Glacial Acetic Acid in High-Performance Liquid Chromatography (HPLC)
AUG 5, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HPLC and GAA Overview
High-Performance Liquid Chromatography (HPLC) is a powerful analytical technique widely used in various fields, including pharmaceutical, environmental, and food industries. It offers high resolution, sensitivity, and versatility in separating and analyzing complex mixtures of compounds. HPLC operates by pumping a liquid mobile phase at high pressure through a column packed with stationary phase particles, allowing for the separation of components based on their interactions with both phases.
Glacial Acetic Acid (GAA) plays a crucial role in HPLC as a common component of mobile phases and buffer solutions. Its unique properties, including its ability to act as both a proton donor and acceptor, make it valuable in adjusting pH and improving peak resolution. GAA's low UV absorbance also contributes to its suitability for HPLC applications, particularly when UV detection is employed.
The use of GAA in HPLC has evolved over time, with researchers continually exploring its potential to enhance chromatographic performance. Its application extends beyond simple pH adjustment, as it can influence selectivity, retention times, and peak shapes. The concentration of GAA in mobile phases can be optimized to achieve desired separations for specific analytes.
Recent advancements in HPLC technology have led to the development of ultra-high-performance liquid chromatography (UHPLC) systems, which operate at even higher pressures and use smaller particle sizes. These developments have further expanded the potential applications of GAA in chromatographic separations, allowing for faster analyses and improved resolution.
The combination of HPLC and GAA has proven particularly effective in the analysis of various compounds, including pharmaceuticals, organic acids, and biomolecules. For instance, GAA-containing mobile phases have been successfully employed in the separation and quantification of active pharmaceutical ingredients and their impurities, demonstrating the technique's importance in quality control and drug development processes.
As research in this field continues, scientists are exploring novel applications of GAA in HPLC, such as its use in two-dimensional liquid chromatography and its potential in green chromatography approaches. The ongoing investigation into the fundamental interactions between GAA, analytes, and stationary phases promises to unlock new possibilities for improving chromatographic performance and expanding the range of analyzable compounds.
Glacial Acetic Acid (GAA) plays a crucial role in HPLC as a common component of mobile phases and buffer solutions. Its unique properties, including its ability to act as both a proton donor and acceptor, make it valuable in adjusting pH and improving peak resolution. GAA's low UV absorbance also contributes to its suitability for HPLC applications, particularly when UV detection is employed.
The use of GAA in HPLC has evolved over time, with researchers continually exploring its potential to enhance chromatographic performance. Its application extends beyond simple pH adjustment, as it can influence selectivity, retention times, and peak shapes. The concentration of GAA in mobile phases can be optimized to achieve desired separations for specific analytes.
Recent advancements in HPLC technology have led to the development of ultra-high-performance liquid chromatography (UHPLC) systems, which operate at even higher pressures and use smaller particle sizes. These developments have further expanded the potential applications of GAA in chromatographic separations, allowing for faster analyses and improved resolution.
The combination of HPLC and GAA has proven particularly effective in the analysis of various compounds, including pharmaceuticals, organic acids, and biomolecules. For instance, GAA-containing mobile phases have been successfully employed in the separation and quantification of active pharmaceutical ingredients and their impurities, demonstrating the technique's importance in quality control and drug development processes.
As research in this field continues, scientists are exploring novel applications of GAA in HPLC, such as its use in two-dimensional liquid chromatography and its potential in green chromatography approaches. The ongoing investigation into the fundamental interactions between GAA, analytes, and stationary phases promises to unlock new possibilities for improving chromatographic performance and expanding the range of analyzable compounds.
Market Demand Analysis
The market demand for glacial acetic acid in High-Performance Liquid Chromatography (HPLC) has been steadily growing due to its crucial role in various analytical applications. This organic compound serves as a key component in mobile phases, buffer solutions, and sample preparation processes, making it indispensable in HPLC workflows across multiple industries.
In the pharmaceutical sector, the increasing emphasis on drug development and quality control has led to a surge in HPLC usage, consequently driving the demand for glacial acetic acid. The stringent regulatory requirements for drug purity and safety have further amplified this trend, as HPLC remains the gold standard for pharmaceutical analysis.
The food and beverage industry has also contributed significantly to the market growth of glacial acetic acid in HPLC applications. With rising consumer awareness about food safety and quality, there is a growing need for precise analytical methods to detect contaminants, additives, and nutritional components in food products. HPLC, utilizing glacial acetic acid, plays a crucial role in these analyses.
Environmental monitoring and water quality testing represent another substantial market segment for glacial acetic acid in HPLC. As global concerns about pollution and environmental degradation intensify, there is an increased demand for accurate and sensitive analytical techniques to detect and quantify various pollutants in water, soil, and air samples.
The biotechnology and life sciences sectors have emerged as rapidly growing markets for HPLC applications, further boosting the demand for glacial acetic acid. These fields require high-precision analytical tools for protein and peptide analysis, DNA sequencing, and metabolomics studies, all of which often involve HPLC methods utilizing glacial acetic acid.
In terms of geographical distribution, North America and Europe currently dominate the market for glacial acetic acid in HPLC applications, primarily due to their well-established pharmaceutical and biotechnology industries. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing investments in research and development, expanding pharmaceutical manufacturing capabilities, and growing awareness about food safety and environmental issues.
The market for glacial acetic acid in HPLC is also influenced by technological advancements in chromatography techniques. The development of ultra-high-performance liquid chromatography (UHPLC) and its growing adoption in various industries have created new opportunities for glacial acetic acid usage, as these advanced systems often require specialized mobile phase compositions.
In the pharmaceutical sector, the increasing emphasis on drug development and quality control has led to a surge in HPLC usage, consequently driving the demand for glacial acetic acid. The stringent regulatory requirements for drug purity and safety have further amplified this trend, as HPLC remains the gold standard for pharmaceutical analysis.
The food and beverage industry has also contributed significantly to the market growth of glacial acetic acid in HPLC applications. With rising consumer awareness about food safety and quality, there is a growing need for precise analytical methods to detect contaminants, additives, and nutritional components in food products. HPLC, utilizing glacial acetic acid, plays a crucial role in these analyses.
Environmental monitoring and water quality testing represent another substantial market segment for glacial acetic acid in HPLC. As global concerns about pollution and environmental degradation intensify, there is an increased demand for accurate and sensitive analytical techniques to detect and quantify various pollutants in water, soil, and air samples.
The biotechnology and life sciences sectors have emerged as rapidly growing markets for HPLC applications, further boosting the demand for glacial acetic acid. These fields require high-precision analytical tools for protein and peptide analysis, DNA sequencing, and metabolomics studies, all of which often involve HPLC methods utilizing glacial acetic acid.
In terms of geographical distribution, North America and Europe currently dominate the market for glacial acetic acid in HPLC applications, primarily due to their well-established pharmaceutical and biotechnology industries. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing investments in research and development, expanding pharmaceutical manufacturing capabilities, and growing awareness about food safety and environmental issues.
The market for glacial acetic acid in HPLC is also influenced by technological advancements in chromatography techniques. The development of ultra-high-performance liquid chromatography (UHPLC) and its growing adoption in various industries have created new opportunities for glacial acetic acid usage, as these advanced systems often require specialized mobile phase compositions.
GAA in HPLC Challenges
The use of Glacial Acetic Acid (GAA) in High-Performance Liquid Chromatography (HPLC) presents several significant challenges that researchers and analysts must address. One of the primary issues is the corrosive nature of GAA, which can potentially damage HPLC system components, particularly those made of stainless steel or other susceptible materials. This corrosion risk necessitates careful selection of compatible materials for system components and regular maintenance to prevent degradation.
Another challenge is the high viscosity of GAA, which can lead to increased back pressure in the HPLC system. This elevated pressure may cause issues with pump performance, column efficiency, and overall system stability. Analysts must carefully optimize flow rates and consider using specialized pumps or system configurations to mitigate these effects.
The strong odor and potential health hazards associated with GAA pose safety concerns for laboratory personnel. Proper ventilation, personal protective equipment, and handling protocols are essential to ensure a safe working environment. Additionally, the disposal of GAA-containing waste requires specific procedures to comply with environmental regulations.
GAA's high UV absorbance can interfere with the detection of analytes, particularly at lower wavelengths. This limitation may necessitate the use of alternative detection methods or careful method development to minimize interference. Furthermore, the strong acidity of GAA can affect the stability and ionization of certain analytes, potentially leading to peak shape distortions or reduced sensitivity.
Temperature control becomes crucial when working with GAA in HPLC, as its physical properties can vary significantly with temperature changes. Maintaining consistent temperature throughout the system is essential for reproducible results, which may require specialized column ovens or temperature-controlled environments.
The preparation and storage of mobile phases containing GAA present additional challenges. The acid's hygroscopic nature can lead to changes in concentration over time, affecting method reproducibility. Careful preparation, storage, and regular verification of mobile phase composition are necessary to maintain consistent chromatographic performance.
Lastly, the compatibility of GAA with certain HPLC column stationary phases can be problematic. Some bonded phases may degrade or exhibit reduced lifetime when exposed to high concentrations of GAA. This necessitates careful selection of column chemistry and thorough method validation to ensure long-term column stability and consistent chromatographic performance.
Another challenge is the high viscosity of GAA, which can lead to increased back pressure in the HPLC system. This elevated pressure may cause issues with pump performance, column efficiency, and overall system stability. Analysts must carefully optimize flow rates and consider using specialized pumps or system configurations to mitigate these effects.
The strong odor and potential health hazards associated with GAA pose safety concerns for laboratory personnel. Proper ventilation, personal protective equipment, and handling protocols are essential to ensure a safe working environment. Additionally, the disposal of GAA-containing waste requires specific procedures to comply with environmental regulations.
GAA's high UV absorbance can interfere with the detection of analytes, particularly at lower wavelengths. This limitation may necessitate the use of alternative detection methods or careful method development to minimize interference. Furthermore, the strong acidity of GAA can affect the stability and ionization of certain analytes, potentially leading to peak shape distortions or reduced sensitivity.
Temperature control becomes crucial when working with GAA in HPLC, as its physical properties can vary significantly with temperature changes. Maintaining consistent temperature throughout the system is essential for reproducible results, which may require specialized column ovens or temperature-controlled environments.
The preparation and storage of mobile phases containing GAA present additional challenges. The acid's hygroscopic nature can lead to changes in concentration over time, affecting method reproducibility. Careful preparation, storage, and regular verification of mobile phase composition are necessary to maintain consistent chromatographic performance.
Lastly, the compatibility of GAA with certain HPLC column stationary phases can be problematic. Some bonded phases may degrade or exhibit reduced lifetime when exposed to high concentrations of GAA. This necessitates careful selection of column chemistry and thorough method validation to ensure long-term column stability and consistent chromatographic performance.
Current GAA Applications
01 Production methods of glacial acetic acid
Various methods are employed to produce glacial acetic acid, including oxidation of acetaldehyde, fermentation processes, and catalytic reactions. These methods often involve specific reaction conditions, catalysts, and purification steps to achieve high purity acetic acid.- Production methods of glacial acetic acid: Various methods are employed to produce glacial acetic acid, including oxidation of acetaldehyde, fermentation processes, and catalytic reactions. These methods often involve specific reaction conditions, catalysts, and purification steps to achieve high purity acetic acid.
- Purification and concentration techniques: Purification and concentration of acetic acid to achieve glacial grade involves processes such as distillation, crystallization, and membrane separation. These techniques aim to remove impurities and increase the acid concentration to near 100%.
- Applications in chemical synthesis: Glacial acetic acid serves as a crucial reagent and solvent in various chemical synthesis processes. It is used in the production of vinyl acetate monomer, acetic anhydride, and other organic compounds, playing a vital role in industrial chemistry.
- Storage and handling equipment: Specialized equipment is required for the safe storage and handling of glacial acetic acid due to its corrosive nature. This includes corrosion-resistant tanks, piping systems, and safety measures to prevent leaks and protect workers.
- Environmental and safety considerations: The production and use of glacial acetic acid involve environmental and safety considerations. This includes waste treatment, emission control, and implementing safety protocols to handle the corrosive and potentially hazardous nature of the compound.
02 Purification and concentration techniques
Purification and concentration of acetic acid to achieve glacial grade often involves distillation, crystallization, and membrane separation processes. These techniques aim to remove impurities and increase the acid concentration to near 100%.Expand Specific Solutions03 Applications in chemical synthesis
Glacial acetic acid serves as a crucial reagent and solvent in various chemical synthesis processes. It is used in the production of vinyl acetate monomer, acetic anhydride, and other organic compounds, playing a vital role in industrial chemistry.Expand Specific Solutions04 Storage and handling equipment
Specialized equipment is required for the safe storage and handling of glacial acetic acid due to its corrosive nature. This includes corrosion-resistant tanks, piping systems, and safety measures to prevent leaks and protect workers.Expand Specific Solutions05 Environmental and safety considerations
The production and use of glacial acetic acid involve environmental and safety considerations. This includes waste treatment, emission control, and implementing safety protocols to handle the corrosive and potentially hazardous nature of the compound.Expand Specific Solutions
Key HPLC Manufacturers
The research on Glacial Acetic Acid in High-Performance Liquid Chromatography (HPLC) is in a mature stage of development, with a growing market driven by increasing demand for analytical techniques in various industries. The global HPLC market size is substantial, expected to reach billions of dollars in the coming years. Technologically, the field is well-established, with companies like Agilent Technologies, Waters Technology, and Phenomenex leading in innovation. These firms, along with others such as Daicel Corp. and Dionex Corp., are continuously improving HPLC systems and developing specialized columns for enhanced separation and analysis of glacial acetic acid and related compounds. The competition is intense, with players focusing on product differentiation, R&D investments, and strategic partnerships to maintain their market positions.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has developed advanced HPLC systems optimized for glacial acetic acid applications. Their InfinityLab LC Series offers high-pressure capabilities up to 1300 bar, allowing for the use of smaller particle size columns and faster analysis times with glacial acetic acid mobile phases[1]. The company has also introduced specialized column chemistries, such as the ZORBAX RRHD StableBond series, which provide excellent peak shape and resolution when using acidic mobile phases like glacial acetic acid[2]. Agilent's OpenLab CDS software includes features for method optimization and troubleshooting specifically for challenging acidic conditions[3].
Strengths: Comprehensive HPLC solutions optimized for acidic conditions, high-pressure capabilities, specialized column chemistries. Weaknesses: Potentially higher cost compared to general-purpose systems, may require specialized training for optimal use.
Daicel Corp.
Technical Solution: Daicel Corporation, a leading manufacturer of chiral HPLC columns, has developed specialized stationary phases for use with glacial acetic acid mobile phases. Their CHIRALPAK series of columns, particularly the CHIRALPAK IB and IC, demonstrate excellent stability and enantioselectivity in the presence of acetic acid[13]. Daicel has also introduced the CHIRALPAK IG column, which shows improved performance in highly acidic conditions, making it suitable for glacial acetic acid applications[14]. The company's immobilized chiral stationary phases offer extended column lifetime and broader solvent compatibility, including with acidic mobile phases containing glacial acetic acid[15].
Strengths: Expertise in chiral separations, specialized columns for acidic conditions, broad range of chiral selectivities. Weaknesses: Focus primarily on chiral separations may limit applicability in some HPLC applications, may require specialized knowledge for optimal use.
GAA-HPLC Innovations
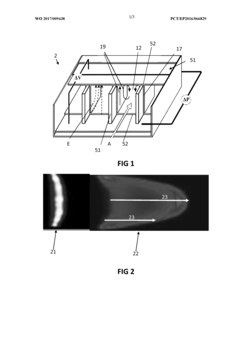
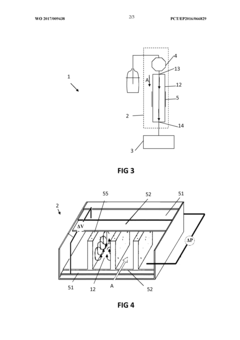
High-performance liquid chromatography with a controllable transverse flow inducer
PatentWO2017009438A1
Innovation
- The use of a controllable transverse flow inducer, such as an array of electrodes generating an alternating current electrokinetic field, to create micro-scale vortices that reduce dispersion and enhance mass transfer between support structures in the chromatography column, allowing for efficient separation without permanent surface charges and minimizing direct contact with electrodes.
A method for producing a separating agent
PatentInactiveIN202048033219A
Innovation
- A new separating medium is developed using a supported ligand derived from a (meth)acrylic polymer with a constituent unit from compounds like aminotetrazole, which enhances retention through ion interactions and hydrogen bonding, specifically formed by radical polymerization on a silica gel or silica monolith support without metal catalysts.
Environmental Impact
The use of glacial acetic acid in High-Performance Liquid Chromatography (HPLC) has significant environmental implications that warrant careful consideration. As a widely used solvent in HPLC mobile phases, glacial acetic acid plays a crucial role in achieving optimal separation and detection of analytes. However, its production, usage, and disposal can have substantial environmental impacts.
The manufacturing process of glacial acetic acid involves energy-intensive procedures and potential emissions of volatile organic compounds (VOCs). These emissions can contribute to air pollution and the formation of ground-level ozone, which has adverse effects on human health and ecosystems. Additionally, the production of glacial acetic acid often relies on fossil fuel-based feedstocks, further contributing to greenhouse gas emissions and climate change concerns.
In HPLC applications, the use of glacial acetic acid as a mobile phase component results in the generation of acidic waste streams. These waste streams, if not properly managed, can pose risks to aquatic ecosystems and wastewater treatment systems. The low pH of acetic acid-containing effluents can disrupt the balance of natural water bodies and potentially harm aquatic organisms.
Proper disposal of HPLC waste containing glacial acetic acid is crucial to mitigate environmental risks. Many laboratories implement waste management protocols that involve neutralization or dilution of acidic waste before disposal. However, these practices may not completely eliminate the environmental impact and can still contribute to the overall chemical burden in wastewater treatment facilities.
The environmental footprint of glacial acetic acid extends beyond its direct use in HPLC. The transportation and storage of this chemical also present potential risks, including accidental spills or leaks that could contaminate soil and water resources. Furthermore, the production of high-purity glacial acetic acid for HPLC applications often requires additional purification steps, which may involve energy-intensive processes and the use of additional chemicals.
As environmental awareness grows within the scientific community, there is an increasing focus on developing more sustainable alternatives to traditional HPLC solvents. Green chemistry initiatives are exploring the use of bio-based acetic acid sources and other environmentally friendly mobile phase additives. These efforts aim to reduce the reliance on petrochemical-derived acetic acid and minimize the overall environmental impact of HPLC techniques.
In conclusion, while glacial acetic acid remains an essential component in many HPLC applications, its environmental impact cannot be overlooked. Balancing analytical performance with environmental responsibility is crucial for the sustainable development of chromatographic techniques. Ongoing research and innovation in this field are essential to identify and implement more eco-friendly alternatives without compromising the quality and reliability of HPLC analyses.
The manufacturing process of glacial acetic acid involves energy-intensive procedures and potential emissions of volatile organic compounds (VOCs). These emissions can contribute to air pollution and the formation of ground-level ozone, which has adverse effects on human health and ecosystems. Additionally, the production of glacial acetic acid often relies on fossil fuel-based feedstocks, further contributing to greenhouse gas emissions and climate change concerns.
In HPLC applications, the use of glacial acetic acid as a mobile phase component results in the generation of acidic waste streams. These waste streams, if not properly managed, can pose risks to aquatic ecosystems and wastewater treatment systems. The low pH of acetic acid-containing effluents can disrupt the balance of natural water bodies and potentially harm aquatic organisms.
Proper disposal of HPLC waste containing glacial acetic acid is crucial to mitigate environmental risks. Many laboratories implement waste management protocols that involve neutralization or dilution of acidic waste before disposal. However, these practices may not completely eliminate the environmental impact and can still contribute to the overall chemical burden in wastewater treatment facilities.
The environmental footprint of glacial acetic acid extends beyond its direct use in HPLC. The transportation and storage of this chemical also present potential risks, including accidental spills or leaks that could contaminate soil and water resources. Furthermore, the production of high-purity glacial acetic acid for HPLC applications often requires additional purification steps, which may involve energy-intensive processes and the use of additional chemicals.
As environmental awareness grows within the scientific community, there is an increasing focus on developing more sustainable alternatives to traditional HPLC solvents. Green chemistry initiatives are exploring the use of bio-based acetic acid sources and other environmentally friendly mobile phase additives. These efforts aim to reduce the reliance on petrochemical-derived acetic acid and minimize the overall environmental impact of HPLC techniques.
In conclusion, while glacial acetic acid remains an essential component in many HPLC applications, its environmental impact cannot be overlooked. Balancing analytical performance with environmental responsibility is crucial for the sustainable development of chromatographic techniques. Ongoing research and innovation in this field are essential to identify and implement more eco-friendly alternatives without compromising the quality and reliability of HPLC analyses.
Method Validation
Method validation is a critical step in the development and implementation of analytical methods using High-Performance Liquid Chromatography (HPLC) with glacial acetic acid. This process ensures that the method is reliable, reproducible, and fit for its intended purpose. The validation of HPLC methods involving glacial acetic acid typically includes several key parameters.
Selectivity is a crucial aspect of method validation, ensuring that the analyte of interest can be accurately identified and quantified in the presence of other components. For HPLC methods using glacial acetic acid, this involves demonstrating that the acetic acid does not interfere with the detection of target compounds and that it enhances the separation of analytes.
Linearity is another important parameter, which assesses the method's ability to produce results directly proportional to the concentration of the analyte. In the context of glacial acetic acid in HPLC, linearity studies would involve preparing calibration curves using standard solutions with varying concentrations of the analyte in the presence of acetic acid.
Accuracy and precision are fundamental to method validation. Accuracy is typically evaluated through recovery studies, where known amounts of the analyte are added to samples and the percentage recovery is calculated. Precision is assessed through repeatability and intermediate precision studies, involving multiple injections of the same sample under various conditions.
The limit of detection (LOD) and limit of quantification (LOQ) are crucial parameters in method validation, particularly for trace analysis. These limits are determined by analyzing samples with decreasing concentrations of the analyte until the lowest detectable and quantifiable levels are established.
Robustness testing is essential to ensure that the method remains reliable under slight variations in experimental conditions. For HPLC methods using glacial acetic acid, this may involve studying the effects of small changes in acetic acid concentration, pH, or column temperature on the method's performance.
Stability studies are also an integral part of method validation, especially when working with glacial acetic acid. These studies assess the stability of the analyte and internal standards in the mobile phase and sample solutions over time, ensuring that the method remains accurate throughout the analysis period.
Selectivity is a crucial aspect of method validation, ensuring that the analyte of interest can be accurately identified and quantified in the presence of other components. For HPLC methods using glacial acetic acid, this involves demonstrating that the acetic acid does not interfere with the detection of target compounds and that it enhances the separation of analytes.
Linearity is another important parameter, which assesses the method's ability to produce results directly proportional to the concentration of the analyte. In the context of glacial acetic acid in HPLC, linearity studies would involve preparing calibration curves using standard solutions with varying concentrations of the analyte in the presence of acetic acid.
Accuracy and precision are fundamental to method validation. Accuracy is typically evaluated through recovery studies, where known amounts of the analyte are added to samples and the percentage recovery is calculated. Precision is assessed through repeatability and intermediate precision studies, involving multiple injections of the same sample under various conditions.
The limit of detection (LOD) and limit of quantification (LOQ) are crucial parameters in method validation, particularly for trace analysis. These limits are determined by analyzing samples with decreasing concentrations of the analyte until the lowest detectable and quantifiable levels are established.
Robustness testing is essential to ensure that the method remains reliable under slight variations in experimental conditions. For HPLC methods using glacial acetic acid, this may involve studying the effects of small changes in acetic acid concentration, pH, or column temperature on the method's performance.
Stability studies are also an integral part of method validation, especially when working with glacial acetic acid. These studies assess the stability of the analyte and internal standards in the mobile phase and sample solutions over time, ensuring that the method remains accurate throughout the analysis period.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!