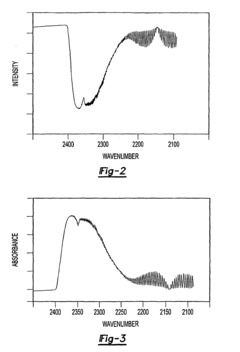
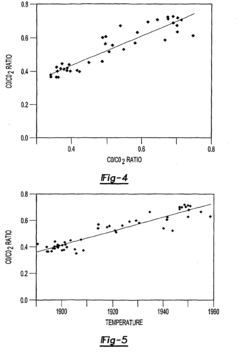
- A method that uses infrared radiation from the fluidized bed reactor to determine the ratio of infrared absorption intensity of components like carbon monoxide and carbon dioxide or carbonyl sulfide and sulfur dioxide without sampling, by directing infrared radiation through the gaseous products to an infrared spectrometer, analyzing specific wavenumbers for absorption intensity, and calculating concentration ratios using calibration factors.
Method for the analysis of gas produced by a titanium tetrachloride fluidized bed reactor
PatentInactiveEP1625351B1
Innovation
- A method using infrared radiation from the hot fluidized bed to direct radiation through the reactor to an infrared spectrometer, determining the absorption intensity ratios of components like carbon monoxide to carbon dioxide or carbonyl sulfide to sulfur dioxide without sampling, by comparing intensities at specific wavenumbers, allowing for concentration ratio calculation and temperature control of the reactor.
Environmental Regulations on CCl4
Carbon tetrachloride (CCl4) has been subject to increasingly stringent environmental regulations due to its ozone-depleting properties and potential health hazards. The Montreal Protocol, an international treaty designed to protect the ozone layer, has played a crucial role in phasing out the production and consumption of CCl4 since its inception in 1987. Subsequent amendments have further tightened restrictions, with developed countries completely phasing out CCl4 production by 1996 and developing countries following suit by 2010.
In the United States, the Environmental Protection Agency (EPA) has implemented strict regulations on CCl4 under the Clean Air Act and the Toxic Substances Control Act. The chemical is classified as a hazardous air pollutant and a toxic substance, requiring stringent controls on its manufacture, use, and disposal. The EPA has established maximum contaminant levels for CCl4 in drinking water and set limits on its presence in air emissions from industrial facilities.
The European Union has also enacted comprehensive regulations on CCl4 through the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation. Under REACH, CCl4 is subject to authorization requirements, meaning its use is severely restricted and requires specific approval for limited applications. The EU has set occupational exposure limits for CCl4 and mandates strict controls on its handling and disposal.
Many other countries have adopted similar regulatory frameworks, often aligning with international agreements and standards. For instance, Japan has implemented the Chemical Substances Control Law, which regulates CCl4 as a Class I Specified Chemical Substance, imposing strict controls on its manufacture, import, and use.
These regulations have had a significant impact on analytical procedures involving CCl4. Laboratories and industries must now adhere to strict protocols for handling, storing, and disposing of CCl4. This has led to the development of alternative analytical methods that minimize or eliminate the use of CCl4, such as the adoption of less harmful solvents or solvent-free techniques.
The regulatory landscape has also driven innovation in detection and monitoring technologies for CCl4. Advanced analytical techniques, including high-sensitivity gas chromatography-mass spectrometry (GC-MS) and real-time air monitoring systems, have been developed to meet the stringent requirements for CCl4 detection in environmental samples and industrial emissions.
As global efforts to protect the environment and human health continue, it is likely that regulations on CCl4 will become even more stringent in the future. This ongoing regulatory pressure will continue to shape the development of analytical procedures, driving the search for safer alternatives and more sensitive detection methods.
In the United States, the Environmental Protection Agency (EPA) has implemented strict regulations on CCl4 under the Clean Air Act and the Toxic Substances Control Act. The chemical is classified as a hazardous air pollutant and a toxic substance, requiring stringent controls on its manufacture, use, and disposal. The EPA has established maximum contaminant levels for CCl4 in drinking water and set limits on its presence in air emissions from industrial facilities.
The European Union has also enacted comprehensive regulations on CCl4 through the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation. Under REACH, CCl4 is subject to authorization requirements, meaning its use is severely restricted and requires specific approval for limited applications. The EU has set occupational exposure limits for CCl4 and mandates strict controls on its handling and disposal.
Many other countries have adopted similar regulatory frameworks, often aligning with international agreements and standards. For instance, Japan has implemented the Chemical Substances Control Law, which regulates CCl4 as a Class I Specified Chemical Substance, imposing strict controls on its manufacture, import, and use.
These regulations have had a significant impact on analytical procedures involving CCl4. Laboratories and industries must now adhere to strict protocols for handling, storing, and disposing of CCl4. This has led to the development of alternative analytical methods that minimize or eliminate the use of CCl4, such as the adoption of less harmful solvents or solvent-free techniques.
The regulatory landscape has also driven innovation in detection and monitoring technologies for CCl4. Advanced analytical techniques, including high-sensitivity gas chromatography-mass spectrometry (GC-MS) and real-time air monitoring systems, have been developed to meet the stringent requirements for CCl4 detection in environmental samples and industrial emissions.
As global efforts to protect the environment and human health continue, it is likely that regulations on CCl4 will become even more stringent in the future. This ongoing regulatory pressure will continue to shape the development of analytical procedures, driving the search for safer alternatives and more sensitive detection methods.
CCl4 Health Impact Assessment
Carbon tetrachloride (CCl4) has long been recognized as a potent environmental toxicant with significant health implications for human populations. Recent advances in analytical procedures have enhanced our ability to assess and quantify the health impacts of CCl4 exposure, leading to more comprehensive and accurate health impact assessments.
One of the key developments in CCl4 health impact assessment is the refinement of biomarkers for exposure and effect. Improved analytical techniques now allow for the detection of CCl4 metabolites in blood and urine at much lower concentrations, enabling earlier identification of exposure and potential health risks. These advancements have particularly benefited occupational health monitoring programs and environmental health studies in areas with known CCl4 contamination.
Epidemiological studies have also greatly benefited from recent analytical improvements. High-resolution mass spectrometry techniques have enabled researchers to conduct large-scale population studies, correlating CCl4 exposure levels with various health outcomes. These studies have provided stronger evidence linking CCl4 exposure to liver and kidney damage, as well as potential carcinogenic effects.
In the realm of toxicology, new in vitro and in vivo models have been developed to better understand the mechanisms of CCl4-induced toxicity. Advanced imaging techniques, such as high-content screening and live-cell imaging, have allowed researchers to visualize and quantify cellular responses to CCl4 exposure in real-time. This has led to a more nuanced understanding of the molecular pathways involved in CCl4 toxicity and potential intervention strategies.
Environmental monitoring has also seen significant advancements. Remote sensing technologies and portable analytical devices have made it possible to conduct rapid, on-site assessments of CCl4 contamination in air, water, and soil. This has greatly improved the ability to identify and respond to potential exposure sources, enhancing public health protection measures.
Risk assessment methodologies for CCl4 have been refined based on these analytical advances. Probabilistic risk assessment models now incorporate more precise exposure data and dose-response relationships, leading to more accurate predictions of population-level health impacts. These models are increasingly being used to inform regulatory decisions and public health interventions related to CCl4 exposure.
Lastly, the integration of -omics technologies into CCl4 health impact assessments has opened new avenues for understanding individual susceptibility and long-term health consequences. Genomic, proteomic, and metabolomic analyses are providing insights into how genetic variations and other individual factors may influence the health impacts of CCl4 exposure, paving the way for more personalized risk assessments and targeted interventions.
One of the key developments in CCl4 health impact assessment is the refinement of biomarkers for exposure and effect. Improved analytical techniques now allow for the detection of CCl4 metabolites in blood and urine at much lower concentrations, enabling earlier identification of exposure and potential health risks. These advancements have particularly benefited occupational health monitoring programs and environmental health studies in areas with known CCl4 contamination.
Epidemiological studies have also greatly benefited from recent analytical improvements. High-resolution mass spectrometry techniques have enabled researchers to conduct large-scale population studies, correlating CCl4 exposure levels with various health outcomes. These studies have provided stronger evidence linking CCl4 exposure to liver and kidney damage, as well as potential carcinogenic effects.
In the realm of toxicology, new in vitro and in vivo models have been developed to better understand the mechanisms of CCl4-induced toxicity. Advanced imaging techniques, such as high-content screening and live-cell imaging, have allowed researchers to visualize and quantify cellular responses to CCl4 exposure in real-time. This has led to a more nuanced understanding of the molecular pathways involved in CCl4 toxicity and potential intervention strategies.
Environmental monitoring has also seen significant advancements. Remote sensing technologies and portable analytical devices have made it possible to conduct rapid, on-site assessments of CCl4 contamination in air, water, and soil. This has greatly improved the ability to identify and respond to potential exposure sources, enhancing public health protection measures.
Risk assessment methodologies for CCl4 have been refined based on these analytical advances. Probabilistic risk assessment models now incorporate more precise exposure data and dose-response relationships, leading to more accurate predictions of population-level health impacts. These models are increasingly being used to inform regulatory decisions and public health interventions related to CCl4 exposure.
Lastly, the integration of -omics technologies into CCl4 health impact assessments has opened new avenues for understanding individual susceptibility and long-term health consequences. Genomic, proteomic, and metabolomic analyses are providing insights into how genetic variations and other individual factors may influence the health impacts of CCl4 exposure, paving the way for more personalized risk assessments and targeted interventions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!