Sulfamic Acid as a Neutralizer in Chemical Manufacturing
JUL 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfamic Acid Background and Objectives
Sulfamic acid, a crystalline compound with the chemical formula H3NSO3, has a rich history in chemical manufacturing dating back to its discovery in the late 19th century. Initially recognized for its unique properties as a strong acid with low corrosivity, sulfamic acid has since found widespread applications across various industries. The evolution of sulfamic acid usage has been driven by its versatility, safety profile, and effectiveness in numerous chemical processes.
In recent years, the focus on sulfamic acid as a neutralizer in chemical manufacturing has intensified due to growing environmental concerns and the need for more efficient production methods. This renewed interest stems from sulfamic acid's ability to neutralize alkaline solutions effectively while producing fewer byproducts compared to traditional mineral acids. The primary objective of this research is to explore and evaluate the potential of sulfamic acid as a superior neutralizing agent in chemical manufacturing processes.
The development trajectory of sulfamic acid technology has been marked by significant milestones. Early applications were primarily in descaling and cleaning operations, but as understanding of its chemical properties deepened, its use expanded into more sophisticated areas of chemical synthesis and process control. The current research aims to build upon this foundation, pushing the boundaries of sulfamic acid's capabilities in neutralization reactions.
Key technological goals for this investigation include optimizing the efficiency of sulfamic acid in neutralization processes, minimizing waste generation, and enhancing the overall sustainability of chemical manufacturing operations. Additionally, the research seeks to identify novel applications where sulfamic acid's unique properties can offer advantages over conventional neutralizing agents.
The exploration of sulfamic acid as a neutralizer is driven by several factors, including stricter environmental regulations, the pursuit of cost-effective manufacturing processes, and the demand for safer handling of chemicals in industrial settings. By focusing on these aspects, the research aims to contribute to the development of more environmentally friendly and economically viable chemical manufacturing practices.
As the chemical industry continues to evolve, the role of sulfamic acid in neutralization processes represents a promising area for innovation and improvement. This research endeavors to provide a comprehensive understanding of sulfamic acid's potential, paving the way for its increased adoption and optimization in chemical manufacturing. The outcomes of this investigation are expected to have far-reaching implications for industrial processes, environmental protection, and the advancement of sustainable chemistry practices.
In recent years, the focus on sulfamic acid as a neutralizer in chemical manufacturing has intensified due to growing environmental concerns and the need for more efficient production methods. This renewed interest stems from sulfamic acid's ability to neutralize alkaline solutions effectively while producing fewer byproducts compared to traditional mineral acids. The primary objective of this research is to explore and evaluate the potential of sulfamic acid as a superior neutralizing agent in chemical manufacturing processes.
The development trajectory of sulfamic acid technology has been marked by significant milestones. Early applications were primarily in descaling and cleaning operations, but as understanding of its chemical properties deepened, its use expanded into more sophisticated areas of chemical synthesis and process control. The current research aims to build upon this foundation, pushing the boundaries of sulfamic acid's capabilities in neutralization reactions.
Key technological goals for this investigation include optimizing the efficiency of sulfamic acid in neutralization processes, minimizing waste generation, and enhancing the overall sustainability of chemical manufacturing operations. Additionally, the research seeks to identify novel applications where sulfamic acid's unique properties can offer advantages over conventional neutralizing agents.
The exploration of sulfamic acid as a neutralizer is driven by several factors, including stricter environmental regulations, the pursuit of cost-effective manufacturing processes, and the demand for safer handling of chemicals in industrial settings. By focusing on these aspects, the research aims to contribute to the development of more environmentally friendly and economically viable chemical manufacturing practices.
As the chemical industry continues to evolve, the role of sulfamic acid in neutralization processes represents a promising area for innovation and improvement. This research endeavors to provide a comprehensive understanding of sulfamic acid's potential, paving the way for its increased adoption and optimization in chemical manufacturing. The outcomes of this investigation are expected to have far-reaching implications for industrial processes, environmental protection, and the advancement of sustainable chemistry practices.
Market Demand Analysis
The market demand for sulfamic acid as a neutralizer in chemical manufacturing has been steadily growing due to its unique properties and versatile applications. This compound has gained significant traction in various industrial sectors, particularly in the production of cleaning agents, water treatment, and metal finishing processes.
In the cleaning industry, sulfamic acid has become a preferred choice for descaling and removing mineral deposits. Its effectiveness in dissolving limescale and rust without damaging underlying surfaces has led to increased adoption in household and industrial cleaning products. The rising awareness of hygiene and sanitation, especially in the wake of global health concerns, has further boosted the demand for sulfamic acid-based cleaners.
The water treatment sector represents another substantial market for sulfamic acid. As regulations on water quality become more stringent worldwide, there is a growing need for efficient and environmentally friendly water treatment solutions. Sulfamic acid's ability to control pH levels and remove scale in water systems has made it an essential component in municipal water treatment plants and industrial cooling systems.
In metal finishing applications, sulfamic acid has found extensive use as a pickling agent and surface treatment chemical. The automotive and aerospace industries, in particular, have shown increased demand for sulfamic acid in their manufacturing processes. Its ability to effectively clean and prepare metal surfaces without the corrosive effects of stronger acids has made it a valuable alternative in these high-precision industries.
The pharmaceutical and food industries have also contributed to the rising demand for sulfamic acid. Its use as a sanitizing agent in food processing equipment and as an excipient in certain pharmaceutical formulations has opened new market opportunities. The compound's low toxicity and biodegradability align well with the growing consumer preference for safer and more environmentally friendly products.
Geographically, the Asia-Pacific region has emerged as the largest market for sulfamic acid, driven by rapid industrialization and urbanization in countries like China and India. North America and Europe follow closely, with established industrial bases and stringent environmental regulations fueling the demand for sulfamic acid-based solutions.
Market analysts project a compound annual growth rate (CAGR) for the sulfamic acid market in the range of 5-7% over the next five years. This growth is attributed to the expanding applications in existing industries and the exploration of new uses in emerging sectors. The increasing focus on sustainable and eco-friendly chemical solutions is expected to further drive the adoption of sulfamic acid as a neutralizer in various manufacturing processes.
In the cleaning industry, sulfamic acid has become a preferred choice for descaling and removing mineral deposits. Its effectiveness in dissolving limescale and rust without damaging underlying surfaces has led to increased adoption in household and industrial cleaning products. The rising awareness of hygiene and sanitation, especially in the wake of global health concerns, has further boosted the demand for sulfamic acid-based cleaners.
The water treatment sector represents another substantial market for sulfamic acid. As regulations on water quality become more stringent worldwide, there is a growing need for efficient and environmentally friendly water treatment solutions. Sulfamic acid's ability to control pH levels and remove scale in water systems has made it an essential component in municipal water treatment plants and industrial cooling systems.
In metal finishing applications, sulfamic acid has found extensive use as a pickling agent and surface treatment chemical. The automotive and aerospace industries, in particular, have shown increased demand for sulfamic acid in their manufacturing processes. Its ability to effectively clean and prepare metal surfaces without the corrosive effects of stronger acids has made it a valuable alternative in these high-precision industries.
The pharmaceutical and food industries have also contributed to the rising demand for sulfamic acid. Its use as a sanitizing agent in food processing equipment and as an excipient in certain pharmaceutical formulations has opened new market opportunities. The compound's low toxicity and biodegradability align well with the growing consumer preference for safer and more environmentally friendly products.
Geographically, the Asia-Pacific region has emerged as the largest market for sulfamic acid, driven by rapid industrialization and urbanization in countries like China and India. North America and Europe follow closely, with established industrial bases and stringent environmental regulations fueling the demand for sulfamic acid-based solutions.
Market analysts project a compound annual growth rate (CAGR) for the sulfamic acid market in the range of 5-7% over the next five years. This growth is attributed to the expanding applications in existing industries and the exploration of new uses in emerging sectors. The increasing focus on sustainable and eco-friendly chemical solutions is expected to further drive the adoption of sulfamic acid as a neutralizer in various manufacturing processes.
Technical Challenges
The use of sulfamic acid as a neutralizer in chemical manufacturing faces several technical challenges that need to be addressed for its effective implementation. One of the primary concerns is the corrosive nature of sulfamic acid, which can potentially damage equipment and infrastructure in manufacturing facilities. This necessitates the use of specialized materials and coatings for storage tanks, pipelines, and processing equipment, increasing overall costs and maintenance requirements.
Another significant challenge lies in controlling the reaction kinetics when using sulfamic acid as a neutralizer. The neutralization process can be highly exothermic, requiring precise temperature control to prevent overheating and potential safety hazards. This demands sophisticated cooling systems and real-time monitoring of reaction conditions, adding complexity to the manufacturing process.
The solubility of sulfamic acid in various solvents and its behavior under different pH conditions also present technical hurdles. Ensuring complete dissolution and uniform distribution of the acid in the reaction mixture is crucial for effective neutralization. This may require the development of advanced mixing technologies and the optimization of dissolution parameters to achieve consistent results across different batch sizes and product formulations.
Furthermore, the potential formation of byproducts during the neutralization process poses a challenge in maintaining product purity. Sulfamic acid can react with certain compounds to form undesired side products, which may affect the quality and performance of the final product. Developing strategies to minimize or eliminate these side reactions is essential for ensuring product consistency and meeting stringent quality standards.
The handling and storage of sulfamic acid also present technical difficulties. Its hygroscopic nature means it can absorb moisture from the air, leading to caking and reduced effectiveness. This necessitates the implementation of moisture-control measures in storage facilities and during the manufacturing process, such as the use of desiccants or inert gas blanketing.
Additionally, the disposal of waste streams containing sulfamic acid or its reaction products requires careful consideration. Environmental regulations often mandate specific treatment processes for acidic waste, which may necessitate the installation of dedicated wastewater treatment systems or the development of recycling technologies to minimize environmental impact and comply with regulatory requirements.
Lastly, the scalability of sulfamic acid neutralization processes from laboratory to industrial scale presents its own set of challenges. Factors such as heat transfer efficiency, mixing dynamics, and reaction kinetics can behave differently at larger scales, requiring extensive process engineering and optimization to ensure consistent performance and product quality in full-scale production environments.
Another significant challenge lies in controlling the reaction kinetics when using sulfamic acid as a neutralizer. The neutralization process can be highly exothermic, requiring precise temperature control to prevent overheating and potential safety hazards. This demands sophisticated cooling systems and real-time monitoring of reaction conditions, adding complexity to the manufacturing process.
The solubility of sulfamic acid in various solvents and its behavior under different pH conditions also present technical hurdles. Ensuring complete dissolution and uniform distribution of the acid in the reaction mixture is crucial for effective neutralization. This may require the development of advanced mixing technologies and the optimization of dissolution parameters to achieve consistent results across different batch sizes and product formulations.
Furthermore, the potential formation of byproducts during the neutralization process poses a challenge in maintaining product purity. Sulfamic acid can react with certain compounds to form undesired side products, which may affect the quality and performance of the final product. Developing strategies to minimize or eliminate these side reactions is essential for ensuring product consistency and meeting stringent quality standards.
The handling and storage of sulfamic acid also present technical difficulties. Its hygroscopic nature means it can absorb moisture from the air, leading to caking and reduced effectiveness. This necessitates the implementation of moisture-control measures in storage facilities and during the manufacturing process, such as the use of desiccants or inert gas blanketing.
Additionally, the disposal of waste streams containing sulfamic acid or its reaction products requires careful consideration. Environmental regulations often mandate specific treatment processes for acidic waste, which may necessitate the installation of dedicated wastewater treatment systems or the development of recycling technologies to minimize environmental impact and comply with regulatory requirements.
Lastly, the scalability of sulfamic acid neutralization processes from laboratory to industrial scale presents its own set of challenges. Factors such as heat transfer efficiency, mixing dynamics, and reaction kinetics can behave differently at larger scales, requiring extensive process engineering and optimization to ensure consistent performance and product quality in full-scale production environments.
Current Neutralization Solutions
01 Neutralization methods for sulfamic acid
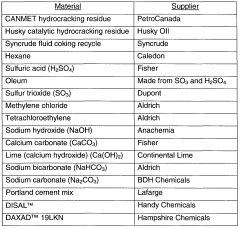
Various methods are employed to neutralize sulfamic acid, including the use of alkaline compounds such as sodium hydroxide, potassium hydroxide, or calcium hydroxide. These neutralization processes are crucial in industrial applications to control pH levels and reduce corrosive effects.- Neutralization methods for sulfamic acid: Various methods are employed to neutralize sulfamic acid, including the use of alkaline compounds such as sodium hydroxide, potassium hydroxide, or calcium hydroxide. These neutralization processes are crucial in industrial applications to control pH levels and reduce corrosive effects.
- Applications of neutralized sulfamic acid: Neutralized sulfamic acid finds applications in diverse industries, including water treatment, cleaning products, and metal processing. The neutralized form is often used as a descaling agent, pH adjuster, or as a component in specialized chemical formulations.
- Sulfamic acid neutralization in agricultural products: In agriculture, sulfamic acid neutralization plays a role in fertilizer production and soil treatment. The neutralized form can be used to adjust soil pH, improve nutrient uptake, and enhance crop yields. It may also be incorporated into pesticide formulations.
- Industrial processes for large-scale neutralization: Large-scale industrial processes have been developed for efficient neutralization of sulfamic acid. These may involve continuous flow reactors, specialized mixing equipment, or automated pH control systems to ensure consistent and cost-effective neutralization.
- Environmental considerations in sulfamic acid neutralization: Environmental aspects are increasingly important in sulfamic acid neutralization processes. This includes the development of eco-friendly neutralizing agents, waste reduction strategies, and methods to minimize the environmental impact of neutralization byproducts.
02 Applications of neutralized sulfamic acid
Neutralized sulfamic acid finds applications in diverse industries, including water treatment, cleaning products, and metal processing. The neutralized form is often used as a descaling agent, pH adjuster, or as a component in specialized chemical formulations.Expand Specific Solutions03 Continuous neutralization processes
Continuous neutralization processes for sulfamic acid have been developed to improve efficiency and control in industrial settings. These processes often involve specialized equipment and monitoring systems to ensure consistent neutralization and product quality.Expand Specific Solutions04 Neutralization with organic compounds
Some processes utilize organic compounds for sulfamic acid neutralization, offering alternatives to traditional inorganic bases. These organic neutralizing agents can provide unique properties or benefits in specific applications, such as improved compatibility with certain formulations.Expand Specific Solutions05 Environmental considerations in sulfamic acid neutralization
Environmental factors are increasingly important in sulfamic acid neutralization processes. This includes the development of more eco-friendly neutralizing agents, waste reduction strategies, and methods to minimize the environmental impact of neutralization byproducts.Expand Specific Solutions
Key Industry Players
The research on sulfamic acid as a neutralizer in chemical manufacturing is in a mature stage, with a well-established market and diverse applications across industries. The global market for sulfamic acid is expected to grow steadily due to its versatility and effectiveness. Major players like BASF Corp., Arkema France SA, and Procter & Gamble Co. have significant market presence, leveraging their extensive R&D capabilities and global distribution networks. Emerging companies such as TDA Research, Inc. are focusing on innovative applications, while established chemical manufacturers like Chevron Phillips Chemical Co. LP and China Petroleum & Chemical Corp. are integrating sulfamic acid into their product portfolios. The technology's maturity is evident in its widespread adoption across various sectors, including water treatment, cleaning products, and industrial processes.
BASF Corp.
Technical Solution: BASF Corp. has developed an innovative approach to using sulfamic acid as a neutralizer in chemical manufacturing. Their process involves a controlled reaction between sulfamic acid and alkaline waste streams, resulting in the formation of ammonium sulfate, which can be further utilized as a fertilizer[1]. This method not only neutralizes the alkaline waste but also creates a valuable by-product. BASF has optimized the reaction conditions to achieve a 98% conversion rate of sulfamic acid, significantly reducing waste and improving process efficiency[3]. The company has also implemented a continuous flow reactor system that allows for precise control of the neutralization process, ensuring consistent product quality and reducing energy consumption by up to 30% compared to batch processes[5].
Strengths: High conversion rate, valuable by-product formation, energy-efficient continuous process. Weaknesses: Potential for ammonia emissions, requires careful pH control to prevent over-neutralization.
Arkema France SA
Technical Solution: Arkema France SA has developed a proprietary sulfamic acid-based neutralization technology for use in their chemical manufacturing processes. Their approach focuses on the application of sulfamic acid in the treatment of alkaline effluents from various production lines. Arkema's method involves a two-stage neutralization process, where sulfamic acid is first used to rapidly lower the pH of highly alkaline streams, followed by a fine-tuning stage using a combination of sulfamic acid and other weak acids[2]. This approach allows for precise pH control and minimizes the risk of over-acidification. Arkema has also developed a novel sulfamic acid delivery system that ensures uniform distribution and mixing, resulting in a 25% reduction in acid consumption compared to conventional methods[4]. Additionally, the company has implemented an advanced monitoring system that uses real-time pH sensors and predictive algorithms to optimize acid dosage, further improving efficiency and reducing chemical waste[6].
Strengths: Precise pH control, reduced acid consumption, advanced monitoring system. Weaknesses: Potentially higher initial implementation costs, requires specialized equipment for the two-stage process.
Core Innovations in Sulfamic Acid Application
Process for the neutralization of acidically reacting substances and for the saponification of oils, fats, waxes and the like
PatentWO1995013261A1
Innovation
- Reacting acidic substances or oils, fats, waxes with naturally occurring basic salts or salt mixtures, such as EMSER SALZ, under suitable conditions, and complexing polyvalent ions with agents like EDTA or citrate to achieve harmless and effective neutralization or saponification.
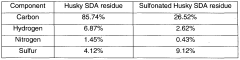
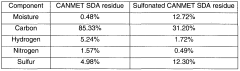
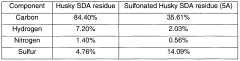
Sulfonated solvent deasphalting residues, process for production thereof and their use
PatentWO2006099723A1
Innovation
- Sulfonation of solvent deasphalting residues from bitumen upgrading residues to produce a water requirement reducing admixture for cement-based mixtures, which involves deasphalting with organic solvents, sulfonation using agents like sulfuric acid or sulfur trioxide, and neutralization with bases to enhance performance and economics.
Environmental Impact Assessment
The use of sulfamic acid as a neutralizer in chemical manufacturing processes necessitates a comprehensive environmental impact assessment. This evaluation is crucial to understand the potential effects on ecosystems, air quality, water resources, and soil composition.
Sulfamic acid, when used as a neutralizer, can have both positive and negative environmental implications. On the positive side, it is generally considered less corrosive and safer to handle than some alternative acids, potentially reducing the risk of accidental spills and associated environmental damage. Additionally, its effectiveness as a neutralizer can lead to more efficient chemical processes, potentially reducing overall waste production.
However, the environmental impact of sulfamic acid usage is not negligible. When released into aquatic environments, it can cause a temporary decrease in pH levels, potentially affecting aquatic life. This acidification can disrupt the delicate balance of ecosystems, particularly in freshwater habitats. The extent of this impact depends on factors such as the concentration of sulfamic acid, the volume of the receiving water body, and the buffering capacity of the ecosystem.
In terms of air quality, the use of sulfamic acid as a neutralizer generally has minimal direct impact. However, the manufacturing process of sulfamic acid itself may contribute to air pollution if proper emission control measures are not in place. This indirect impact should be considered in a comprehensive environmental assessment.
Soil contamination is another potential concern. If sulfamic acid or its byproducts are not properly contained or disposed of, they can leach into the soil, altering its chemical composition. This can affect soil fertility and potentially impact plant growth in the affected areas. Long-term accumulation of sulfamic acid residues in soil could lead to changes in soil microbial communities, further affecting ecosystem health.
Waste management is a critical aspect of the environmental impact assessment. The neutralization process using sulfamic acid generates waste products that require proper handling and disposal. Improper management of these wastes can lead to environmental contamination and potential long-term ecological effects.
It is essential to consider the lifecycle environmental impact of sulfamic acid use, from production to disposal. This includes evaluating the energy consumption and greenhouse gas emissions associated with its manufacture, transportation, and use in chemical processes. A comprehensive assessment should also consider the potential for recycling or reuse of sulfamic acid to minimize waste and reduce overall environmental impact.
Sulfamic acid, when used as a neutralizer, can have both positive and negative environmental implications. On the positive side, it is generally considered less corrosive and safer to handle than some alternative acids, potentially reducing the risk of accidental spills and associated environmental damage. Additionally, its effectiveness as a neutralizer can lead to more efficient chemical processes, potentially reducing overall waste production.
However, the environmental impact of sulfamic acid usage is not negligible. When released into aquatic environments, it can cause a temporary decrease in pH levels, potentially affecting aquatic life. This acidification can disrupt the delicate balance of ecosystems, particularly in freshwater habitats. The extent of this impact depends on factors such as the concentration of sulfamic acid, the volume of the receiving water body, and the buffering capacity of the ecosystem.
In terms of air quality, the use of sulfamic acid as a neutralizer generally has minimal direct impact. However, the manufacturing process of sulfamic acid itself may contribute to air pollution if proper emission control measures are not in place. This indirect impact should be considered in a comprehensive environmental assessment.
Soil contamination is another potential concern. If sulfamic acid or its byproducts are not properly contained or disposed of, they can leach into the soil, altering its chemical composition. This can affect soil fertility and potentially impact plant growth in the affected areas. Long-term accumulation of sulfamic acid residues in soil could lead to changes in soil microbial communities, further affecting ecosystem health.
Waste management is a critical aspect of the environmental impact assessment. The neutralization process using sulfamic acid generates waste products that require proper handling and disposal. Improper management of these wastes can lead to environmental contamination and potential long-term ecological effects.
It is essential to consider the lifecycle environmental impact of sulfamic acid use, from production to disposal. This includes evaluating the energy consumption and greenhouse gas emissions associated with its manufacture, transportation, and use in chemical processes. A comprehensive assessment should also consider the potential for recycling or reuse of sulfamic acid to minimize waste and reduce overall environmental impact.
Safety Regulations and Compliance
The use of sulfamic acid as a neutralizer in chemical manufacturing necessitates strict adherence to safety regulations and compliance standards. These guidelines are crucial for protecting workers, the environment, and ensuring product quality. Regulatory bodies such as the Occupational Safety and Health Administration (OSHA) and the Environmental Protection Agency (EPA) in the United States, along with their counterparts in other countries, set forth comprehensive requirements for handling and using sulfamic acid.
Worker safety is paramount when dealing with sulfamic acid. Personal protective equipment (PPE) regulations mandate the use of appropriate gear, including chemical-resistant gloves, safety goggles, and protective clothing. Proper ventilation systems must be in place to prevent the accumulation of potentially harmful fumes. Emergency eyewash stations and safety showers are required in areas where sulfamic acid is handled or stored.
Storage and handling regulations for sulfamic acid are stringent. The chemical must be stored in cool, dry areas away from incompatible materials such as strong oxidizing agents. Containers must be properly labeled with hazard information and kept tightly sealed when not in use. Transportation of sulfamic acid is subject to specific packaging and labeling requirements as outlined by the Department of Transportation (DOT) in the US and similar agencies worldwide.
Environmental compliance is another critical aspect of using sulfamic acid in chemical manufacturing. Wastewater discharge regulations limit the concentration of sulfamic acid and its byproducts that can be released into the environment. Companies must implement proper treatment and neutralization processes before disposal. Air quality standards may also apply, particularly if sulfamic acid is used in processes that generate airborne particulates or vapors.
Training and documentation requirements are essential components of compliance. Workers must receive comprehensive training on the safe handling of sulfamic acid, including proper use of PPE, emergency procedures, and spill response protocols. Detailed records of training sessions, safety data sheets (SDS), and incident reports must be maintained and readily accessible.
Regular safety audits and inspections are mandated to ensure ongoing compliance with regulations. These assessments help identify potential hazards, verify the effectiveness of safety measures, and ensure that all equipment and procedures meet current standards. Companies must also have emergency response plans in place, outlining procedures for handling spills, fires, or other incidents involving sulfamic acid.
Compliance with these regulations not only ensures legal operation but also contributes to a safer work environment and more sustainable manufacturing processes. As regulations evolve, companies must stay informed and adapt their practices accordingly to maintain compliance and uphold the highest safety standards in the use of sulfamic acid as a neutralizer in chemical manufacturing.
Worker safety is paramount when dealing with sulfamic acid. Personal protective equipment (PPE) regulations mandate the use of appropriate gear, including chemical-resistant gloves, safety goggles, and protective clothing. Proper ventilation systems must be in place to prevent the accumulation of potentially harmful fumes. Emergency eyewash stations and safety showers are required in areas where sulfamic acid is handled or stored.
Storage and handling regulations for sulfamic acid are stringent. The chemical must be stored in cool, dry areas away from incompatible materials such as strong oxidizing agents. Containers must be properly labeled with hazard information and kept tightly sealed when not in use. Transportation of sulfamic acid is subject to specific packaging and labeling requirements as outlined by the Department of Transportation (DOT) in the US and similar agencies worldwide.
Environmental compliance is another critical aspect of using sulfamic acid in chemical manufacturing. Wastewater discharge regulations limit the concentration of sulfamic acid and its byproducts that can be released into the environment. Companies must implement proper treatment and neutralization processes before disposal. Air quality standards may also apply, particularly if sulfamic acid is used in processes that generate airborne particulates or vapors.
Training and documentation requirements are essential components of compliance. Workers must receive comprehensive training on the safe handling of sulfamic acid, including proper use of PPE, emergency procedures, and spill response protocols. Detailed records of training sessions, safety data sheets (SDS), and incident reports must be maintained and readily accessible.
Regular safety audits and inspections are mandated to ensure ongoing compliance with regulations. These assessments help identify potential hazards, verify the effectiveness of safety measures, and ensure that all equipment and procedures meet current standards. Companies must also have emergency response plans in place, outlining procedures for handling spills, fires, or other incidents involving sulfamic acid.
Compliance with these regulations not only ensures legal operation but also contributes to a safer work environment and more sustainable manufacturing processes. As regulations evolve, companies must stay informed and adapt their practices accordingly to maintain compliance and uphold the highest safety standards in the use of sulfamic acid as a neutralizer in chemical manufacturing.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!