Biodegradable Nitinol Composites for Sustained Drug Release
AUG 6, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biodegradable Nitinol Composites: Background and Objectives
Nitinol, an alloy of nickel and titanium, has been widely used in medical devices due to its unique shape memory and superelastic properties. However, its non-biodegradable nature has limited its application in certain medical fields. The development of biodegradable Nitinol composites represents a significant advancement in biomaterials, offering the potential for temporary medical implants that can dissolve over time, eliminating the need for removal surgeries.
The concept of biodegradable Nitinol composites emerged from the need to combine the advantageous properties of Nitinol with the ability to degrade safely within the body. This innovative approach aims to address the limitations of traditional Nitinol implants while expanding their potential applications, particularly in drug delivery systems.
The evolution of this technology can be traced back to the early 2000s when researchers began exploring ways to modify Nitinol's surface properties to enhance its biocompatibility. As material science and nanotechnology progressed, efforts shifted towards developing composite materials that could maintain Nitinol's desirable characteristics while introducing biodegradability.
A key milestone in this field was the successful creation of Nitinol-based composites incorporating biodegradable polymers or ceramics. These composites demonstrated the ability to degrade over time while still exhibiting shape memory and superelastic behaviors. This breakthrough opened up new possibilities for controlled drug release applications, where the degradation of the material could be synchronized with drug elution rates.
The primary objective of research in biodegradable Nitinol composites for sustained drug release is to develop a versatile platform that can address various medical needs. This includes creating implants that can deliver drugs locally over extended periods, reducing systemic side effects and improving treatment efficacy. The technology aims to combine the mechanical advantages of Nitinol with precise control over material degradation and drug release kinetics.
Another crucial goal is to optimize the composition and manufacturing processes of these composites to ensure predictable and controllable degradation rates. This involves extensive research into the interactions between Nitinol and various biodegradable materials, as well as the effects of different processing techniques on the final product's properties.
Furthermore, researchers are focusing on enhancing the biocompatibility and safety profiles of these composites. This includes studying the potential toxicity of degradation products and developing strategies to mitigate any adverse effects. The ultimate aim is to create a material that can safely degrade within the body without triggering inflammatory responses or other complications.
As the field progresses, there is also a growing emphasis on tailoring these composites for specific medical applications. This involves designing materials with customized degradation profiles, mechanical properties, and drug release characteristics to suit different therapeutic needs, from cardiovascular stents to orthopedic implants and beyond.
The concept of biodegradable Nitinol composites emerged from the need to combine the advantageous properties of Nitinol with the ability to degrade safely within the body. This innovative approach aims to address the limitations of traditional Nitinol implants while expanding their potential applications, particularly in drug delivery systems.
The evolution of this technology can be traced back to the early 2000s when researchers began exploring ways to modify Nitinol's surface properties to enhance its biocompatibility. As material science and nanotechnology progressed, efforts shifted towards developing composite materials that could maintain Nitinol's desirable characteristics while introducing biodegradability.
A key milestone in this field was the successful creation of Nitinol-based composites incorporating biodegradable polymers or ceramics. These composites demonstrated the ability to degrade over time while still exhibiting shape memory and superelastic behaviors. This breakthrough opened up new possibilities for controlled drug release applications, where the degradation of the material could be synchronized with drug elution rates.
The primary objective of research in biodegradable Nitinol composites for sustained drug release is to develop a versatile platform that can address various medical needs. This includes creating implants that can deliver drugs locally over extended periods, reducing systemic side effects and improving treatment efficacy. The technology aims to combine the mechanical advantages of Nitinol with precise control over material degradation and drug release kinetics.
Another crucial goal is to optimize the composition and manufacturing processes of these composites to ensure predictable and controllable degradation rates. This involves extensive research into the interactions between Nitinol and various biodegradable materials, as well as the effects of different processing techniques on the final product's properties.
Furthermore, researchers are focusing on enhancing the biocompatibility and safety profiles of these composites. This includes studying the potential toxicity of degradation products and developing strategies to mitigate any adverse effects. The ultimate aim is to create a material that can safely degrade within the body without triggering inflammatory responses or other complications.
As the field progresses, there is also a growing emphasis on tailoring these composites for specific medical applications. This involves designing materials with customized degradation profiles, mechanical properties, and drug release characteristics to suit different therapeutic needs, from cardiovascular stents to orthopedic implants and beyond.
Market Analysis for Sustained Drug Release Systems
The sustained drug release systems market has been experiencing significant growth due to the increasing prevalence of chronic diseases and the need for more effective drug delivery methods. This market segment is driven by the demand for improved patient compliance, reduced dosing frequency, and enhanced therapeutic outcomes. The global market for sustained release drug delivery systems is projected to expand at a compound annual growth rate (CAGR) of 7.8% from 2021 to 2028.
Several factors contribute to the growing demand for sustained drug release systems. Firstly, the aging population worldwide is more susceptible to chronic conditions that require long-term medication management. Secondly, there is a rising awareness among healthcare providers and patients about the benefits of controlled release formulations in improving treatment efficacy and reducing side effects. Additionally, the pharmaceutical industry's focus on developing innovative drug delivery technologies has further propelled market growth.
The market for sustained drug release systems can be segmented based on technology, route of administration, and therapeutic application. Polymer-based systems dominate the technology segment, accounting for the largest market share due to their versatility and biocompatibility. Oral route of administration holds the majority market share, followed by injectable and transdermal systems. In terms of therapeutic applications, cardiovascular diseases, diabetes, and central nervous system disorders represent the largest market segments for sustained release formulations.
Geographically, North America leads the global market for sustained drug release systems, followed by Europe and Asia-Pacific. The United States, in particular, holds a significant market share due to its advanced healthcare infrastructure, high healthcare expenditure, and strong presence of pharmaceutical companies. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth in the coming years, driven by improving healthcare access and rising disposable incomes.
Key market players in the sustained drug release systems industry include Johnson & Johnson, Pfizer, Merck & Co., Novartis AG, and AstraZeneca. These companies are investing heavily in research and development to introduce novel drug delivery technologies and expand their product portfolios. Strategic collaborations and partnerships between pharmaceutical companies and drug delivery technology providers are becoming increasingly common to leverage expertise and accelerate product development.
The integration of nanotechnology and smart materials in sustained release systems represents a promising trend in the market. These advanced technologies offer enhanced control over drug release kinetics and improved targeting capabilities. Furthermore, the development of biodegradable and biocompatible materials for sustained release formulations aligns with the growing emphasis on sustainability in healthcare.
Several factors contribute to the growing demand for sustained drug release systems. Firstly, the aging population worldwide is more susceptible to chronic conditions that require long-term medication management. Secondly, there is a rising awareness among healthcare providers and patients about the benefits of controlled release formulations in improving treatment efficacy and reducing side effects. Additionally, the pharmaceutical industry's focus on developing innovative drug delivery technologies has further propelled market growth.
The market for sustained drug release systems can be segmented based on technology, route of administration, and therapeutic application. Polymer-based systems dominate the technology segment, accounting for the largest market share due to their versatility and biocompatibility. Oral route of administration holds the majority market share, followed by injectable and transdermal systems. In terms of therapeutic applications, cardiovascular diseases, diabetes, and central nervous system disorders represent the largest market segments for sustained release formulations.
Geographically, North America leads the global market for sustained drug release systems, followed by Europe and Asia-Pacific. The United States, in particular, holds a significant market share due to its advanced healthcare infrastructure, high healthcare expenditure, and strong presence of pharmaceutical companies. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness the fastest growth in the coming years, driven by improving healthcare access and rising disposable incomes.
Key market players in the sustained drug release systems industry include Johnson & Johnson, Pfizer, Merck & Co., Novartis AG, and AstraZeneca. These companies are investing heavily in research and development to introduce novel drug delivery technologies and expand their product portfolios. Strategic collaborations and partnerships between pharmaceutical companies and drug delivery technology providers are becoming increasingly common to leverage expertise and accelerate product development.
The integration of nanotechnology and smart materials in sustained release systems represents a promising trend in the market. These advanced technologies offer enhanced control over drug release kinetics and improved targeting capabilities. Furthermore, the development of biodegradable and biocompatible materials for sustained release formulations aligns with the growing emphasis on sustainability in healthcare.
Current Challenges in Biodegradable Nitinol Development
The development of biodegradable Nitinol composites for sustained drug release faces several significant challenges. One of the primary obstacles is achieving the delicate balance between the material's biodegradability and its mechanical properties. Nitinol, known for its superelasticity and shape memory effects, typically exhibits excellent durability and corrosion resistance. However, introducing biodegradable components to create a composite material often compromises these desirable characteristics.
Another major challenge lies in controlling the degradation rate of the composite material. The ideal biodegradable Nitinol composite should degrade at a rate that matches the healing process of the targeted tissue while maintaining its structural integrity long enough to fulfill its intended function. This requires precise engineering of the material composition and microstructure, which is particularly challenging given the complex interactions between Nitinol and biodegradable components.
The biocompatibility of the degradation products poses yet another hurdle. As the composite material breaks down, it must not release any toxic substances that could harm surrounding tissues or interfere with the drug release mechanism. This necessitates careful selection of biodegradable components and thorough testing of their degradation byproducts in physiological conditions.
Furthermore, ensuring uniform drug distribution within the composite and achieving controlled, sustained release presents significant technical difficulties. The drug release kinetics must be carefully tailored to maintain therapeutic levels over extended periods, which requires a deep understanding of drug-material interactions and diffusion mechanisms within the composite structure.
Manufacturing scalability and reproducibility also present considerable challenges. Producing biodegradable Nitinol composites with consistent properties and performance across different batches demands highly controlled and sophisticated manufacturing processes. This is particularly challenging given the sensitivity of Nitinol's properties to processing conditions and the added complexity of incorporating biodegradable components and drugs.
Lastly, the long-term stability and shelf life of these composites remain a concern. Ensuring that the material maintains its properties and drug efficacy during storage and sterilization processes is crucial for practical applications. This requires extensive stability studies and potentially the development of specialized packaging and storage solutions.
Overcoming these challenges requires interdisciplinary collaboration among materials scientists, biomedical engineers, pharmacologists, and manufacturing experts. Advances in nanotechnology, surface modification techniques, and computational modeling may provide promising avenues for addressing these issues and realizing the full potential of biodegradable Nitinol composites for sustained drug release applications.
Another major challenge lies in controlling the degradation rate of the composite material. The ideal biodegradable Nitinol composite should degrade at a rate that matches the healing process of the targeted tissue while maintaining its structural integrity long enough to fulfill its intended function. This requires precise engineering of the material composition and microstructure, which is particularly challenging given the complex interactions between Nitinol and biodegradable components.
The biocompatibility of the degradation products poses yet another hurdle. As the composite material breaks down, it must not release any toxic substances that could harm surrounding tissues or interfere with the drug release mechanism. This necessitates careful selection of biodegradable components and thorough testing of their degradation byproducts in physiological conditions.
Furthermore, ensuring uniform drug distribution within the composite and achieving controlled, sustained release presents significant technical difficulties. The drug release kinetics must be carefully tailored to maintain therapeutic levels over extended periods, which requires a deep understanding of drug-material interactions and diffusion mechanisms within the composite structure.
Manufacturing scalability and reproducibility also present considerable challenges. Producing biodegradable Nitinol composites with consistent properties and performance across different batches demands highly controlled and sophisticated manufacturing processes. This is particularly challenging given the sensitivity of Nitinol's properties to processing conditions and the added complexity of incorporating biodegradable components and drugs.
Lastly, the long-term stability and shelf life of these composites remain a concern. Ensuring that the material maintains its properties and drug efficacy during storage and sterilization processes is crucial for practical applications. This requires extensive stability studies and potentially the development of specialized packaging and storage solutions.
Overcoming these challenges requires interdisciplinary collaboration among materials scientists, biomedical engineers, pharmacologists, and manufacturing experts. Advances in nanotechnology, surface modification techniques, and computational modeling may provide promising avenues for addressing these issues and realizing the full potential of biodegradable Nitinol composites for sustained drug release applications.
Existing Biodegradable Nitinol Composite Solutions
01 Biodegradable nitinol composites for controlled drug release
Biodegradable nitinol composites are developed for controlled drug release applications. These composites combine the shape memory properties of nitinol with biodegradable materials to create implants or devices that can deliver drugs over time and then safely degrade in the body. The drug release rate can be tailored by adjusting the composition and structure of the composite.- Biodegradable nitinol composites for controlled drug release: Biodegradable nitinol composites are developed for controlled drug release applications. These composites combine the shape memory properties of nitinol with biodegradable materials to create implants that can deliver drugs over time and then degrade safely in the body. The drug release rate can be tailored by adjusting the composition and structure of the composite.
- Surface modification of nitinol for enhanced drug loading: Surface modification techniques are applied to nitinol to enhance its drug loading capacity and control release kinetics. Methods such as plasma treatment, chemical etching, or coating with biocompatible polymers can create a porous or functionalized surface on nitinol, allowing for increased drug adsorption and more controlled release profiles.
- Incorporation of biodegradable polymers with nitinol: Biodegradable polymers are incorporated with nitinol to create composite materials for drug delivery. These polymers, such as polylactic acid (PLA) or polycaprolactone (PCL), can be loaded with drugs and combined with nitinol to form implants or stents that provide both structural support and controlled drug release over time as the polymer degrades.
- Nanostructured nitinol composites for drug delivery: Nanostructured nitinol composites are developed to enhance drug delivery capabilities. These composites incorporate nanoparticles or nanostructures within or on the surface of nitinol, creating a high surface area for drug loading and allowing for more precise control over drug release kinetics. The nanostructures can also improve the biocompatibility and integration of the implant.
- Stimuli-responsive drug release from nitinol composites: Stimuli-responsive drug release mechanisms are incorporated into nitinol composites. These systems can respond to external stimuli such as temperature changes, magnetic fields, or ultrasound to trigger or modulate drug release. The shape memory properties of nitinol can be utilized in conjunction with smart polymers or other responsive materials to create advanced drug delivery implants.
02 Surface modification of nitinol for drug loading and release
The surface of nitinol can be modified to enhance drug loading capacity and control release kinetics. Techniques such as creating porous structures, applying coatings, or attaching functional groups to the nitinol surface are used to improve drug retention and provide sustained release profiles. These modifications can be tailored to specific drug molecules and therapeutic applications.Expand Specific Solutions03 Polymer-nitinol composite systems for drug delivery
Polymer-nitinol composite systems are developed to combine the mechanical properties of nitinol with the drug-carrying capabilities of biodegradable polymers. These composites can be formed into various shapes and structures, such as stents or scaffolds, that provide both structural support and controlled drug release. The polymer component degrades over time, releasing the drug and leaving behind the nitinol framework.Expand Specific Solutions04 Nanostructured nitinol composites for enhanced drug delivery
Nanostructured nitinol composites are engineered to improve drug loading capacity and release kinetics. These composites incorporate nanoscale features such as nanoparticles, nanofibers, or nanoporous structures that increase the surface area for drug adsorption and provide more precise control over release rates. The nanostructured design can also enhance the biocompatibility and integration of the composite with surrounding tissues.Expand Specific Solutions05 Stimuli-responsive drug release from nitinol composites
Stimuli-responsive drug release systems are developed using nitinol composites. These systems can respond to external stimuli such as temperature changes, magnetic fields, or ultrasound to trigger or modulate drug release. The shape memory properties of nitinol are utilized to create devices that can change shape or structure in response to stimuli, enabling on-demand or targeted drug delivery in specific physiological conditions.Expand Specific Solutions
Key Players in Biodegradable Alloy and Drug Delivery
The research on biodegradable Nitinol composites for sustained drug release is in an emerging stage, with significant potential for growth. The market size is expanding as the demand for advanced drug delivery systems increases. Technologically, the field is progressing rapidly, with various companies and institutions contributing to its development. Yale University, Takeda Pharmaceutical, and PolyPid Ltd. are at the forefront, leveraging their expertise in materials science and drug delivery. Genentech and Novartis AG are also making strides in this area, bringing their pharmaceutical prowess to bear. The involvement of these major players indicates a growing maturity in the technology, though it is still evolving. As the field advances, we can expect to see more innovative solutions and potential breakthroughs in sustained drug release mechanisms.
Yale University
Technical Solution: Yale University has developed a novel approach to biodegradable Nitinol composites for sustained drug release. Their research focuses on creating a composite material that combines the shape memory properties of Nitinol with biodegradable polymers. This composite is designed to provide controlled drug release over extended periods. The university's team has engineered a porous Nitinol scaffold coated with a biodegradable polymer matrix containing the drug. As the polymer degrades, it releases the drug in a sustained manner. The shape memory effect of Nitinol allows for minimally invasive deployment and precise positioning of the drug delivery system[1][3]. Yale's research also explores the use of surface modifications to enhance biocompatibility and control degradation rates, ensuring optimal drug release kinetics[5].
Strengths: Combines shape memory properties with controlled drug release, allows for minimally invasive deployment. Weaknesses: Potential challenges in scaling up production, long-term biocompatibility concerns with Nitinol degradation products.
PolyPid Ltd.
Technical Solution: PolyPid Ltd. has developed a proprietary technology platform called PLEX (Polymer-Lipid Encapsulation matriX) for sustained drug release, which could be adapted for use with biodegradable Nitinol composites. Their approach involves encapsulating drugs within a polymer-lipid matrix that provides controlled release over extended periods. While not specifically focused on Nitinol, their technology could potentially be integrated with biodegradable Nitinol scaffolds to create a hybrid drug delivery system. PolyPid's PLEX technology allows for customization of drug release profiles and has shown promise in various therapeutic applications, including wound healing and infection prevention[2][4]. The company has conducted clinical trials demonstrating the efficacy of their sustained release formulations in reducing surgical site infections[6].
Strengths: Proven technology for sustained drug release, customizable release profiles. Weaknesses: May require additional research to integrate with Nitinol composites, limited experience with metal-based delivery systems.
Core Innovations in Nitinol-based Drug Release
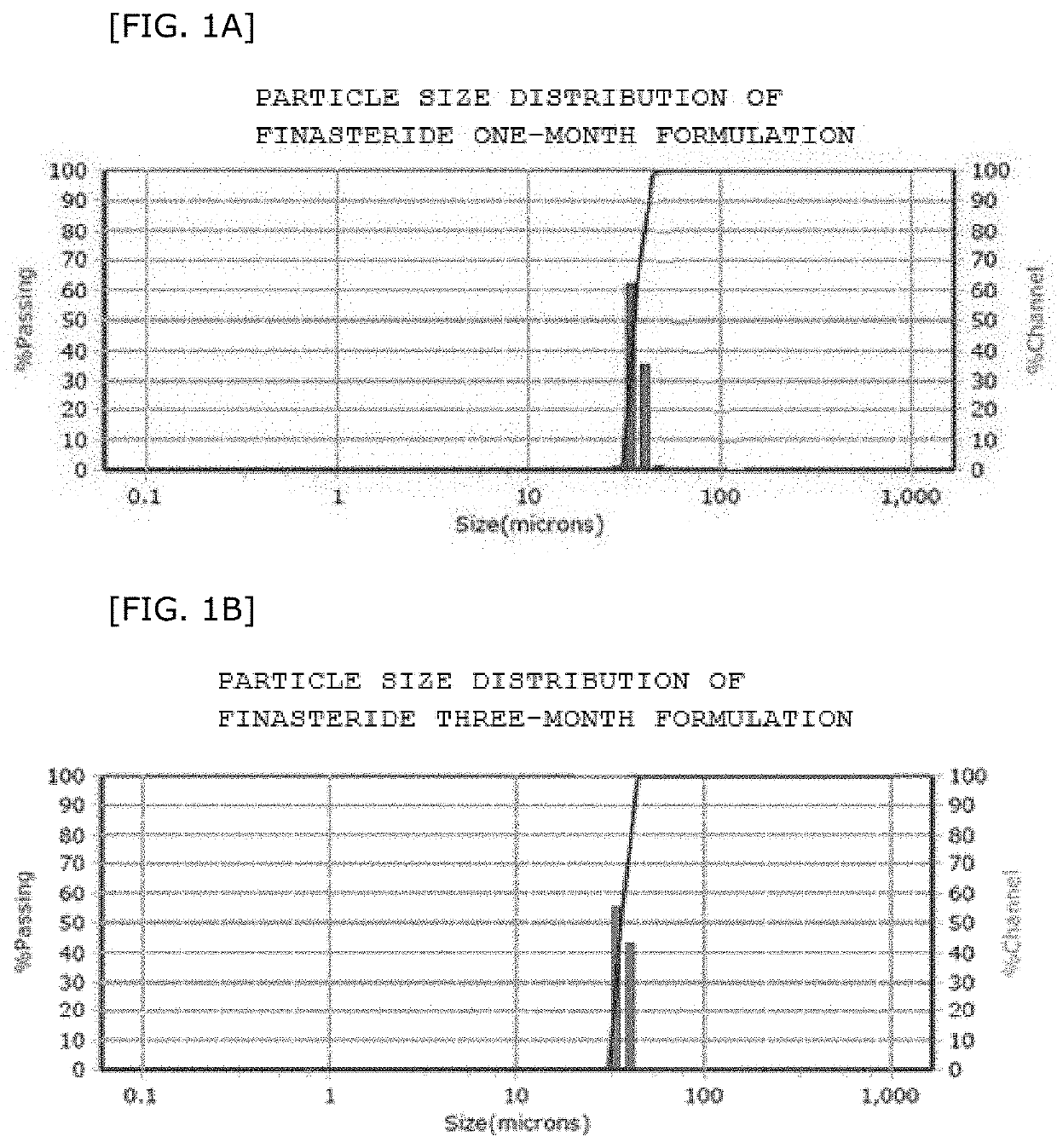
Biodegradable microparticles for sustained drug delivery, methods of preparation and use thereof
PatentWO2024173668A1
Innovation
- The development of covalently and three-dimensionally crosslinked biodegradable microparticles that incorporate a polymer matrix, including crosslinked polylactic acid, polyglycolic acid, or their copolymers, to control drug release independently of solubility in physiologic fluids, providing a zero-order release profile and heat stability for use in high-temperature processes like hot melt extrusion.
Sustained-release microparticles for sustained release of drug
PatentPendingUS20220249452A1
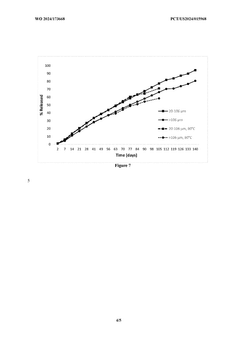
Innovation
- The development of sustained-release microparticles with a biodegradable polymer and drug combination, characterized by uniform particle size distribution, specific surface area, and a controlled mixing ratio, ensuring consistent and prolonged drug release without initial excessive release.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount in the development of biodegradable Nitinol composites for sustained drug release. The unique properties of Nitinol, combined with biodegradable materials, present both opportunities and challenges in ensuring patient safety and optimal therapeutic outcomes.
The primary concern in using Nitinol-based composites is the potential release of nickel ions during degradation. Nickel is known to cause allergic reactions in some individuals and may have toxic effects at high concentrations. Therefore, extensive in vitro and in vivo studies are necessary to evaluate the rate and extent of nickel release from these composites under physiological conditions.
Surface modification techniques, such as passivation or coating with biocompatible polymers, can be employed to enhance the biocompatibility of Nitinol composites. These modifications can create a barrier between the metal and surrounding tissues, reducing the risk of adverse reactions and improving overall safety.
The biodegradable component of the composite must also be carefully selected to ensure its degradation products are non-toxic and easily metabolized by the body. Common biodegradable polymers like polylactic acid (PLA) or poly(lactic-co-glycolic acid) (PLGA) have well-established safety profiles and may be suitable candidates for this application.
Long-term safety studies are crucial to assess the potential for chronic inflammation, foreign body reactions, or systemic toxicity associated with the prolonged presence of the composite in the body. These studies should evaluate tissue response, organ function, and potential accumulation of degradation products over extended periods.
The mechanical properties of the composite must be carefully engineered to maintain structural integrity throughout the drug release period while gradually degrading. Premature failure or unexpected changes in mechanical behavior could lead to device migration or fragmentation, posing significant safety risks.
Sterilization methods for these composites must be thoroughly validated to ensure they do not compromise the material properties or drug stability. Conventional sterilization techniques may not be suitable for all biodegradable materials, necessitating the development of novel sterilization protocols.
Regulatory considerations play a crucial role in the development of these composites. Comprehensive documentation of biocompatibility testing, safety assessments, and clinical data will be required to obtain regulatory approval for medical use. Adherence to international standards, such as ISO 10993 for biological evaluation of medical devices, is essential.
In conclusion, while biodegradable Nitinol composites offer promising potential for sustained drug release, their development must prioritize biocompatibility and safety. Rigorous testing, careful material selection, and adherence to regulatory guidelines are essential to ensure these innovative materials can be safely and effectively used in medical applications.
The primary concern in using Nitinol-based composites is the potential release of nickel ions during degradation. Nickel is known to cause allergic reactions in some individuals and may have toxic effects at high concentrations. Therefore, extensive in vitro and in vivo studies are necessary to evaluate the rate and extent of nickel release from these composites under physiological conditions.
Surface modification techniques, such as passivation or coating with biocompatible polymers, can be employed to enhance the biocompatibility of Nitinol composites. These modifications can create a barrier between the metal and surrounding tissues, reducing the risk of adverse reactions and improving overall safety.
The biodegradable component of the composite must also be carefully selected to ensure its degradation products are non-toxic and easily metabolized by the body. Common biodegradable polymers like polylactic acid (PLA) or poly(lactic-co-glycolic acid) (PLGA) have well-established safety profiles and may be suitable candidates for this application.
Long-term safety studies are crucial to assess the potential for chronic inflammation, foreign body reactions, or systemic toxicity associated with the prolonged presence of the composite in the body. These studies should evaluate tissue response, organ function, and potential accumulation of degradation products over extended periods.
The mechanical properties of the composite must be carefully engineered to maintain structural integrity throughout the drug release period while gradually degrading. Premature failure or unexpected changes in mechanical behavior could lead to device migration or fragmentation, posing significant safety risks.
Sterilization methods for these composites must be thoroughly validated to ensure they do not compromise the material properties or drug stability. Conventional sterilization techniques may not be suitable for all biodegradable materials, necessitating the development of novel sterilization protocols.
Regulatory considerations play a crucial role in the development of these composites. Comprehensive documentation of biocompatibility testing, safety assessments, and clinical data will be required to obtain regulatory approval for medical use. Adherence to international standards, such as ISO 10993 for biological evaluation of medical devices, is essential.
In conclusion, while biodegradable Nitinol composites offer promising potential for sustained drug release, their development must prioritize biocompatibility and safety. Rigorous testing, careful material selection, and adherence to regulatory guidelines are essential to ensure these innovative materials can be safely and effectively used in medical applications.
Regulatory Pathway for Biodegradable Medical Implants
The regulatory pathway for biodegradable medical implants, including biodegradable Nitinol composites for sustained drug release, involves a complex process of approval and oversight by regulatory agencies such as the FDA in the United States and the EMA in Europe. These agencies are responsible for ensuring the safety and efficacy of medical devices before they can be marketed and used in patients.
For biodegradable Nitinol composites, the regulatory process typically begins with preclinical studies to assess the material's biocompatibility, degradation profile, and potential toxicity. These studies must demonstrate that the material breaks down safely in the body without causing adverse effects. Additionally, the drug release kinetics and the impact of degradation on drug delivery must be thoroughly characterized.
Once preclinical data is collected, manufacturers must submit an Investigational Device Exemption (IDE) application to the FDA to begin clinical trials. These trials are crucial for demonstrating the safety and effectiveness of the biodegradable implant in human subjects. The trials are typically conducted in phases, starting with small-scale studies and progressing to larger, more comprehensive trials.
Following successful clinical trials, a Premarket Approval (PMA) application must be submitted to the FDA. This application includes all preclinical and clinical data, manufacturing information, and proposed labeling. The FDA reviews this information to determine if the benefits of the device outweigh its risks.
In Europe, manufacturers must obtain CE marking through a Notified Body. This process involves demonstrating compliance with the Medical Device Regulation (MDR), which includes rigorous safety and performance requirements. The MDR places particular emphasis on post-market surveillance, requiring manufacturers to continuously monitor the performance and safety of their devices after they are released to the market.
For biodegradable implants, long-term follow-up studies are often required to monitor the complete degradation process and any potential long-term effects. This is particularly important for Nitinol composites, as the degradation products and their interactions with the body over time must be carefully assessed.
Regulatory agencies also require manufacturers to implement quality management systems to ensure consistent production and ongoing safety monitoring. This includes processes for handling adverse events, conducting recalls if necessary, and making any required modifications to the device or its labeling.
Given the novel nature of biodegradable Nitinol composites for sustained drug release, regulatory agencies may require additional testing or data beyond standard requirements. This could include specialized degradation studies, detailed analysis of the drug release mechanism, and comprehensive biocompatibility assessments of both the initial implant and its degradation products.
For biodegradable Nitinol composites, the regulatory process typically begins with preclinical studies to assess the material's biocompatibility, degradation profile, and potential toxicity. These studies must demonstrate that the material breaks down safely in the body without causing adverse effects. Additionally, the drug release kinetics and the impact of degradation on drug delivery must be thoroughly characterized.
Once preclinical data is collected, manufacturers must submit an Investigational Device Exemption (IDE) application to the FDA to begin clinical trials. These trials are crucial for demonstrating the safety and effectiveness of the biodegradable implant in human subjects. The trials are typically conducted in phases, starting with small-scale studies and progressing to larger, more comprehensive trials.
Following successful clinical trials, a Premarket Approval (PMA) application must be submitted to the FDA. This application includes all preclinical and clinical data, manufacturing information, and proposed labeling. The FDA reviews this information to determine if the benefits of the device outweigh its risks.
In Europe, manufacturers must obtain CE marking through a Notified Body. This process involves demonstrating compliance with the Medical Device Regulation (MDR), which includes rigorous safety and performance requirements. The MDR places particular emphasis on post-market surveillance, requiring manufacturers to continuously monitor the performance and safety of their devices after they are released to the market.
For biodegradable implants, long-term follow-up studies are often required to monitor the complete degradation process and any potential long-term effects. This is particularly important for Nitinol composites, as the degradation products and their interactions with the body over time must be carefully assessed.
Regulatory agencies also require manufacturers to implement quality management systems to ensure consistent production and ongoing safety monitoring. This includes processes for handling adverse events, conducting recalls if necessary, and making any required modifications to the device or its labeling.
Given the novel nature of biodegradable Nitinol composites for sustained drug release, regulatory agencies may require additional testing or data beyond standard requirements. This could include specialized degradation studies, detailed analysis of the drug release mechanism, and comprehensive biocompatibility assessments of both the initial implant and its degradation products.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!