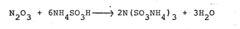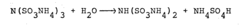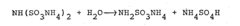Sulfamic Acid as a Precursor for Ammonium Sulfamate Production
JUL 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfamic Acid Background and Objectives
Sulfamic acid, a crystalline compound with the chemical formula NH2SO3H, has been a subject of significant interest in the chemical industry for decades. Its unique properties and versatile applications have made it a valuable precursor in various industrial processes, particularly in the production of ammonium sulfamate. The evolution of sulfamic acid as a key component in chemical synthesis can be traced back to the early 20th century, with its importance growing steadily over time.
The primary objective of researching sulfamic acid as a precursor for ammonium sulfamate production is to optimize the synthesis process, enhance yield, and improve the overall efficiency of the manufacturing chain. This research aims to address the increasing demand for ammonium sulfamate in various sectors, including agriculture, flame retardants, and water treatment. By focusing on sulfamic acid as the starting material, researchers seek to develop more cost-effective and environmentally friendly production methods.
The technical landscape surrounding sulfamic acid has seen significant advancements in recent years. These developments have been driven by the need for more sustainable chemical processes and the growing applications of ammonium sulfamate. The research objectives extend beyond mere production optimization, encompassing the exploration of novel reaction pathways, catalysts, and process conditions that could revolutionize the synthesis of ammonium sulfamate from sulfamic acid.
One of the key trends in this field is the pursuit of green chemistry principles. Researchers are increasingly focusing on developing processes that minimize waste, reduce energy consumption, and utilize safer reagents. This aligns with the broader industry trend towards sustainability and environmental responsibility. The exploration of sulfamic acid as a precursor fits well within this paradigm, as it offers potential advantages in terms of atom economy and process simplicity compared to alternative routes.
Another significant aspect of the research objectives is the investigation of the reaction mechanisms involved in the conversion of sulfamic acid to ammonium sulfamate. Understanding these mechanisms at a molecular level is crucial for designing more efficient catalysts and optimizing reaction conditions. This fundamental research has the potential to unlock new possibilities in the synthesis of not only ammonium sulfamate but also other related compounds.
The technological evolution in this field is expected to have far-reaching implications. Improved production methods for ammonium sulfamate could lead to its increased adoption in various applications, potentially displacing less environmentally friendly alternatives. This research also contributes to the broader goal of developing more sustainable chemical processes, which is a critical challenge facing the industry in the 21st century.
The primary objective of researching sulfamic acid as a precursor for ammonium sulfamate production is to optimize the synthesis process, enhance yield, and improve the overall efficiency of the manufacturing chain. This research aims to address the increasing demand for ammonium sulfamate in various sectors, including agriculture, flame retardants, and water treatment. By focusing on sulfamic acid as the starting material, researchers seek to develop more cost-effective and environmentally friendly production methods.
The technical landscape surrounding sulfamic acid has seen significant advancements in recent years. These developments have been driven by the need for more sustainable chemical processes and the growing applications of ammonium sulfamate. The research objectives extend beyond mere production optimization, encompassing the exploration of novel reaction pathways, catalysts, and process conditions that could revolutionize the synthesis of ammonium sulfamate from sulfamic acid.
One of the key trends in this field is the pursuit of green chemistry principles. Researchers are increasingly focusing on developing processes that minimize waste, reduce energy consumption, and utilize safer reagents. This aligns with the broader industry trend towards sustainability and environmental responsibility. The exploration of sulfamic acid as a precursor fits well within this paradigm, as it offers potential advantages in terms of atom economy and process simplicity compared to alternative routes.
Another significant aspect of the research objectives is the investigation of the reaction mechanisms involved in the conversion of sulfamic acid to ammonium sulfamate. Understanding these mechanisms at a molecular level is crucial for designing more efficient catalysts and optimizing reaction conditions. This fundamental research has the potential to unlock new possibilities in the synthesis of not only ammonium sulfamate but also other related compounds.
The technological evolution in this field is expected to have far-reaching implications. Improved production methods for ammonium sulfamate could lead to its increased adoption in various applications, potentially displacing less environmentally friendly alternatives. This research also contributes to the broader goal of developing more sustainable chemical processes, which is a critical challenge facing the industry in the 21st century.
Market Analysis for Ammonium Sulfamate
The global market for ammonium sulfamate has been experiencing steady growth, driven by its diverse applications across various industries. As a versatile compound, ammonium sulfamate finds extensive use in agriculture, flame retardants, and industrial cleaning products. The agricultural sector remains the primary consumer, utilizing ammonium sulfamate as an effective herbicide and plant growth regulator.
In recent years, the market has witnessed a surge in demand from the flame retardant industry, particularly in the construction and automotive sectors. This growth is attributed to stringent fire safety regulations and increased awareness of fire prevention measures. The compound's ability to inhibit flame spread and reduce smoke generation has made it a preferred choice among manufacturers.
The industrial cleaning segment also contributes significantly to the market growth, with ammonium sulfamate being used in specialized cleaning formulations for metal surfaces and industrial equipment. Its effectiveness in removing scale and rust has led to increased adoption in maintenance operations across various industries.
Geographically, North America and Europe have traditionally been the largest markets for ammonium sulfamate, owing to their well-established agricultural and industrial sectors. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by expanding agricultural activities, industrialization, and increasing fire safety awareness in countries like China and India.
Market analysts project a compound annual growth rate (CAGR) for the global ammonium sulfamate market in the range of 4-6% over the next five years. This growth is expected to be fueled by increasing agricultural productivity demands, stricter fire safety regulations, and the expansion of industrial cleaning applications.
The market landscape is characterized by a mix of established players and new entrants, with ongoing research and development efforts focused on improving production efficiency and exploring novel applications. The use of sulfamic acid as a precursor for ammonium sulfamate production is gaining attention due to its potential to enhance production yields and reduce manufacturing costs.
Challenges facing the market include environmental concerns related to the use of chemical herbicides and the need for sustainable production methods. These factors are driving research into eco-friendly alternatives and more efficient production processes. Additionally, fluctuations in raw material prices and regulatory changes in different regions can impact market dynamics.
In recent years, the market has witnessed a surge in demand from the flame retardant industry, particularly in the construction and automotive sectors. This growth is attributed to stringent fire safety regulations and increased awareness of fire prevention measures. The compound's ability to inhibit flame spread and reduce smoke generation has made it a preferred choice among manufacturers.
The industrial cleaning segment also contributes significantly to the market growth, with ammonium sulfamate being used in specialized cleaning formulations for metal surfaces and industrial equipment. Its effectiveness in removing scale and rust has led to increased adoption in maintenance operations across various industries.
Geographically, North America and Europe have traditionally been the largest markets for ammonium sulfamate, owing to their well-established agricultural and industrial sectors. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by expanding agricultural activities, industrialization, and increasing fire safety awareness in countries like China and India.
Market analysts project a compound annual growth rate (CAGR) for the global ammonium sulfamate market in the range of 4-6% over the next five years. This growth is expected to be fueled by increasing agricultural productivity demands, stricter fire safety regulations, and the expansion of industrial cleaning applications.
The market landscape is characterized by a mix of established players and new entrants, with ongoing research and development efforts focused on improving production efficiency and exploring novel applications. The use of sulfamic acid as a precursor for ammonium sulfamate production is gaining attention due to its potential to enhance production yields and reduce manufacturing costs.
Challenges facing the market include environmental concerns related to the use of chemical herbicides and the need for sustainable production methods. These factors are driving research into eco-friendly alternatives and more efficient production processes. Additionally, fluctuations in raw material prices and regulatory changes in different regions can impact market dynamics.
Technical Challenges in Precursor Conversion
The conversion of sulfamic acid to ammonium sulfamate presents several technical challenges that researchers and manufacturers must overcome. One of the primary difficulties lies in controlling the reaction conditions to ensure high yield and product purity. The process typically involves the neutralization of sulfamic acid with ammonia, but achieving the optimal pH and temperature range for this reaction can be complex.
Maintaining precise temperature control during the conversion process is crucial. Excessive heat can lead to the decomposition of sulfamic acid or the formation of unwanted by-products, while insufficient heat may result in incomplete conversion. The exothermic nature of the neutralization reaction further complicates temperature management, requiring sophisticated cooling systems and precise process control.
Another significant challenge is the handling and storage of raw materials, particularly ammonia. As a corrosive and toxic gas, ammonia requires specialized equipment and safety protocols. The storage and transportation of anhydrous ammonia or concentrated ammonium hydroxide solutions pose additional logistical and safety concerns.
The purity of the sulfamic acid precursor is also a critical factor. Impurities in the starting material can lead to side reactions, reducing the yield and quality of the final ammonium sulfamate product. Developing efficient purification methods for sulfamic acid or sourcing high-purity raw materials is essential for overcoming this challenge.
Scale-up from laboratory to industrial production presents its own set of difficulties. Processes that work well at small scales may encounter unforeseen issues when implemented in large-scale reactors. Heat transfer, mixing efficiency, and reaction kinetics can all be affected by the increased volume, potentially leading to reduced yields or product quality.
Environmental concerns and regulatory compliance add another layer of complexity to the conversion process. The production of ammonium sulfamate must adhere to strict environmental regulations regarding emissions, waste disposal, and worker safety. Developing green chemistry approaches and implementing effective pollution control measures are ongoing challenges in this field.
Lastly, the economic viability of the conversion process remains a significant hurdle. The cost of raw materials, energy requirements, and capital investment in specialized equipment must be balanced against the market value of ammonium sulfamate. Improving process efficiency, reducing energy consumption, and optimizing resource utilization are key areas of focus for making the conversion economically feasible on an industrial scale.
Maintaining precise temperature control during the conversion process is crucial. Excessive heat can lead to the decomposition of sulfamic acid or the formation of unwanted by-products, while insufficient heat may result in incomplete conversion. The exothermic nature of the neutralization reaction further complicates temperature management, requiring sophisticated cooling systems and precise process control.
Another significant challenge is the handling and storage of raw materials, particularly ammonia. As a corrosive and toxic gas, ammonia requires specialized equipment and safety protocols. The storage and transportation of anhydrous ammonia or concentrated ammonium hydroxide solutions pose additional logistical and safety concerns.
The purity of the sulfamic acid precursor is also a critical factor. Impurities in the starting material can lead to side reactions, reducing the yield and quality of the final ammonium sulfamate product. Developing efficient purification methods for sulfamic acid or sourcing high-purity raw materials is essential for overcoming this challenge.
Scale-up from laboratory to industrial production presents its own set of difficulties. Processes that work well at small scales may encounter unforeseen issues when implemented in large-scale reactors. Heat transfer, mixing efficiency, and reaction kinetics can all be affected by the increased volume, potentially leading to reduced yields or product quality.
Environmental concerns and regulatory compliance add another layer of complexity to the conversion process. The production of ammonium sulfamate must adhere to strict environmental regulations regarding emissions, waste disposal, and worker safety. Developing green chemistry approaches and implementing effective pollution control measures are ongoing challenges in this field.
Lastly, the economic viability of the conversion process remains a significant hurdle. The cost of raw materials, energy requirements, and capital investment in specialized equipment must be balanced against the market value of ammonium sulfamate. Improving process efficiency, reducing energy consumption, and optimizing resource utilization are key areas of focus for making the conversion economically feasible on an industrial scale.
Current Production Methods Overview
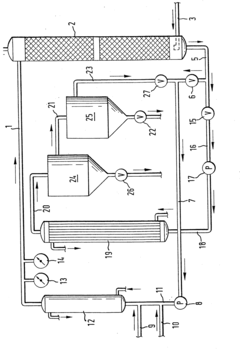
01 Synthesis and production of sulfamic acid
Various methods for synthesizing and producing sulfamic acid are described. These processes often involve reactions between sulfur-containing compounds and ammonia or other nitrogen sources. The production methods aim to improve yield, purity, and efficiency in industrial-scale manufacturing of sulfamic acid.- Synthesis and production of sulfamic acid: Various methods for synthesizing and producing sulfamic acid are described. These processes often involve reactions between sulfur-containing compounds and ammonia or other nitrogen sources. The production methods aim to improve yield, purity, and efficiency in industrial-scale manufacturing of sulfamic acid.
- Applications in cleaning and descaling: Sulfamic acid is widely used in cleaning and descaling formulations. It is effective in removing mineral deposits, rust, and other stubborn stains from various surfaces. These applications often involve combining sulfamic acid with other ingredients to enhance its cleaning power and reduce potential corrosion.
- Use in water treatment and purification: Sulfamic acid plays a role in water treatment and purification processes. It can be used to adjust pH levels, remove scale buildup in water systems, and as a component in water treatment chemicals. These applications aim to improve water quality and maintain the efficiency of water-related equipment.
- Agricultural and horticultural applications: Sulfamic acid finds use in agricultural and horticultural settings. It can be employed as a component in fertilizers, soil conditioners, or plant growth regulators. These applications leverage the acid's properties to enhance nutrient availability or adjust soil conditions for optimal plant growth.
- Industrial and manufacturing processes: Sulfamic acid is utilized in various industrial and manufacturing processes. It can serve as a catalyst, reagent, or intermediate in chemical reactions. Applications include metal processing, polymer production, and as a component in specialized industrial formulations.
02 Applications in cleaning and descaling
Sulfamic acid is widely used in cleaning and descaling formulations. It is effective in removing mineral deposits, rust, and other stubborn stains from various surfaces. These applications often involve combining sulfamic acid with other ingredients to enhance its cleaning power and tailor it for specific uses.Expand Specific Solutions03 Use in water treatment and purification
Sulfamic acid plays a role in water treatment and purification processes. It can be used for pH adjustment, scale prevention, and as a component in water treatment chemicals. The applications extend to both industrial and municipal water treatment systems.Expand Specific Solutions04 Agricultural and horticultural applications
Sulfamic acid finds use in agricultural and horticultural settings. It can be employed as a component in fertilizers, soil conditioners, and plant growth regulators. These applications leverage the acid's properties to improve soil quality and enhance plant growth.Expand Specific Solutions05 Industrial processing and manufacturing
Sulfamic acid is utilized in various industrial processes and manufacturing applications. It serves as a raw material or intermediate in the production of other chemicals, dyes, and materials. The acid's properties make it valuable in metal processing, textile manufacturing, and other industrial sectors.Expand Specific Solutions
Key Industry Players and Competitors
The research on sulfamic acid as a precursor for ammonium sulfamate production is in a developing stage, with growing market potential due to increasing applications in various industries. The market size is expanding, driven by demand in sectors such as agriculture, water treatment, and chemical manufacturing. Technologically, the field is moderately mature, with established players like Procter & Gamble, Arkema France, and Cargill leading research efforts. Academic institutions such as Zhejiang University and East China Normal University are contributing to advancements. Companies like SK Innovation and thyssenkrupp Industrial Solutions are also actively involved, indicating a competitive landscape with both industrial and academic players striving for innovation in this niche chemical sector.
Arkema France SA
Technical Solution: Arkema France SA has developed an innovative process for the production of ammonium sulfamate using sulfamic acid as a precursor. Their method involves a controlled reaction between sulfamic acid and ammonia in an aqueous medium under specific temperature and pressure conditions. The process utilizes a proprietary catalyst system that enhances the reaction efficiency and selectivity[1]. Arkema's approach also incorporates a novel purification step, employing advanced membrane technology to remove impurities and achieve high-purity ammonium sulfamate[3]. The company has optimized the reaction parameters to maximize yield while minimizing energy consumption, resulting in a more sustainable production process[5].
Strengths: High purity product, improved reaction efficiency, and reduced environmental impact. Weaknesses: Potentially higher production costs due to specialized equipment and catalyst requirements.
thyssenkrupp Industrial Solutions AG
Technical Solution: thyssenkrupp Industrial Solutions AG has developed a large-scale continuous process for ammonium sulfamate production using sulfamic acid. Their technology employs a series of specially designed reactors that allow for precise control of reaction conditions. The process begins with the dissolution of sulfamic acid in water, followed by controlled addition of ammonia gas. A unique mixing system ensures uniform distribution of reactants, improving conversion rates[2]. The company has also implemented an advanced heat recovery system, significantly reducing energy consumption. Additionally, thyssenkrupp's process incorporates in-line quality control measures, utilizing spectroscopic techniques for real-time monitoring of product composition[4].
Strengths: Scalable continuous process, energy-efficient, and consistent product quality. Weaknesses: High initial capital investment and complexity in process control.
Innovative Approaches in Precursor Utilization
Processes for producing ammonium sulfamate and ammonium nitriletrisulfonate
PatentInactiveEP0016430A1
Innovation
- A process involving the production of an ammonium sulfite solution with a pH between 5 and 7.5, conversion to ammonium nitrilotrisulfonate, and subsequent reaction with ammonia at elevated temperature and pressure below 30°C to form ammonium sulfamate, while maintaining low water content and using crystallizers to optimize crystallization conditions.
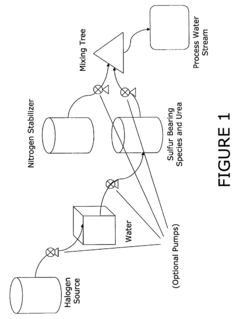
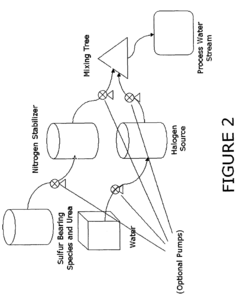
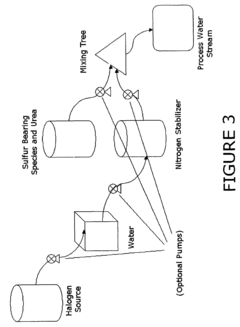
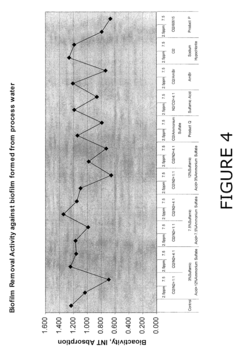
Use of sulfamic acid or its salts as stabilizers especially in combination with ammonium salt and/or ammine for bleach or other halogen containing biocides in the paper area
PatentActiveUS9265259B2
Innovation
- A composition comprising a halogen source, urea, and an additional halogen stabilizer, optionally with an alkali to maintain a pH greater than 10, which includes a mixture of sulfur-bearing species like sulfamic acid and nitrogen stabilizers, such as ammonium sulfate, to enhance the persistence and compatibility of halogen biocides in papermaking processes.
Environmental Impact Assessment
The production of ammonium sulfamate using sulfamic acid as a precursor has significant environmental implications that require careful assessment. The process involves the reaction of sulfamic acid with ammonia, which can potentially lead to various environmental impacts throughout the production cycle.
Air quality is a primary concern in this production process. The release of ammonia gas during the reaction can contribute to air pollution if not properly controlled. Ammonia is a respiratory irritant and can form particulate matter in the atmosphere, affecting local air quality and potentially contributing to smog formation. Additionally, the production and handling of sulfamic acid may release sulfur dioxide, another air pollutant that can cause respiratory issues and contribute to acid rain.
Water resources are also at risk from this production process. The use of sulfamic acid and the potential for spills or improper disposal of waste products can lead to water contamination. Sulfamic acid is highly soluble in water and can lower the pH of aquatic environments, potentially harming aquatic ecosystems. Furthermore, the release of ammonium ions into water bodies can contribute to eutrophication, leading to algal blooms and oxygen depletion in aquatic systems.
Soil contamination is another environmental concern associated with ammonium sulfamate production. Accidental spills or improper storage of raw materials and finished products can lead to soil acidification, affecting plant growth and soil microbial communities. The persistence of ammonium sulfamate in soil can also impact soil fertility and potentially enter the food chain through plant uptake.
Energy consumption and greenhouse gas emissions are important factors to consider in the environmental impact assessment. The production process requires energy for heating, mixing, and purification steps, which may contribute to carbon dioxide emissions depending on the energy source used. The transportation of raw materials and finished products also adds to the overall carbon footprint of ammonium sulfamate production.
Waste management is a critical aspect of the environmental impact assessment. The production process may generate solid and liquid waste streams that require proper treatment and disposal. Improper handling of these wastes can lead to soil and water contamination, as well as potential health risks for workers and nearby communities.
To mitigate these environmental impacts, several measures can be implemented. These include the use of closed-loop systems to minimize ammonia emissions, installation of scrubbers and filters to reduce air pollutants, implementation of wastewater treatment systems, and adoption of spill prevention and containment measures. Additionally, optimizing the production process to improve energy efficiency and reduce waste generation can significantly lower the overall environmental footprint of ammonium sulfamate production.
Air quality is a primary concern in this production process. The release of ammonia gas during the reaction can contribute to air pollution if not properly controlled. Ammonia is a respiratory irritant and can form particulate matter in the atmosphere, affecting local air quality and potentially contributing to smog formation. Additionally, the production and handling of sulfamic acid may release sulfur dioxide, another air pollutant that can cause respiratory issues and contribute to acid rain.
Water resources are also at risk from this production process. The use of sulfamic acid and the potential for spills or improper disposal of waste products can lead to water contamination. Sulfamic acid is highly soluble in water and can lower the pH of aquatic environments, potentially harming aquatic ecosystems. Furthermore, the release of ammonium ions into water bodies can contribute to eutrophication, leading to algal blooms and oxygen depletion in aquatic systems.
Soil contamination is another environmental concern associated with ammonium sulfamate production. Accidental spills or improper storage of raw materials and finished products can lead to soil acidification, affecting plant growth and soil microbial communities. The persistence of ammonium sulfamate in soil can also impact soil fertility and potentially enter the food chain through plant uptake.
Energy consumption and greenhouse gas emissions are important factors to consider in the environmental impact assessment. The production process requires energy for heating, mixing, and purification steps, which may contribute to carbon dioxide emissions depending on the energy source used. The transportation of raw materials and finished products also adds to the overall carbon footprint of ammonium sulfamate production.
Waste management is a critical aspect of the environmental impact assessment. The production process may generate solid and liquid waste streams that require proper treatment and disposal. Improper handling of these wastes can lead to soil and water contamination, as well as potential health risks for workers and nearby communities.
To mitigate these environmental impacts, several measures can be implemented. These include the use of closed-loop systems to minimize ammonia emissions, installation of scrubbers and filters to reduce air pollutants, implementation of wastewater treatment systems, and adoption of spill prevention and containment measures. Additionally, optimizing the production process to improve energy efficiency and reduce waste generation can significantly lower the overall environmental footprint of ammonium sulfamate production.
Regulatory Framework for Chemical Production
The regulatory framework for chemical production plays a crucial role in ensuring the safety, environmental protection, and quality standards of chemical manufacturing processes. In the context of sulfamic acid as a precursor for ammonium sulfamate production, several key regulations and guidelines must be considered.
At the international level, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to hazard communication. This system is particularly relevant for sulfamic acid and ammonium sulfamate, as it ensures consistent safety information across borders.
In the United States, the Environmental Protection Agency (EPA) regulates chemical production under the Toxic Substances Control Act (TSCA). The TSCA inventory listing is essential for both sulfamic acid and ammonium sulfamate. Additionally, the Occupational Safety and Health Administration (OSHA) sets workplace safety standards, including permissible exposure limits for these chemicals.
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation is another significant framework. It requires manufacturers and importers to register chemicals and provide safety data. For sulfamic acid and ammonium sulfamate production, compliance with REACH is mandatory for companies operating in or exporting to the EU.
Specific to the production process, Good Manufacturing Practices (GMP) guidelines are crucial. These guidelines, often industry-specific, ensure consistent quality and safety in chemical production. For ammonium sulfamate, which has applications in agriculture, adherence to agricultural chemical regulations is also necessary.
Environmental regulations are equally important. The Clean Air Act and Clean Water Act in the US, and similar legislation in other countries, set limits on emissions and effluents from chemical production facilities. Waste management regulations, such as the Resource Conservation and Recovery Act (RCRA), govern the disposal of chemical byproducts.
Transportation of sulfamic acid and ammonium sulfamate is regulated by agencies like the Department of Transportation (DOT) in the US and the International Maritime Dangerous Goods (IMDG) Code for international shipping. These regulations dictate packaging, labeling, and handling requirements during transport.
Compliance with these regulatory frameworks is not only a legal requirement but also essential for maintaining public trust and ensuring sustainable business practices in the chemical industry. As regulations evolve, ongoing monitoring and adaptation of production processes are necessary to maintain compliance and optimize safety and environmental performance.
At the international level, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to hazard communication. This system is particularly relevant for sulfamic acid and ammonium sulfamate, as it ensures consistent safety information across borders.
In the United States, the Environmental Protection Agency (EPA) regulates chemical production under the Toxic Substances Control Act (TSCA). The TSCA inventory listing is essential for both sulfamic acid and ammonium sulfamate. Additionally, the Occupational Safety and Health Administration (OSHA) sets workplace safety standards, including permissible exposure limits for these chemicals.
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation is another significant framework. It requires manufacturers and importers to register chemicals and provide safety data. For sulfamic acid and ammonium sulfamate production, compliance with REACH is mandatory for companies operating in or exporting to the EU.
Specific to the production process, Good Manufacturing Practices (GMP) guidelines are crucial. These guidelines, often industry-specific, ensure consistent quality and safety in chemical production. For ammonium sulfamate, which has applications in agriculture, adherence to agricultural chemical regulations is also necessary.
Environmental regulations are equally important. The Clean Air Act and Clean Water Act in the US, and similar legislation in other countries, set limits on emissions and effluents from chemical production facilities. Waste management regulations, such as the Resource Conservation and Recovery Act (RCRA), govern the disposal of chemical byproducts.
Transportation of sulfamic acid and ammonium sulfamate is regulated by agencies like the Department of Transportation (DOT) in the US and the International Maritime Dangerous Goods (IMDG) Code for international shipping. These regulations dictate packaging, labeling, and handling requirements during transport.
Compliance with these regulatory frameworks is not only a legal requirement but also essential for maintaining public trust and ensuring sustainable business practices in the chemical industry. As regulations evolve, ongoing monitoring and adaptation of production processes are necessary to maintain compliance and optimize safety and environmental performance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!