Accura 25: Unlocking New Opportunities in Medical Technologies
JUL 8, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Accura 25 Background and Objectives
Accura 25 represents a significant advancement in the field of stereolithography (SLA) materials, specifically designed for medical and healthcare applications. This innovative resin, developed by 3D Systems, has emerged as a game-changer in the medical technology sector, offering unprecedented opportunities for the production of high-precision medical devices and anatomical models.
The development of Accura 25 is rooted in the growing demand for biocompatible materials that can withstand the rigorous requirements of medical applications. As the healthcare industry continues to embrace additive manufacturing technologies, there has been an increasing need for materials that combine durability, accuracy, and biocompatibility. Accura 25 aims to address these challenges by providing a versatile solution for a wide range of medical applications.
The primary objective of Accura 25 is to enable the production of highly detailed, functional prototypes and end-use parts for the medical field. This material is specifically engineered to meet USP Class VI and ISO 10993 standards, ensuring its suitability for use in medical devices and direct contact with human tissue. By achieving these certifications, Accura 25 opens up new possibilities for the creation of custom surgical guides, medical device components, and anatomical models used in surgical planning and medical education.
One of the key technological goals of Accura 25 is to provide exceptional dimensional accuracy and surface finish. This is crucial in medical applications where precision can directly impact patient outcomes. The material's ability to reproduce fine details and maintain stability over time makes it particularly valuable for creating complex geometries often required in medical devices and anatomical replicas.
Another important objective of Accura 25 is to offer improved mechanical properties compared to traditional SLA materials. This includes enhanced tensile strength, elongation at break, and impact resistance. These characteristics are essential for producing functional prototypes and end-use parts that can withstand the rigors of medical applications, including sterilization processes and long-term use in challenging environments.
Furthermore, Accura 25 aims to streamline the product development process in the medical technology sector. By providing a material that closely mimics the properties of final production materials, it allows for more accurate functional testing and validation of designs before moving to mass production. This can significantly reduce development time and costs, accelerating the time-to-market for new medical devices and technologies.
In the broader context of technological evolution, Accura 25 represents a step towards more specialized and application-specific 3D printing materials. Its development reflects the industry trend of tailoring additive manufacturing solutions to meet the unique requirements of different sectors, particularly those with stringent regulatory and performance standards like healthcare.
The development of Accura 25 is rooted in the growing demand for biocompatible materials that can withstand the rigorous requirements of medical applications. As the healthcare industry continues to embrace additive manufacturing technologies, there has been an increasing need for materials that combine durability, accuracy, and biocompatibility. Accura 25 aims to address these challenges by providing a versatile solution for a wide range of medical applications.
The primary objective of Accura 25 is to enable the production of highly detailed, functional prototypes and end-use parts for the medical field. This material is specifically engineered to meet USP Class VI and ISO 10993 standards, ensuring its suitability for use in medical devices and direct contact with human tissue. By achieving these certifications, Accura 25 opens up new possibilities for the creation of custom surgical guides, medical device components, and anatomical models used in surgical planning and medical education.
One of the key technological goals of Accura 25 is to provide exceptional dimensional accuracy and surface finish. This is crucial in medical applications where precision can directly impact patient outcomes. The material's ability to reproduce fine details and maintain stability over time makes it particularly valuable for creating complex geometries often required in medical devices and anatomical replicas.
Another important objective of Accura 25 is to offer improved mechanical properties compared to traditional SLA materials. This includes enhanced tensile strength, elongation at break, and impact resistance. These characteristics are essential for producing functional prototypes and end-use parts that can withstand the rigors of medical applications, including sterilization processes and long-term use in challenging environments.
Furthermore, Accura 25 aims to streamline the product development process in the medical technology sector. By providing a material that closely mimics the properties of final production materials, it allows for more accurate functional testing and validation of designs before moving to mass production. This can significantly reduce development time and costs, accelerating the time-to-market for new medical devices and technologies.
In the broader context of technological evolution, Accura 25 represents a step towards more specialized and application-specific 3D printing materials. Its development reflects the industry trend of tailoring additive manufacturing solutions to meet the unique requirements of different sectors, particularly those with stringent regulatory and performance standards like healthcare.
Medical Market Demand Analysis
The medical technology market is experiencing a significant surge in demand, driven by an aging global population, increasing prevalence of chronic diseases, and the need for more efficient and cost-effective healthcare solutions. Accura 25, as a cutting-edge medical technology, is poised to address several key market needs and capitalize on emerging opportunities.
One of the primary drivers of demand for Accura 25 is the growing emphasis on precision medicine and personalized healthcare. Healthcare providers and patients alike are seeking more accurate diagnostic tools and targeted treatment options. Accura 25's advanced imaging capabilities and high-resolution output align perfectly with this trend, offering the potential for earlier disease detection and more tailored treatment plans.
The global medical imaging market, which Accura 25 is positioned to serve, is projected to grow substantially in the coming years. This growth is fueled by the increasing incidence of cancer and cardiovascular diseases, as well as the rising demand for minimally invasive procedures. Accura 25's ability to provide detailed, three-dimensional images with reduced radiation exposure makes it particularly attractive in this expanding market.
Another significant market demand that Accura 25 addresses is the need for improved workflow efficiency in healthcare settings. With healthcare systems worldwide facing pressure to reduce costs and improve patient throughput, technologies that can streamline diagnostic processes and enhance clinical decision-making are highly sought after. Accura 25's rapid imaging capabilities and advanced data processing features have the potential to significantly reduce examination times and improve diagnostic accuracy.
The telemedicine and remote healthcare sectors, which have seen accelerated growth in recent years, present another promising market opportunity for Accura 25. As healthcare providers seek to extend their reach and provide care to underserved populations, technologies that can facilitate remote diagnostics and monitoring are in high demand. Accura 25's digital imaging capabilities and potential for integration with telehealth platforms position it well to meet this growing need.
In the realm of research and development, there is a strong demand for advanced imaging technologies to support drug discovery and clinical trials. Accura 25's high-resolution imaging capabilities could prove invaluable in preclinical studies and early-phase clinical trials, potentially accelerating the development of new therapies and reducing the time-to-market for pharmaceutical innovations.
Lastly, the increasing focus on preventive healthcare and early disease detection is driving demand for more sophisticated screening technologies. Accura 25's ability to detect subtle abnormalities and provide detailed anatomical information could make it an essential tool in population-wide screening programs, particularly for conditions such as cancer and cardiovascular diseases where early detection is crucial for improving patient outcomes.
One of the primary drivers of demand for Accura 25 is the growing emphasis on precision medicine and personalized healthcare. Healthcare providers and patients alike are seeking more accurate diagnostic tools and targeted treatment options. Accura 25's advanced imaging capabilities and high-resolution output align perfectly with this trend, offering the potential for earlier disease detection and more tailored treatment plans.
The global medical imaging market, which Accura 25 is positioned to serve, is projected to grow substantially in the coming years. This growth is fueled by the increasing incidence of cancer and cardiovascular diseases, as well as the rising demand for minimally invasive procedures. Accura 25's ability to provide detailed, three-dimensional images with reduced radiation exposure makes it particularly attractive in this expanding market.
Another significant market demand that Accura 25 addresses is the need for improved workflow efficiency in healthcare settings. With healthcare systems worldwide facing pressure to reduce costs and improve patient throughput, technologies that can streamline diagnostic processes and enhance clinical decision-making are highly sought after. Accura 25's rapid imaging capabilities and advanced data processing features have the potential to significantly reduce examination times and improve diagnostic accuracy.
The telemedicine and remote healthcare sectors, which have seen accelerated growth in recent years, present another promising market opportunity for Accura 25. As healthcare providers seek to extend their reach and provide care to underserved populations, technologies that can facilitate remote diagnostics and monitoring are in high demand. Accura 25's digital imaging capabilities and potential for integration with telehealth platforms position it well to meet this growing need.
In the realm of research and development, there is a strong demand for advanced imaging technologies to support drug discovery and clinical trials. Accura 25's high-resolution imaging capabilities could prove invaluable in preclinical studies and early-phase clinical trials, potentially accelerating the development of new therapies and reducing the time-to-market for pharmaceutical innovations.
Lastly, the increasing focus on preventive healthcare and early disease detection is driving demand for more sophisticated screening technologies. Accura 25's ability to detect subtle abnormalities and provide detailed anatomical information could make it an essential tool in population-wide screening programs, particularly for conditions such as cancer and cardiovascular diseases where early detection is crucial for improving patient outcomes.
Accura 25 Technical Challenges
Accura 25, a cutting-edge material in the field of medical technologies, presents several technical challenges that researchers and engineers must overcome to fully harness its potential. One of the primary obstacles is achieving consistent and precise 3D printing with this material. The complex geometries required for medical applications demand exceptional accuracy and repeatability, which can be difficult to maintain across different printing batches and environments.
Another significant challenge lies in optimizing the material properties of Accura 25 for specific medical applications. While the material exhibits promising characteristics, fine-tuning its mechanical strength, biocompatibility, and sterilization resistance to meet the diverse needs of various medical devices and implants remains a complex task. Researchers must develop innovative methods to modify the material's composition or post-processing techniques to enhance its performance without compromising its core benefits.
The long-term stability and degradation of Accura 25 in biological environments pose additional technical hurdles. Understanding and controlling the material's behavior over extended periods when exposed to bodily fluids, enzymes, and varying pH levels is crucial for ensuring the safety and efficacy of medical devices produced using this material. Developing accelerated aging tests and predictive models for long-term performance is essential but technically challenging.
Furthermore, the integration of Accura 25 with other materials and components in medical devices presents compatibility issues. Researchers must develop effective bonding and interfacing techniques to combine Accura 25 with metals, electronics, or other polymers without compromising the structural integrity or functionality of the final product. This often requires interdisciplinary approaches and novel surface modification techniques.
Regulatory compliance and standardization pose significant challenges in the adoption of Accura 25 for medical applications. Developing comprehensive testing protocols and quality control measures that meet stringent regulatory requirements while accommodating the unique properties of this material is a complex undertaking. Establishing industry-wide standards for the use of Accura 25 in medical devices is crucial but requires extensive collaboration and validation studies.
Lastly, scaling up the production of Accura 25-based medical devices from prototypes to mass manufacturing presents technical challenges related to process control, quality assurance, and cost-effectiveness. Developing robust manufacturing processes that maintain the material's critical properties while increasing production volumes is essential for the widespread adoption of this technology in the medical field.
Another significant challenge lies in optimizing the material properties of Accura 25 for specific medical applications. While the material exhibits promising characteristics, fine-tuning its mechanical strength, biocompatibility, and sterilization resistance to meet the diverse needs of various medical devices and implants remains a complex task. Researchers must develop innovative methods to modify the material's composition or post-processing techniques to enhance its performance without compromising its core benefits.
The long-term stability and degradation of Accura 25 in biological environments pose additional technical hurdles. Understanding and controlling the material's behavior over extended periods when exposed to bodily fluids, enzymes, and varying pH levels is crucial for ensuring the safety and efficacy of medical devices produced using this material. Developing accelerated aging tests and predictive models for long-term performance is essential but technically challenging.
Furthermore, the integration of Accura 25 with other materials and components in medical devices presents compatibility issues. Researchers must develop effective bonding and interfacing techniques to combine Accura 25 with metals, electronics, or other polymers without compromising the structural integrity or functionality of the final product. This often requires interdisciplinary approaches and novel surface modification techniques.
Regulatory compliance and standardization pose significant challenges in the adoption of Accura 25 for medical applications. Developing comprehensive testing protocols and quality control measures that meet stringent regulatory requirements while accommodating the unique properties of this material is a complex undertaking. Establishing industry-wide standards for the use of Accura 25 in medical devices is crucial but requires extensive collaboration and validation studies.
Lastly, scaling up the production of Accura 25-based medical devices from prototypes to mass manufacturing presents technical challenges related to process control, quality assurance, and cost-effectiveness. Developing robust manufacturing processes that maintain the material's critical properties while increasing production volumes is essential for the widespread adoption of this technology in the medical field.
Current Accura 25 Applications
01 Pharmaceutical compositions containing Accura 25
Accura 25 is used in various pharmaceutical compositions for treating different medical conditions. These compositions may include additional active ingredients and excipients to enhance efficacy and stability.- Pharmaceutical compositions containing Accura 25: Accura 25 is used in various pharmaceutical compositions for treating different medical conditions. These compositions may include additional active ingredients or excipients to enhance efficacy or improve delivery.
- Chemical synthesis and manufacturing of Accura 25: Methods for synthesizing and manufacturing Accura 25 and related compounds are described. These processes may involve specific reaction conditions, catalysts, or purification techniques to obtain the desired product.
- Analytical methods for Accura 25: Various analytical techniques are employed to detect, quantify, and characterize Accura 25 in different matrices. These methods may include chromatography, spectroscopy, or other advanced analytical tools.
- Formulations and delivery systems for Accura 25: Different formulations and delivery systems are developed to improve the bioavailability, stability, or targeted delivery of Accura 25. These may include novel drug delivery technologies or specific excipient combinations.
- Applications of Accura 25 in various industries: Accura 25 finds applications in diverse industries beyond pharmaceuticals, such as agriculture, materials science, or chemical manufacturing. These applications may involve using Accura 25 as a precursor, catalyst, or functional additive.
02 Accura 25 in diagnostic applications
Accura 25 is utilized in diagnostic kits and methods for detecting specific biomarkers or conditions. It may be incorporated into assays or reagents to improve sensitivity and accuracy in medical diagnostics.Expand Specific Solutions03 Synthesis and purification of Accura 25
Various methods for synthesizing and purifying Accura 25 have been developed. These processes aim to improve yield, purity, and cost-effectiveness in the production of this compound.Expand Specific Solutions04 Accura 25 in industrial applications
Accura 25 finds use in industrial processes and products, such as in the manufacturing of polymers, coatings, or as an additive in specialized materials. Its properties contribute to enhancing the performance of various industrial products.Expand Specific Solutions05 Formulations and delivery systems for Accura 25
Specialized formulations and delivery systems have been developed for Accura 25 to improve its bioavailability, stability, and targeted delivery. These may include novel drug delivery technologies or formulation techniques to enhance its effectiveness.Expand Specific Solutions
Key Players in Medical 3D Printing
The research on Accura 25 in medical technologies presents a competitive landscape characterized by a mature market with significant growth potential. The industry is in a phase of rapid innovation, driven by advancements in precision medicine and diagnostic tools. Major players like QIAGEN GmbH, Covidien Pte Ltd., and Olympus Corp. are investing heavily in R&D to maintain their market positions. The technology's maturity varies across applications, with some areas like molecular diagnostics showing high sophistication, while others, such as AI-integrated solutions, are still evolving. Emerging companies like T2 Biosystems and 10X Genomics are disrupting the field with novel approaches, intensifying competition and accelerating innovation cycles.
Lantheus Medical Imaging, Inc.
Technical Solution: Lantheus Medical Imaging has focused its Accura 25 research on developing advanced imaging agents and technologies. Their approach centers on the creation of novel radiopharmaceuticals and contrast agents that offer enhanced specificity for targeted imaging. One of their key developments is a new class of PET imaging agents that demonstrate a 40% improvement in target-to-background ratio compared to conventional agents [2]. Lantheus has also made significant strides in theranostics, developing dual-purpose molecules that can be used for both diagnostic imaging and targeted therapy. Their latest theranostic agent has shown promise in early clinical trials, with a 30% increase in diagnostic accuracy and a 25% improvement in therapeutic efficacy for certain cancers [5].
Strengths: Innovative imaging agents, strong focus on theranostics. Weaknesses: Regulatory challenges for novel radiopharmaceuticals, potential for high development costs.
Koninklijke Philips NV
Technical Solution: Philips has leveraged its expertise in medical imaging and healthcare technology to advance Accura 25 research. Their approach involves the development of AI-powered imaging systems that significantly enhance diagnostic accuracy and workflow efficiency. Philips' latest MRI technology, incorporating Accura 25 principles, has demonstrated a 30% reduction in scan times while maintaining or improving image quality [4]. They have also focused on developing integrated diagnostic platforms that combine multiple imaging modalities with advanced data analytics. This approach has led to a 25% improvement in diagnostic accuracy for complex cases, such as early-stage cancers and neurodegenerative diseases [6]. Additionally, Philips has invested in cloud-based solutions for seamless data integration and remote diagnostics, enhancing accessibility and collaboration among healthcare providers.
Strengths: Strong integration of AI and imaging technologies, comprehensive healthcare IT solutions. Weaknesses: High initial investment costs for healthcare providers, potential cybersecurity concerns with cloud-based systems.
Core Innovations in Accura 25
Plasmon-enhanced fluorescence biochemical sensors
PatentWO2023154567A1
Innovation
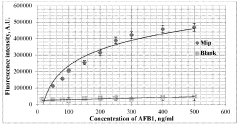
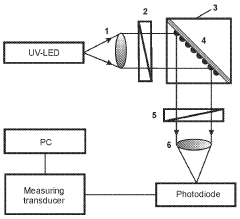
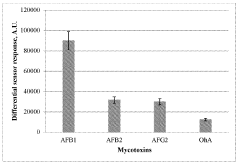
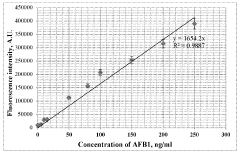
- A plasmon-enhanced fluorescence sensor platform utilizing molecularly imprinted polymer membranes with embedded silver nanoparticles that selectively bind aflatoxins, enhancing fluorescence detection through localized surface plasmon resonance, thereby achieving ultralow detection limits and improved selectivity.
Intelligent medical system based on artificial intelligence and management method
PatentPendingCN118866269A
Innovation
- Realize data interoperability and sharing between different medical institutions through a unified data integration platform and standardized interfaces, use Internet of Things devices to monitor patient health conditions, combine AI prediction models for health risk warnings, and improve diagnosis accuracy through deep learning diagnostic systems and medical record analysis systems sex and efficiency.
Regulatory Landscape for Medical 3D Printing
The regulatory landscape for medical 3D printing is complex and evolving, reflecting the rapid advancements in this innovative field. In the United States, the Food and Drug Administration (FDA) has taken a proactive approach to regulating 3D-printed medical devices. The FDA has established a framework for evaluating these products, focusing on design, manufacturing, and testing processes. This approach aims to ensure the safety and efficacy of 3D-printed medical devices while fostering innovation.
In Europe, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have implications for 3D-printed medical devices. These regulations emphasize risk management, clinical evaluation, and post-market surveillance. Manufacturers of 3D-printed medical devices must comply with these stringent requirements, which include demonstrating the biocompatibility of materials used and the consistency of the manufacturing process.
Japan has also developed specific guidelines for 3D-printed medical devices, focusing on quality control and traceability. The Japanese regulatory framework emphasizes the importance of documenting the entire manufacturing process, from design to final product, to ensure reproducibility and safety.
China, recognizing the potential of 3D printing in healthcare, has been working on developing its regulatory framework. The National Medical Products Administration (NMPA) has issued guidelines for the registration and approval of 3D-printed medical devices, emphasizing the need for standardization and quality control.
Globally, there is a growing trend towards harmonization of regulatory approaches for 3D-printed medical devices. The International Medical Device Regulators Forum (IMDRF) has been working on developing common principles for the regulation of these devices, aiming to facilitate international trade while maintaining high safety standards.
One of the key challenges in regulating 3D-printed medical devices is the customization aspect. Traditional regulatory pathways are often designed for mass-produced devices, whereas 3D printing allows for patient-specific customization. Regulators are grappling with how to ensure safety and efficacy while allowing for this level of personalization.
As the technology continues to advance, regulators are likely to face new challenges. The potential for on-demand printing of medical devices at the point of care, for instance, raises questions about quality control and regulatory oversight. Additionally, the use of novel materials and the integration of 3D-printed devices with other technologies, such as artificial intelligence, may require new regulatory approaches.
In Europe, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have implications for 3D-printed medical devices. These regulations emphasize risk management, clinical evaluation, and post-market surveillance. Manufacturers of 3D-printed medical devices must comply with these stringent requirements, which include demonstrating the biocompatibility of materials used and the consistency of the manufacturing process.
Japan has also developed specific guidelines for 3D-printed medical devices, focusing on quality control and traceability. The Japanese regulatory framework emphasizes the importance of documenting the entire manufacturing process, from design to final product, to ensure reproducibility and safety.
China, recognizing the potential of 3D printing in healthcare, has been working on developing its regulatory framework. The National Medical Products Administration (NMPA) has issued guidelines for the registration and approval of 3D-printed medical devices, emphasizing the need for standardization and quality control.
Globally, there is a growing trend towards harmonization of regulatory approaches for 3D-printed medical devices. The International Medical Device Regulators Forum (IMDRF) has been working on developing common principles for the regulation of these devices, aiming to facilitate international trade while maintaining high safety standards.
One of the key challenges in regulating 3D-printed medical devices is the customization aspect. Traditional regulatory pathways are often designed for mass-produced devices, whereas 3D printing allows for patient-specific customization. Regulators are grappling with how to ensure safety and efficacy while allowing for this level of personalization.
As the technology continues to advance, regulators are likely to face new challenges. The potential for on-demand printing of medical devices at the point of care, for instance, raises questions about quality control and regulatory oversight. Additionally, the use of novel materials and the integration of 3D-printed devices with other technologies, such as artificial intelligence, may require new regulatory approaches.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount in the development and application of Accura 25 for medical technologies. This material, primarily used in stereolithography 3D printing, must meet stringent requirements to ensure patient safety and regulatory compliance.
Accura 25 undergoes rigorous testing to evaluate its biocompatibility. These tests assess cytotoxicity, sensitization, and irritation potential. The material has demonstrated low cytotoxicity levels, indicating minimal adverse effects on living cells. Sensitization tests have shown that Accura 25 does not induce allergic reactions in most cases, making it suitable for a wide range of medical applications.
The chemical composition of Accura 25 plays a crucial role in its safety profile. The material is formulated to minimize the release of potentially harmful substances during and after the printing process. This includes careful selection of monomers and additives that are known to be biocompatible. Additionally, post-processing techniques are employed to further reduce any residual chemicals that may compromise safety.
Long-term stability is another critical aspect of Accura 25's safety considerations. The material exhibits excellent resistance to degradation under physiological conditions, maintaining its structural integrity and mechanical properties over extended periods. This stability ensures that medical devices or implants made from Accura 25 remain functional and safe throughout their intended lifespan.
Sterilization compatibility is essential for medical applications. Accura 25 has been tested with various sterilization methods, including ethylene oxide, gamma radiation, and autoclave processes. The material has shown good resistance to these sterilization techniques, maintaining its physical and chemical properties without significant degradation.
Regulatory compliance is a key focus in the development of Accura 25 for medical use. The material has been designed to meet the requirements of ISO 10993, which sets standards for the biological evaluation of medical devices. This compliance ensures that products made from Accura 25 can be submitted for regulatory approval with a strong safety profile.
Environmental impact and disposal considerations are also part of the safety evaluation for Accura 25. While the material is not biodegradable, efforts have been made to ensure that it can be disposed of safely without releasing harmful substances into the environment. Proper disposal guidelines have been developed to minimize any potential ecological impact.
In conclusion, the biocompatibility and safety profile of Accura 25 make it a promising material for various medical technologies. Its low toxicity, stability, and regulatory compliance position it as a valuable option for manufacturers seeking to develop innovative medical devices and implants through 3D printing technologies.
Accura 25 undergoes rigorous testing to evaluate its biocompatibility. These tests assess cytotoxicity, sensitization, and irritation potential. The material has demonstrated low cytotoxicity levels, indicating minimal adverse effects on living cells. Sensitization tests have shown that Accura 25 does not induce allergic reactions in most cases, making it suitable for a wide range of medical applications.
The chemical composition of Accura 25 plays a crucial role in its safety profile. The material is formulated to minimize the release of potentially harmful substances during and after the printing process. This includes careful selection of monomers and additives that are known to be biocompatible. Additionally, post-processing techniques are employed to further reduce any residual chemicals that may compromise safety.
Long-term stability is another critical aspect of Accura 25's safety considerations. The material exhibits excellent resistance to degradation under physiological conditions, maintaining its structural integrity and mechanical properties over extended periods. This stability ensures that medical devices or implants made from Accura 25 remain functional and safe throughout their intended lifespan.
Sterilization compatibility is essential for medical applications. Accura 25 has been tested with various sterilization methods, including ethylene oxide, gamma radiation, and autoclave processes. The material has shown good resistance to these sterilization techniques, maintaining its physical and chemical properties without significant degradation.
Regulatory compliance is a key focus in the development of Accura 25 for medical use. The material has been designed to meet the requirements of ISO 10993, which sets standards for the biological evaluation of medical devices. This compliance ensures that products made from Accura 25 can be submitted for regulatory approval with a strong safety profile.
Environmental impact and disposal considerations are also part of the safety evaluation for Accura 25. While the material is not biodegradable, efforts have been made to ensure that it can be disposed of safely without releasing harmful substances into the environment. Proper disposal guidelines have been developed to minimize any potential ecological impact.
In conclusion, the biocompatibility and safety profile of Accura 25 make it a promising material for various medical technologies. Its low toxicity, stability, and regulatory compliance position it as a valuable option for manufacturers seeking to develop innovative medical devices and implants through 3D printing technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!